Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation?
Abstract
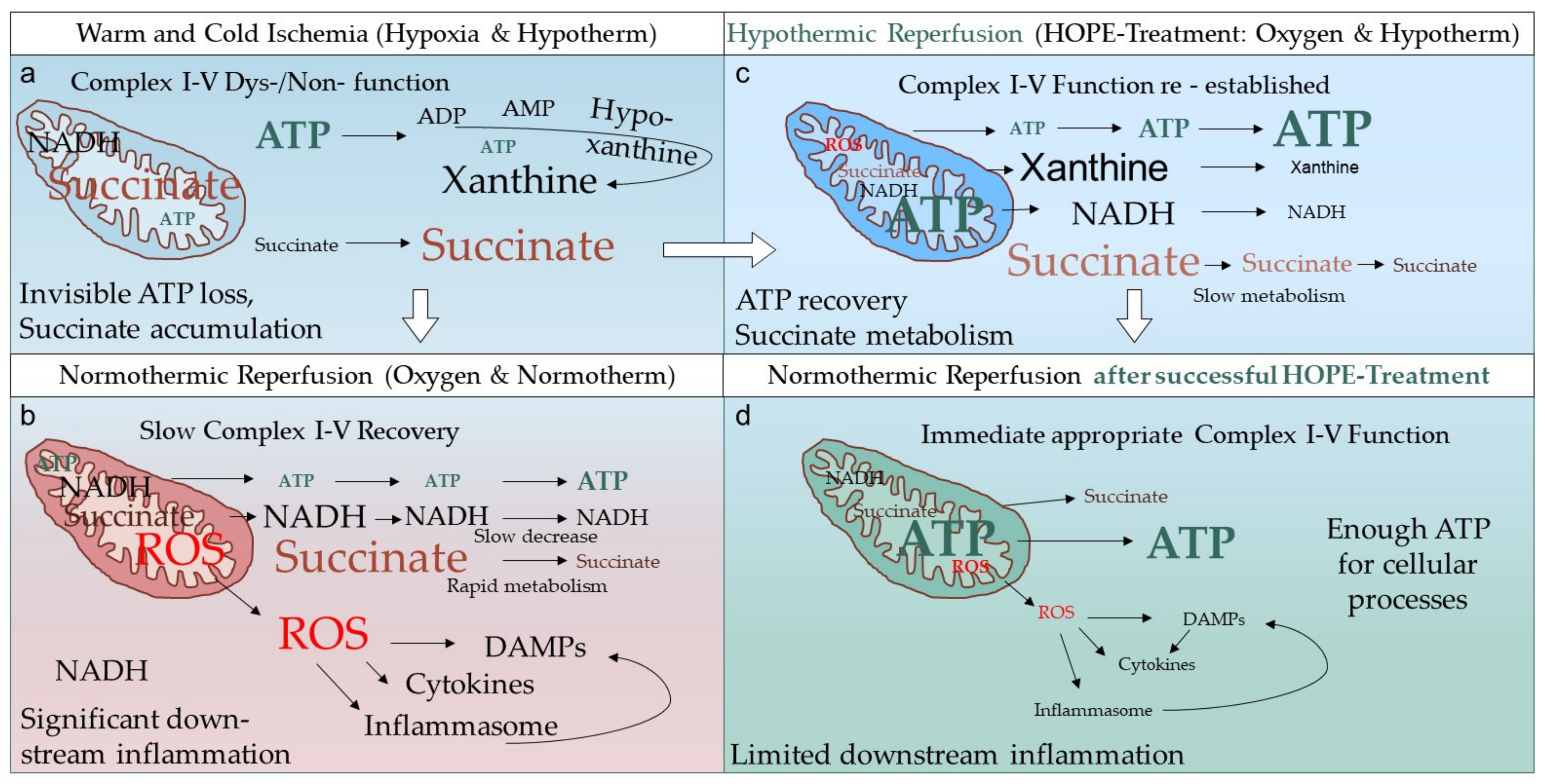
1. Introduction
2. What Is the Real HOPE-Effect in Solid Organ Transplantation?
3. What Do We Know from Clinical Studies with Hypothermic Liver Perfusion?
4. Where Do We Need More Evidence?
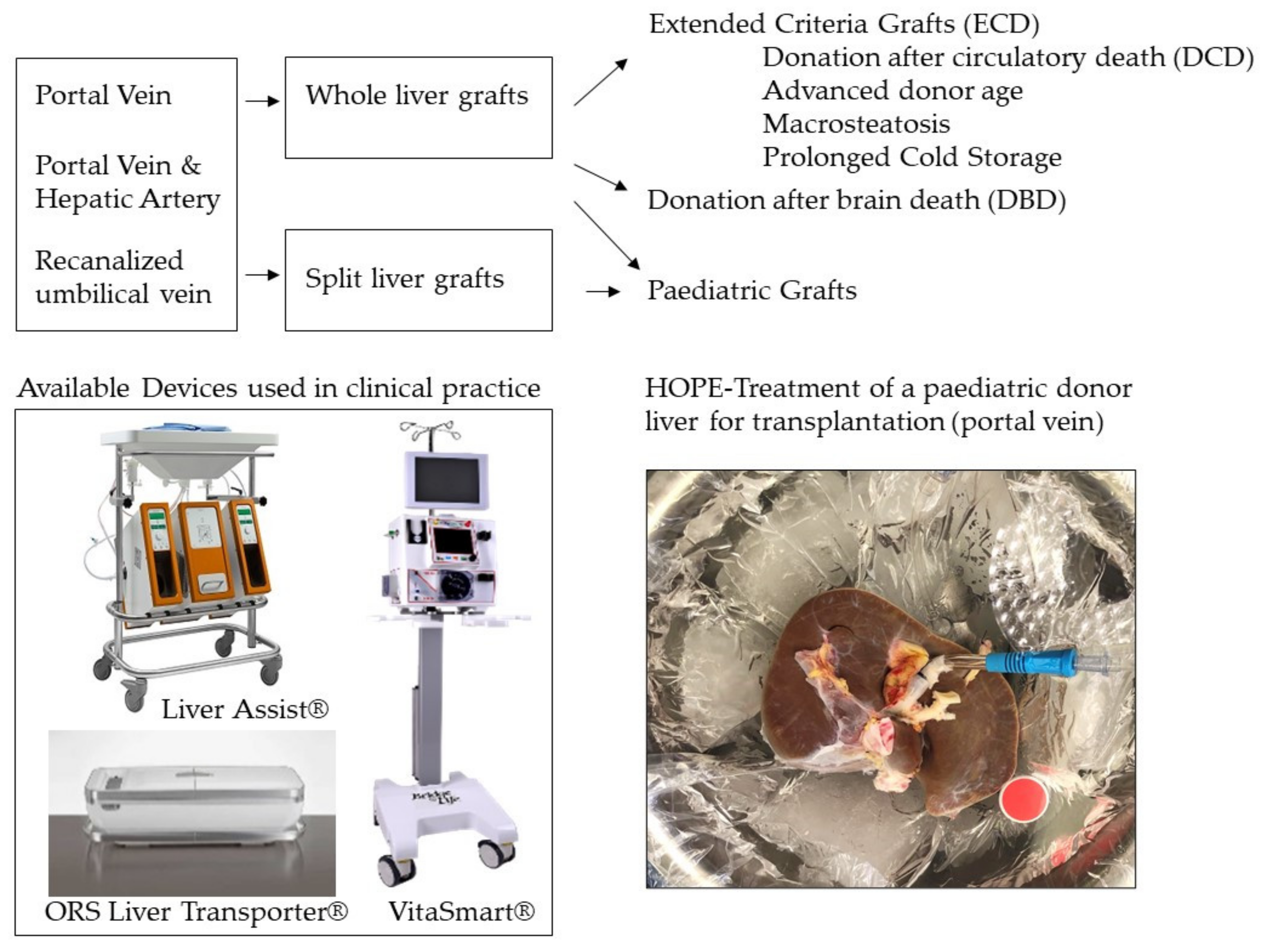
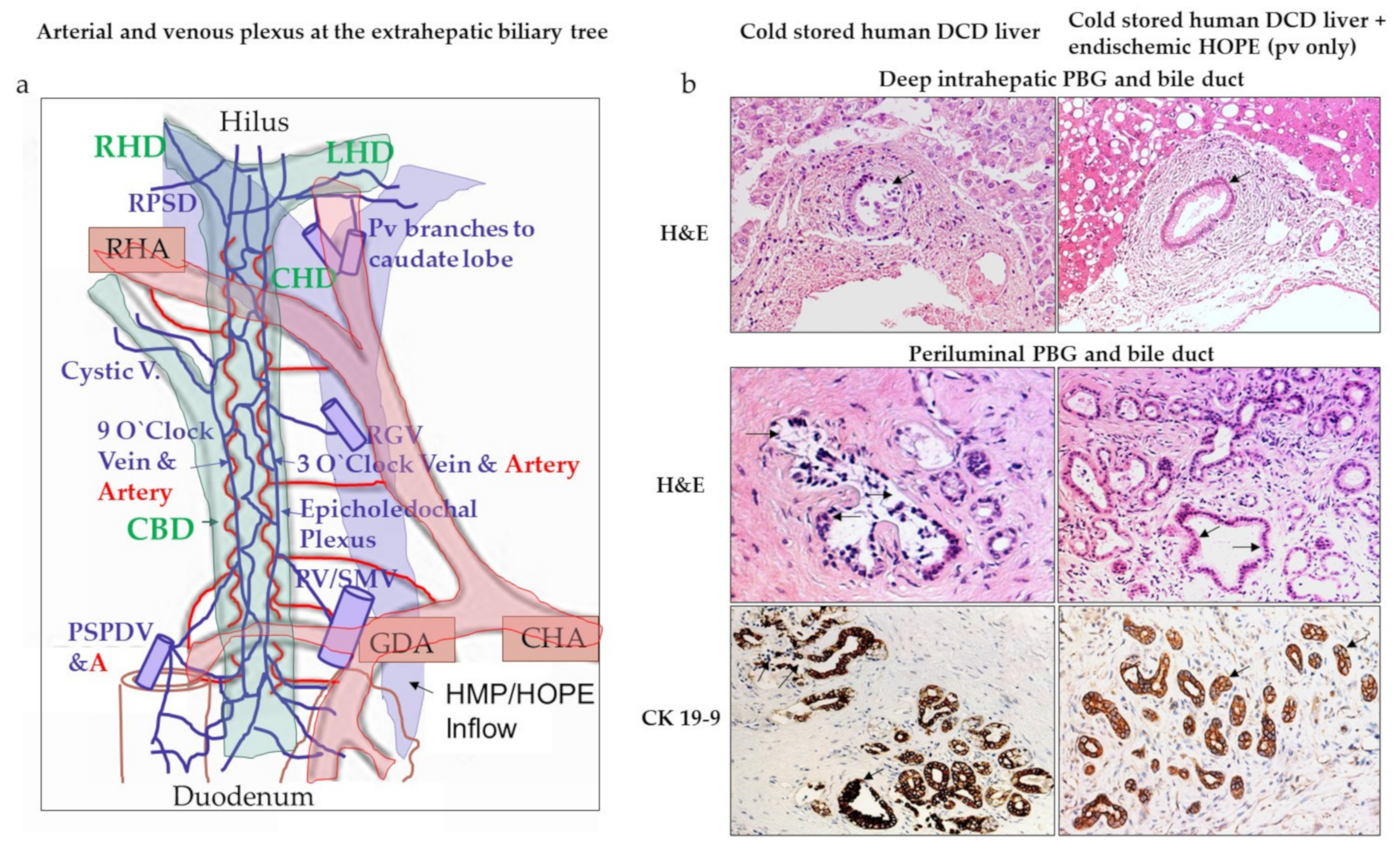
4.1. Do We Need to Perfuse through Portal Vein and Hepatic Artery?
4.2. Do We Really Need a Transportable Device for Hypothermic Perfusion?
4.3. How Much Cold Storage Time Can We Afford before Hypothermic Liver Perfusion?
4.4. Is FMN the Best Mitochondrial Parameter to Predict Liver Function?
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jameel, N.M.; Thirunavukkarasu, C.; Murase, N.; Cascio, M.; Prelich, J.; Yang, S.; Harvey, S.A.K.; Gandhi, C.R. Constitutive release of powerful antioxidant-scavenging activity by hepatic stellate cells: Protection of hepatocytes from ischemia/reperfusion injury. Liver Transpl. 2010, 16, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lindell, S.L.; Klahn, S.L.; Piazza, T.M.; Mangino, M.J.; Torrealba, J.R.; Southard, J.H.; Carey, H.V. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G473–G480. [Google Scholar] [CrossRef]
- Dutkowski, P.; Clavien, P. Uploading cellular batteries: Caring for mitochondria is key. Liver Transpl. 2018, 24, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020. [Google Scholar] [CrossRef] [PubMed]
- Speijer, D. Being right on Q: Shaping eukaryotic evolution. Biochem. J. 2016. [Google Scholar] [CrossRef]
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019. [Google Scholar] [CrossRef]
- Kim, M.; Stepanova, A.; Niatsetskaya, Z.; Sosunov, S.; Arndt, S.; Murphy, M.P.; Galkin, A.; Ten, V.S. Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury. Free Radic. Biol. Med. 2018. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Burlage, L.C.; Karimian, N.; Westerkamp, A.C.; Visser, N.; Matton, A.P.M.; van Rijn, R.; Adelmeijer, J.; Wiersema-Buist, J.; Gouw, A.S.H.; Lisman, T.; et al. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB 2017, 19, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Rougemont ODe Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Schlegel, A.; Dutkowski, P. Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. Int. J. Mol. Sci. 2018, 19, 3567. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.-A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shen XDa Gao, F.; Zhao, A.; Freitas, M.C.; Lassman, C.; Luster, A.D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology 2008. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Martin, J.; Costa, A.; Gruszczyk, A.; Beach, T.; Allen, F.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef]
- Saeb-Parsy, K.; Martin, J.L.; Summers, D.M.; Watson, C.J.E.; Krieg, T.; Murphy, M.P. Mitochondria as Therapeutic Targets in Transplantation. Trends. Mol. Med. 2021, 27, 185–198. [Google Scholar] [CrossRef]
- Berman, D.I.; Bulakhova, N.A.; Meshcheryakova, E.N. The Siberian wood frog survives for months underwater without oxygen. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Boteon, Y.; Laing, R.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.H.; Hübscher, S.; Perera, M.T.P.R.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018, 24, 1699–1715. [Google Scholar] [CrossRef]
- Van Golen, R.F.; Van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402. [Google Scholar] [CrossRef]
- Longatto Boteon, Y.; Schlegel, A.; Laing, R.; Attard, J.; Bhogal, R.; Wallace, L.; Reynolds, G.; Mirza, D.; Mergental, H.; Afford, S.; et al. Combination of hypothermic oxygenated machine perfusion followed by normothermic machine perfusion optimises the reconditioning of marginal human donor livers. HPB 2018. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transpl. 2020. [Google Scholar] [CrossRef]
- Wyss, R.; Méndez Carmona, N.; Arnold, M.; Segiser, A.; Mueller, M.; Dutkowski, P.; Carrel, T.P.; Longnus, S.L. Hypothermic, oxygenated perfusion (HOPE) provides cardioprotection via succinate oxidation prior to normothermic perfusion in a rat model of donation after circulatory death (DCD). Am. J. Transpl. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.; Afford, S.; Mergental, H. Pushing the Limits: Machine Preservation of the Liver as a Tool to Recondition High-Risk Grafts. Curr. Transpl. Rep. 2018, 5, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.; Mergental, H.; Yap, C.; Kirkham, A.; Whilku, M.; Barton, D.; Curbishley, S.; Boteon, Y.L.; Neil, D.A.; Hübscher, S.G.; et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: Study protocol for an open-label, non-randomised, prospective, single-arm trial. BMJ Open 2017. [Google Scholar] [CrossRef]
- Panconesi, R.; Flores Carvalho, M.; Mueller, M.; Meierhofer, D.; Dutkowski, P.; Muiesan, P.; Schlegel, A. Viability Assessment in Liver Transplantation—What Is the Impact of Dynamic Organ Preservation? Biomedicines 2021, 9, 161. [Google Scholar] [CrossRef]
- Watson, C.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transpl. 2018. [Google Scholar] [CrossRef]
- Van Leeuwen, O.B.; De Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.N.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.M.; Reyntjens, K.M.E.; van den Berg, A.P.; de Boer, M.T.; et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- A nd normothermic machine perfusion: A prospective clinical trial. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Matton, A.P.M.; de Vries, Y.; Burlage, L.C.; van Rijn, R.; Fujiyoshi, M.; de Meijer, V.E.; de Boer, M.T.; de Kleine, R.H.J.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, pH, and Glucose Are Suitable Biomarkers of Biliary Viability during Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transpl. Rep. 2018. [Google Scholar] [CrossRef]
- Stepanova, A.; Sosunov, S.; Niatsetskaya, Z.; Konrad, C.; Starkov, A.; Manfredi, G.; Wittig, I.; Ten, V.; Galkin, A. Redox-Dependent Loss of Flavin by Mitochondrial Complex I in Brain Ischemia/Reperfusion Injury. Antioxid Redox Signal 2019, 20, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.-A.; Dutkowski, P. Novel real time prediction of liver graft function during hypothermic oxygenated machine perfusion prior to liver transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.E.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin mononucleotide as a biomarker of organ quality—A pilot study. Transpl. Direct. 2020. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S.; et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transpl. 2010, 10, 372–381. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Reznik, E.; Musat, C.; Lukose, T.I.; Ratner, L.E.; Brown, R.S.; Kato, T.; Emond, J.C. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am. J. Transpl. 2015, 15, 161–169. [Google Scholar] [CrossRef]
- Van Rijn, R.; Karimian, N.; Matton, A.; Burlage, L.; Wetserkamp, A.; Van den Berg, A.; de Kleine, R.H.J.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 907–917. [Google Scholar] [CrossRef]
- Dutkowski, P.; Schlegel, A.; De Oliveira, M.; Müllhaupt, B.; Neff, F.; Clavien, P.A. HOPE for human liver grafts obtained from donors after cardiac death. J. Hepatol. 2014, 60, 765–772. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhang, M.; Ma, Z.; Wu, S. Hypothermic machine perfusion reduces the incidences of early allograft dysfunction and biliary complications and improves 1-year graft survival after human liver transplantation: A meta-analysis. Medicine 2019, 98, e16033. [Google Scholar] [CrossRef]
- Van Rijn, R.; Schurink, I.; de Vries, Y.; van den Berg, A.; Cortes Cerisuelo, M.; Murad, S.D.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Surra, A.; Catalano, G.; Rizza, G.; Berchialla, P.; Martini, S.; Tandoi, F.; Lupo, F.; Mirabella, S.; Stratta, C.; et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci. Rep. 2019, 27, 9. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Catalano, G.; Rizza, G.; Lavorato, N.; Berchialla, P.; Gambella, A.; Caropreso, P.; Mengozzi, G.; Romagnoli, R. Perfusate Analysis during Dual Hypothermic Oxygenated Machine Perfusion of Liver Grafts: Correlations with Donor Factors and Early Outcomes. Transplantation 2020. [Google Scholar] [CrossRef]
- Werner, M.J.M.; van Leeuwen, O.B.; de Jong, I.E.M.; Bodewes, F.A.J.A.; Fujiyoshi, M.; Luhker, O.C.; Scheenstra, R.; de Vries, Y.; de Kleine, R.H.J.; Porte, R.J. First report of successful transplantation of a pediatric donor liver graft after hypothermic machine perfusion. Pediatr Transpl. 2019, 23. [Google Scholar] [CrossRef] [PubMed]
- Cussa, D.; Patrono, D.; Catalano, G.; Rizza, G.; Catalano, S.; Gambella, A.; Tandoi, F.; Romagnoli, R. Use of Dual Hypothermic Oxygenated Machine Perfusion to Recover Extended Criteria Pediatric Liver Grafts. Liver Transpl. 2020, 26, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Thorne, A.M.; Lantinga, V.; Bodewes, S.; de Kleine, R.H.J.; Nijkamp, M.W.; Sprakel, J.; Hartog, H.; Polak, W.G.; Porte, R.J.; de Meijer, V.E. Ex Situ Dual Hypothermic Oxygenated Machine Perfusion for Human Split Liver Transplantation. Transpl. Direct 2021, 7, e666. [Google Scholar] [CrossRef]
- Jia, J.J.; Xie, H.Y.; Li, J.H.; He, Y.; Jiang, L.; He, N.; Zhou, L.; Wang, W.; Zheng, S.-S. Graft protection of the liver by hypothermic machine perfusion involves recovery of graft regeneration in rats. J. Int. Med. Res. 2019, 47, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, O.B.; Fujiyoshi, M.; Ubbink, R.; Werner, M.J.M.; Brüggenwirth, I.M.A.; Porte, R.J.; de Meijer, V.E. Ex Situ Machine Perfusion of Human Donor Livers via the Surgically Reopened Umbilical Vein: A Proof of Concept. Transplantation 2019, 103, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Schlegel, A.; Frassoni, S.; Olivieri, T.; Ravaioli, M.; Camagni, S.; Patrono, D.; Bassi, D.; Pagano, D.; Di Sandro, S.; et al. How to Preserve Liver Grafts From Circulatory Death With Long Warm Ischemia? A Retrospective Italian Cohort Study with Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation 2021. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Mathé, Z.; Gallinat, A.; Canbay, A.C.; Treckmann, J.W.; Rauen, U.; Paul, A.; Minor, T. Controlled Oxygenated Rewarming of Cold Stored Livers Prior to Transplantation: First Clinical Application of a New Concept. Transplantation 2016, 100, 147–152. [Google Scholar] [CrossRef]
- De Vries, Y.; Matton, A.P.M.; Nijsten, M.W.N.; Werner, M.J.M.; van den Berg, A.P.; de Boer, M.T.; Buis, C.I.; Fujiyoshi, M.; de Kleine, R.H.J.; van Leeuwen, O.B.; et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am. J. Transpl. 2019. [Google Scholar] [CrossRef]
- Karangwa, S.; Panayotova, G.; Dutkowski, P.; Porte, R.J.; Guarrera, J.V.; Schlegel, A. Hypothermic Machine Perfusion in Liver Transplantation. Int. J. Surg. 2020, 28, S17. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Hoso, M.; Sanzen, T.; Sasaki, M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc. Res. Tech. 1997. [Google Scholar] [CrossRef]
- Ramesh Babu, C.S.; Sharma, M. Biliary tract anatomy and its relationship with venous drainage. J. Clin. Exp. Hepatol. 2014, 4. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; De Oliveira, M.L.; Clavien, P.A.; Dutkowski, P. Is single portal vein approach sufficient for hypothermic machine perfusion of DCD liver grafts? J. Hepatol. 2016, 64, 239–241. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.R.; Isaac, J.R.; Clavien, P.-A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, G.; Cutler, Q.; Paterno, F.; McCarty, M.; Bailey, A.; Dikdan, G.; Rosado, J.; Schlegel, A.; Dutkowski, P.; Shah, S.; et al. A Novel Biomarker to Predict Ischemia/Reperfusion Injury after Hypothermic Oxygenated Machine Preservation in Human Liver Transplants. ASTS 2019, 20, 78. [Google Scholar]
- Koetting, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation in a large animal model. Transplantation 2011. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, I.M.A.; van Leeuwen, O.B.; de Vries, Y.; Bodewes, S.B.; Adelmeijer, J.; Wiersema-Buist, J.; Lisman, T.; Martins, P.N.; de Meijer, V.E.; Porte, R.J. Extended hypothermic oxygenated machine perfusion enables ex situ preservation of porcine and human livers for up to 24 h. JHEP Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Marcon, F.; Schlegel, A.; Bartlett, D.C.; Kalisvaart, M.; Bishop, D.; Mergental, H.; Roberts, K.J.; Mirza, D.F.; Isaac, J.; Muiesan, P.; et al. Utilisation of declined liver grafts yields comparable transplant outcomes and previous decline should not be a deterrent to graft use. Transplantation 2018, 102, e211–e218. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; De pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic oxygenated new Machine perfusion System in Liver and Kidney transplantation of extended criteria Donors: First italian clinical trial. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Akhtar, M.Z.; Nasralla, D.; Kocabayoglu, P.; Boffa, C.; Kaisar, M.; Brat, A.; O’Callaghan, J.; Pengel, L.H.; Knight, S.; et al. Past, Present, and Future of Dynamic Kidney and Liver Preservation and Resuscitation. Am. J. Transpl. 2016, 16, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.J.; Efremov, R.G.; Nakamaru-Ogiso, E.; Sazanov, L.A. Reversible FMN dissociation from Escherichia coli respiratory complex I. Biochim. Biophys. Acta Bioenerg. 2016. [Google Scholar] [CrossRef]
- Oi, K.; Davies, W.; Tazelaar, H.; Bailey, K.; Federspiel, M.; Russell, S.; McGregor, C.G.A. Ex vivo hypothermic recirculatory adenoviral gene transfer to the transplanted pig heart. J. Gene Med. 2006, 8, 795–803. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Number and Type of Livers | Duration of Cold Storage before HOPE (min or h) | Duration of HMP (min or h) | Device Transport (Yes/No) | Type of Device | HMP through Portal Vein (PV) or Dually (D) | Main Study Findings |
|---|---|---|---|---|---|---|---|
| Van Rijn et al., 2021, RCT, multicenter | DCD livers, n = 78 | 6 h 11 min (IQR: 5 h 16 min–6 h 55 min) | 2 h 12 min (IQR: 2 h–2 h 33 min) | No | Liver Assist® | D | D-HOPE protects from ischemic cholangiopathy, interventions at the biliary tree and early allograft dysfunction. Same anastomotic stricture rate between D-HOPE group and cold storage control |
| Patrono et al., 2020 ¶ | Extended DBD livers, macrosteatotic, n = 50 | 354 min (IQR: 299–390 min) | 122 min (IQR: 103–176 min) | No | Liver Assist® | D | Low rate of graft loss (2%, n = 1); the level of macrosteatosis correlates with the occurrence of EAD |
| Ravaioli et al., 2019 | Extended DBD liver, donor age, and cold storage (n = 10) | 14.5 h (IQR: 10.8–22 h) | 2.2 h (IQR: 1–3.5 h) | No | VitaSmart® | PV | No PNF and significantly lower rate of EAD, significantly lower recipient transaminases after HOPE treatment and 100% graft survival compared to the cold storage control group |
| Schlegel et al., 2019 * | DCD livers, n = 50 | 4.4 h (IQR: 3.5–5.2 h) | 2.0 h (IQR: 1.6–2.4 h) | No | Liver Assist® | PV | Less PNF, HAT and ischemic cholangiopathy result in a significantly improved five-year survival of HOPE-treated extended DCD liver grafts |
| Patrono et al., 2019 ¶ | Extended DBD livers, macrosteatotic, n = 25 | 311 min ±53 (mean, SD) | 186 min ±49 (mean, SD) | No | Liver Assist® | D | Lower rate of postreperfusion syndrome, acute kidney injury grades 2–3, and lower EAD due to lower recipient transaminases |
| Van Rijn et al., 2018 § | DCD livers, n = 20 | 358 min (IQR: 314–398 min) | 163 min (155–194 min) | No | Liver Assist® | D | D-HOPE treatment reduced reperfusion injury of the biliary tree and led to a better 6- and 12-month graft survival |
| Kron et al., 2018 | Extended DBD (n = 1) and DCD (n = 5) livers, macrosteatotic, n = 6 | 4.7 h | 2.3 h | No | Liver Assist® | PV | HOPE treatment improves immediate liver function and reduces complications and graft loss |
| Van Rijn et al., 2017 § | DCD livers, n = 10 | 395 min (346–457 min) | 126 min (123–135) | No | Liver Assist® | D | Restoration of ATP, lower transaminases and protection of the biliary tree from reperfusion injury and complications through D-HOPE |
| Guarrera et al., 2015 | Extended DBD (n = 31) | 9.3 h ± 1.36 (mean, SD) | 3.8 h ± 0.9 (mean, SD) | No | Own Device | D | HMP showed significantly fewer biliary complications, less EAD and shorter hospital stay |
| Dutkowski et al., 2015 * | DCD livers, n = 25 | 188 min (141–264 min) | 129 min | No | Liver Assist® | PV | HOPE protected from biliary complications and achieved similar outcomes compared to a matched DBD cohort |
| Dutkowski et al., 2014 * | DCD livers, n = 8 | 141 min | 118 min | No | Liver Assist® | PV | Equal outcomes compared to standard DBD livers, normal immediate function, no biliary complications 8.5 months after LT |
| Guarrera et al., 2010 | Extended DBD (n = 20) | 9.2 h ± 2.1 (mean, SD) | 4.3 h ± 0.9 | No | Own Device | D | HMP showed lower complications, lower EAD rates, and shorter hospital stay |
| Research Group | Number of Centers | Design | Graft Type | Number of Total Participants | Primary Endpoint |
|---|---|---|---|---|---|
| Zurich (Switzerland) | 13 | Cold storage + HOPE vs. cold storage | DBD (incl. ECD, retransplant) | 170 | Complications 1-year CCI (Clavien III-V) |
| Aachen (Germany) | 4 | Cold storage + HOPE vs. cold storage | DBD (incl. ECD) | 46 | Peak ALT within the first week |
| New Jersey (USA) | 8 | Upfront HMP vs. cold storage | DBD (incl. ECD) | 140 | EAD within the first week |
| Lyon (France) | 8 | Cold storage + HOPE vs. cold storage | DBD (incl. ECD) | 266 | EAD within the first week |
| Bologna (Italy) | 1 | Cold storage + HOPE vs. cold storage | DBD (incl. ECD) | 220 | EAD and DGF within the first 30 days |
| Warsaw (Poland) | 1 | Cold storage + HOPE vs. cold storage | DBD (incl. ECD) | 104 | Model for early graft dysfunction score in first 3 days |
| Bergamo (Italy) | 1 | Cold storage + HOPE with and without cytokine filter | DBD (incl. ECD), DCD | 20 | Postreperfusion syndrome within 5 min after reperfusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panconesi, R.; Flores Carvalho, M.; Mueller, M.; Dutkowski, P.; Muiesan, P.; Schlegel, A. Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation? Transplantology 2021, 2, 149-161. https://doi.org/10.3390/transplantology2020015
Panconesi R, Flores Carvalho M, Mueller M, Dutkowski P, Muiesan P, Schlegel A. Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation? Transplantology. 2021; 2(2):149-161. https://doi.org/10.3390/transplantology2020015
Chicago/Turabian StylePanconesi, Rebecca, Mauricio Flores Carvalho, Matteo Mueller, Philipp Dutkowski, Paolo Muiesan, and Andrea Schlegel. 2021. "Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation?" Transplantology 2, no. 2: 149-161. https://doi.org/10.3390/transplantology2020015
APA StylePanconesi, R., Flores Carvalho, M., Mueller, M., Dutkowski, P., Muiesan, P., & Schlegel, A. (2021). Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation? Transplantology, 2(2), 149-161. https://doi.org/10.3390/transplantology2020015







