1. Introduction
Mitochondrial function is essential to the energy production and, therefore, functionality of all cells. Furthermore, maintaining mitochondrial function is largely dependent on assuring sufficient amounts of functional enzymes. Here, the mitochondrial heat-shock protein 60/10 (HSP60/HSP10) complex is crucial as the major protein folding machinery for mitochondrial matrix proteins, facilitating the folding of several hundred protein targets. This complex is one of the most highly conserved protein complexes throughout the animal kingdom [
1] (Zeilstra-Ryalls et al., 1991). Thus, it is crucial for maintaining mitochondrial protein homeostasis and function, and consequently mitochondrial energy production. Deletion of HSP60 causes embryonic lethality in mice and early postnatal death in humans, while mutations in HSP60 cause neurodegeneration [
2]. Although the presence of functional HSP60 is imperative for health and survival, a 50% reduction in HSP60 expression unexpectedly does not cause development defects, but organ-specific metabolic alterations. Thus, it is not understood why mammals express these high concentrations of a protein, when 50% of its expression is sufficient for normal development. From a metabolic standpoint, it seems futile to invest so much energy to produce high HSP60 protein levels, when a reduced expression does not impact the biological fitness or present a selective disadvantage. Similarly, while aging, mice heterozygous for a deletion of Hsp60 (Hsp60
+/− mice) develop a neurodegeneration, yet at a stage where maximal reproductive capacity and biological fitness has already been attenuated. Furthermore, a previous study shows that the offspring of Hsp60
+/− mice are born at the expected mendelian ratio, arguing against infertility of heterozygous individuals [
3].
Mitochondrial function is linked to spermatozoa quality and can modulate reproductive capacity. Male fertility is a key determinant of reproductive success, and impairments in sperm quality contribute significantly to infertility, a growing concern in both clinical and population health contexts. It has been shown that human spermatozoa quality correlates with expression of mitochondrial transcription factor A and different mitochondrial matrix proteins [
4]. Interestingly, the majority of these proteins have been shown to interact with the HSP60/HSP10 complex [
5]. These data imply that altered HSP60/HSP10 expression alters spermatozoa quality. Moreover, active mitochondria in spermatozoa reflect an important sperm quality parameter in domestic animals [
6]. In line with this, there is ample evidence that mitochondrial dysfunction decreases sperm quality [
7] and causes at least a subset of infertility [
8]. A reduction in HSP60 causes mitochondrial dysfunction [
2,
9], indicating that this could also affect sperm quality.
Interestingly, the ubiquitously expressed HSP60 is not only present in mitochondria but also in non-mitochondrial compartments. HSP60 is expressed throughout spermatozoa development and also in testes, and present in mitochondria and on the surface of human spermatozoa [
10]. In addition, HSP60 resides on the luminal surface of oviduct epithelial cells and interacts with spermatozoa [
11], suggesting an important function of HSP60 for fertilization. Strikingly, it has already been stated that altered HSP60 expression is responsible for an altered sex ratio in the offspring [
3]. The mice used in the study by Christensen et al. carry a heterozygous deletion of Hsp60 (Hsp60
+/−) on a C57BL/6J background. It is noteworthy that C57BL/6J mice do not express the mitochondrial nicotinamide nucleotide transhydrogenase (Nnt) [
12]. The absence of NNT leads to mitochondrial dysfunction [
13], which could confound mitochondrial phenotypes observed in Hsp60
+/− mice on a C57BL/6J background. To mitigate this confounder of a pre-existing mitochondrial dysfunction, we crossed those mice back to a C57BL/6N background, as this strain does express functional Nnt.
In this study, we then bred male Hsp60
+/− on a C57BL/6N background with wild-type females and vice versa [
14] and analyzed the genotype of the offspring to assess any potential alteration in offspring numbers or genetic transmission.
Our analysis revealed that breeding Hsp60+/− mice with wild-type (WT) mice resulted in abnormal genotype distribution, with fewer than expected Hsp60+/− offspring born compared to the wild-type genotype. In males this was linked to less active mitochondria in the spermatozoa of Hsp60+/− males and reduced ATP synthase expression compared to the control. Our data show that reduced HSP60 expression represents a selective disadvantage for genotype transmission, giving an evolutionary rationale for high HSP60 expression in mammals.
2. Materials and Methods
2.1. Animal Studies
The original Hsp60
+/− mouse model is described in detail elsewhere [
3]. Those mice were bred on a C57BL/6J background. To mitigate pre-existing mitochondrial dysfunction of the C57BL/6J mice due to lack of Nnt, we re-derivatized frozen sperm from these mice in C57BL/6N mice and crossed the resulting offspring back on a C57BL/6N background. C57BL/6N WT and Hsp60
+/− mice were then bred in-house and group housed in a temperature-controlled room (22 ± 1 °C) on a 12 h light/dark cycle with free access to food and water in individually ventilated cages (IVC). For the offspring numbers, a total of 35 breeding pas were included in the data analysis and their genotype assessed via PCR. Breedings were started with mice aged 10 weeks and kept to a maximum age of 6 months. Long-term reproductive outcomes were not assessed. Animals were kept on a normal chow diet (NCD), purchased from ssniff Spezialdiäten GmbH (Soest, Germany) #V1124-300 (composition: 12% energy from fat, 27% from protein, 61% from carbohydrates), for 12 weeks, starting at 4 weeks of age. At 16 weeks of age, 6 males per genotype were killed and the testes removed. The testes and surrounding tissue were kept at 4 °C and transported to the Leibniz Institute for Zoo and Wildlife Research (Berlin, Germany). Analyses were performed within 6 h of organ collection. Details and phenotypical characteristics of Hsp60
+/− mice on the C57BL/6N background can be found in [
3,
14] For some parameters, not all samples could be analyzed, details on the final number can be found in the respective figure legends and method descriptions.
2.2. Genotyping
PCR typing for heterozygosity was performed on genomic DNA from tail biopsies, isolated by incubation at 56 °C overnight in lysis buffer (10 mM Tris-HCl (pH 8.0), 10 mM EDTA, 150 mM NaCl, 0.2% SDS, 8 µg/µL Proteinase K) and subsequent isopropanol/ethanol precipitation. Oligonucleotide primers specific for the deletion site (P1: upper, 5′-TAAGACAGCATTTCTCCGGTAG-3′, P2: lower, 5′-CTGAGTGTTGGGATTATGCAG-3′, P3: LTR lower 5′-GCCAGTCCTCCGATTGAC-3′) were used in a multiplex PCR. Wild-type (WT) mice show a single band at 497 bp, whereas Hsp60+/− mice show two bands at 497 and 416 bp, respectively. The resulting DNA products were separated on a 2% agarose gel stained with ethidium bromide and visualized under UV light.
2.3. Cell Culture
Mouse embryonic fibroblasts have been isolated at E12.5, as described in [
15]. Cells were cultured in Gibco DMEM GlutaMAXTM (Thermo Fisher Scientific Inc., Waltham, MA, USA) culture medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Thermo Fisher Scientific Inc.) and 1% pyruvate (Thermo Fisher Scientific Inc.) at 37 °C and 5% CO
2. Cycloheximide (Carl Roth GmbH + Co. KG, Karlsruhe, Germany, #8682.1) was used in a concentration of 5 µg/mL for designated timepoints.
2.4. Testis and Spermatozoa Preparation
Testes and epididymides were freed from the surrounding tissue and separated. Testis weight and volume, as well as the weight of the cauda epididymis (sum of both sides) were determined as described previously [
16]. One testis was frozen at −20°C before spermatogenetic activity was analyzed. Both caudae epididymides were finely minced in a defined volume (500 µL) of M199-k composed of M199 (Sigma-Aldrich (Taufkirchen, Germany) M7528) supplemented with 1 mM sodium pyruvate, 14 mM sodium lactate and 0.4% (
w/v) bovine serum albumin (fraction V, K32491618-406, Merck, Darmstadt, Germany)). The resulting cell solution was filtered through a 30 μm cell filter (Sysmex Partec GmbH, Goerlitz, Germany) and an aliquot was used to determine the sperm concentration in a counting chamber. Note that due to epididymal atrophy, only 5 out of 6 Hsp60
+/− samples could be measured for cauda weight and sperm number.
2.5. Protein Isolation, Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot
Testis, cauda epididymis and spermatocytes extracted from the vas deferens were homogenized in RIPA (Radio-Immunoprecipitation Assay) lysis buffer (50 mM TRIS-HCl, 150 mM NaCl, 1 mM EDTA, 0.25% Na-deoxycholate, Triton X-100, protease inhibitor). After centrifugation, the protein content in the supernatant was measured using Pierce Reagent (Thermo Fisher Scientific). A measure of 20 µg of total protein per sample was separated on a 12% SDS acrylamide gel and afterwards blotted on a (polyvinylidene difluoride) PVDF membrane. Membranes were probed for HSP60 (CST #4870, 1:1000 in 0.2% skim milk), secondary antibody anti-rabbit CST #7074, 1:2000 in 0.2% skim milk), total oxidative phosphorylation (OXPHOS) complexes (Abcam ab110413, 1:1000 in 0.2% skim milk, secondary antibody anti-mouse CST #7076, 1:2000 in 0.2% skim milk) or b-Actin-HRP (Sigma-Aldrich A3854, 1:10,000 in 0.2% skim milk). Chemiluminescence of proteins of interest was visualized on the BioRad ChemiDoc Imaging System (Hercules, CA, USA). Ponceau S staining was used as a normalization factor for proteins of interest. Due to limited samples, technical replicates could not be performed. Band intensities were quantified via densitometric analysis from one Western blot per experiment, using the Gel Analyzer Plugin from ImageJ Fiji Version 1.54p.
2.6. Immunofluorescence Measurements
Spermatocytes extracted from the vas deferens were diluted in 50 µL phosphate-buffered saline (PBS). For analysis of mitochondrial membrane potential, 300 nM MitoTracker™ Orange CMTMRos (Thermo Fisher Scientific) was added and incubated for 30 min at 37 °C. Subsequently, samples were centrifuged, resuspended in 50 µL PBS and smeared on a glass slide. Samples were allowed to air-dry for 20 min, fixed with 4% Formaldehyde in PBS for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 10 min and blocked with a 3% BSA solution for 30 min. Slides were incubated overnight with HSP60 primary antibody (CST #4870, 1:200 in 3% BSA-PBS). Secondary anti-rabbit Alexa Fluor™ 594 (Thermo Fisher #A-11012, 1:500 in 3% BSA-PBS) was added for 1 h at room temperature. Slides were imaged using the Carl Zeiss confocal laser scanning microscope LSM 780 with a 40× Oil DIC Plan-Neofluar objective using an excitation wavelength of 543 nm. Fluorophores were detected in separate channels with emission filters set at 575/62 (Mitotracker Orange) and 622/50 (Alexafluor 594, i.e., HSP60).
2.7. Evaluation of Spermatozoa Morphology
For morphological analysis, 10 µL filtered sperm suspension was fixed with 90 μL 0.5% formaldehyde in PBS Dulbecco (Sigma-Aldrich D 8537, St. Louis, MO, USA). Aliquots were mounted on a wet slide and intactness of heads and tails were evaluated under a microscope (Leica, Wetzlar, Germany) with phase contrast optics and a 1000× magnification using oil immersion. Note that, due to insufficient sperm numbers, only 5 out of 6 Hsp60+/− samples could be measured.
2.8. Spermatozoa Motility Assay
To examine sperm motility, filtered sperm suspension was diluted with prewarmed (38 °C) M199-k medium (final concentration ~10 × 106 cells/mL) and a prewarmed 20 micron Leja 4 chamber slide (Leja Products BV, Nieuw-Vennep, The Netherlands) was loaded with 3 µL sperm suspension. Sperm motility was analyzed by the computer-assisted sperm analysis (CASA) system AndroVision (Minitüb, Tiefenbach, Germany) under negative phase contrast optics at an AXIOScope A1 microscope (Zeiss, Oberkochen, Germany). Pictures were recorded for 0.5 s at 60 Hz by a video camera Basler acA2440-75 µm (Basler AG, Ahrensburg, Germany) and 8 fields with 20–50 spermatozoa per field were evaluated per sample. Progressive sperm comprise sperm with a straight-line velocity (VSL) > 50 µm/s and a curved line velocity (VCL) > 55 µm/s, rapid sperm have a VCL > 95 µm/s. Further motion parameters such as linearity (LIN = VSL/VCL) or beat cross frequency (BCF) were determined. Note that, due to insufficient sperm numbers, only 4 out of 6 Hsp60+/− samples could be measured.
2.9. Evaluation of Sperm Mitochondrial Activity
To assess the percentage of sperm cells with active mitochondria, the filtered sperm suspension was diluted with M199-k to 1–2 × 106 sperm cells in 250 μL M199-k. Aliquots of 1 μL rhodamine 123 (R-302, incorporating in active mitochondria due to their membrane polarization) from a 50 μg/mL stock as well as of 2.5 μL propidium iodide (P 3566) from a 1 mg/mL stock were added followed by an incubation for 20 min at 37 °C in the dark. A volume of 10 μL stained sperm suspension was measured in 2 mL pre-warmed M199-k on a PAS III flow cytometer (Sysmex Partec GmbH, Goerlitz, Germany). Excitation (488 nm) was performed by a 200 mW Argon laser. The green fluorescence of rhodamine 123 accumulating in active mitochondria was recorded using a band pass (500–560 nm). The red fluorescence of propidium iodide accumulating in dead sperm cells nuclei was recorded using a long pass (>610 nm). A number of 15,000 events were counted per sample. Sperm cells were gated in a forward–sideward scatterplot, and the percentage of sperm cells with active mitochondria was determined in the fluorescence dotplot by the FlowMax software (Version 2.9, Sysmex Partec GmbH, Goerlitz, Germany). Note that, due to insufficient sperm numbers, only 5 out of 6 Hsp60+/− samples could be measured.
2.10. Evaluation of Spermatogenetic Activity
Spermatogenetic activity was analyzed in one testis according to [
17]. The frozen testis was thawed, weighed and fine-minced in 0.4 mL 100 mM citric acid containing 0.5% (
v/v) Tween 20 per 25 mg parenchyma. This suspension was agitated for 20 min at room temperature. Per 25 mg treated parenchyma, 2 mL of a 400 mM Na2HPO4 solution containing 5 µM 4′,6-diamidino-2-phenylindol (DAPI) was added and the DNA of dissociated and lysed testis cells was stained for 10 min in the dark. The fluorescence intensity of the cells, corresponding to the DNA content of their nuclei, was recorded on a PAS III flow cytometer (Sysmex Partec GmbH, Goerlitz, Germany)) equipped with an UV LED and an appropriated filter set (excitation: 365 nm; emission: 420 nm). The histograms were analyzed for the proportions of cells in each peak by the FlowMax software (Version 2.9, Sysmex Partec GmbH, Goerlitz, Germany). Postmeiotic germ cell stages like spermatids and sperm cells exhibit haploid signals (1c); spermatogonia, secondary spermatocytes and somatic testicular cells exhibit diploid signals (2c) and tetraploid signals (4c) mainly derive from G2/M phase of cell cycle in meiotic primary spermatocytes but also in mitotic spermatogonia. Between 2c and 4c, cells in the S-phase (s) were detected. The proportion of haploid cells is a measure of spermatogenetic activity.
2.11. Declaration of Approval
All animal procedures were conducted according to German laws for the protection of animals. Breeding of mice was in compliance with protocols approved by local government authorities (Landesamt für Arbeitsschutz, Verbraucherschutz und Gesundheit (LAVG) State Agency of Environment, Health and Consumer Protection, State of Brandenburg, Germany) under the approval numbers 2347-40-2015 (approved 7 February 2016). Tissue collection pertaining to this project was approved by the animal welfare office of the German Institute of Human Nutrition (DIfE) under approval number T-17-18-CRM (approved 7 May 2018) and T-08-24-UNI (approved 18 January 2024).
2.12. Statistical Analysis
Graphs and statistics were generated in GraphPad Prism (version 10.4.1). Differences in genotype or sex distribution of offspring was tested with a binomial test, with an expected mendelian genotype or sex ratio of 0.5. When comparing continuous data between two groups, a Mann–Whitney test was performed except unless stated otherwise. Significance was determined as a p value < 0.05. Data are shown as Box and whiskers plot, min to max, and all points are shown.
3. Results
The 12-week-old male or female Hsp60
+/− mice were bred to wild-type mice and their genotype was confirmed by DNA and mRNA analysis (
Figure 1A and published in [
14]). As already reported, we and others did not detect any obvious developmental or morphological alterations in mice with a 50% reduction in HSP60 gene and protein expression [
3,
14]. Yet, when analyzing offspring in this mouse cohort, differences in genotype frequencies could be observed. Out of 35 different breedings, with a total of 290 offspring, the wild-type genotype was present in 59.7%, while the Hsp60
+/− genotype was present in 40.3% of all offspring. This was a significant decrease in Hsp60
+/− offspring and deviated from the expected Mendelian ratio of 1:1 (179 WT vs. 111 Hsp60
+/− pups,
p < 0.0001,
Figure 1B). Next, we analyzed whether the genotype distribution was altered predominantly due to either parent’s genotype. This analysis revealed the same significant difference in genotype ratio, irrespective of whether the Hsp60
+/− genotype was coming through the paternal or maternal lineage (
Figure 1C, paternal Hsp60
+/− 90 WT vs. 50 Hsp60
+/− pups,
p = 0.0007, 16 breedings, maternal Hsp60
+/− 89 WT vs. 61 Hsp60
+/− pups,
p = 0.0224, 19 breedings).
The observed genotype distribution could be due to embryonal lethality of Hsp60
+/− embryos. However, looking at the average litter size between the breeding pairs of C57BL/6N WT mice to either Hsp60
+/− males or Hsp60
+/− females showed no significant differences, suggesting that embryos bearing one Hsp60 null allele develop normally (
Figure 1D,E).
However, it had been reported previously that HSP60 expression impacts the sex ratio in the offspring, where 60% of offspring are male and 40% female. Analyzing the total number of male and female offspring in our breedings (
Figure 1F), we can confirm the previously published ratio also in our data set, with 172 (59.3%) male and 118 (40.7%) female offspring, which differs from breedings performed with only WT mice (50.4% male, 49.6% female offspring). In terms of the sex of offspring, the genotype of the parental mice seemed irrelevant, as breeding with a maternal or paternal Hsp60
+/− led to almost identical deviation of sex ratio in the offspring. This suggests a selective disadvantage of female offspring when breeding Hsp60
+/− mice (
Figure 1F). Interestingly, stratifying this data further into the genotypes of the resulting offspring reveals a striking difference between paternal and maternal Hsp60
+/− genotype breedings (
Figure 1G). Breedings with male Hsp60
+/− led to a non-significant reduction in female offspring of both WT (49 vs. 41,
p = 0.4018) and heterozygous (32 vs. 18,
p = 0.0649) genotype. On the contrary, breeding with Hsp60
+/− females shows a clear reduction in resulting WT females (61 vs. 28,
p = 0.0006), but almost identical Hsp60
+/− offspring (30 male and 31 female pups).
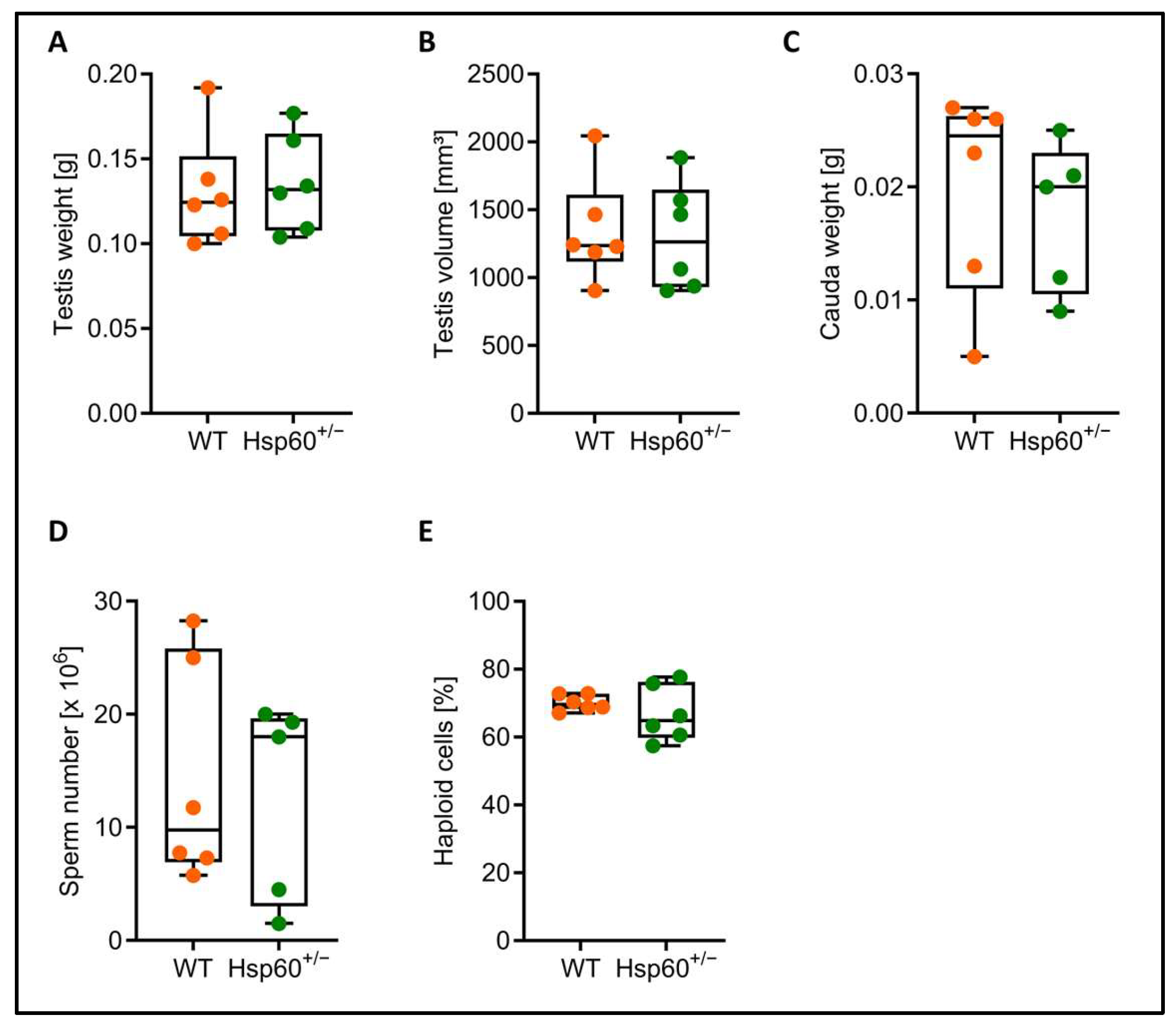
Although the heterozygous genotype rate difference was transmitted via both the male and female lineage, we focused on a potential mechanism in male Hsp60
+/− mice first and further analyzed testis and spermatozoa. There was no difference in testis weight and volume as well as in cauda epididymis weight, and similar numbers of cauda spermatozoa could be collected from Hsp60
+/− and wild-type males (
Figure 2A–D). The percentage of haploid cells within the testis can inform about impaired spermatogenesis; however, we did not find a difference in the proportion of haploid cells between the genotypes (
Figure 2E).
Spermatozoa from both genotypes show similar occurrence of normal morphology, as well as comparable percentages of motile, progressive, and rapid spermatozoa, respectively (
Figure 3A–D). Furthermore, we did not detect any alterations in sperm motion characteristics such as linearity or beat cross frequency (
Supplementary Figure S1A,B). Interestingly, the curved line velocity (VCL) specifically of the rapid spermatozoa is decreased in Hsp60
+/− samples (
Figure 3E,F).
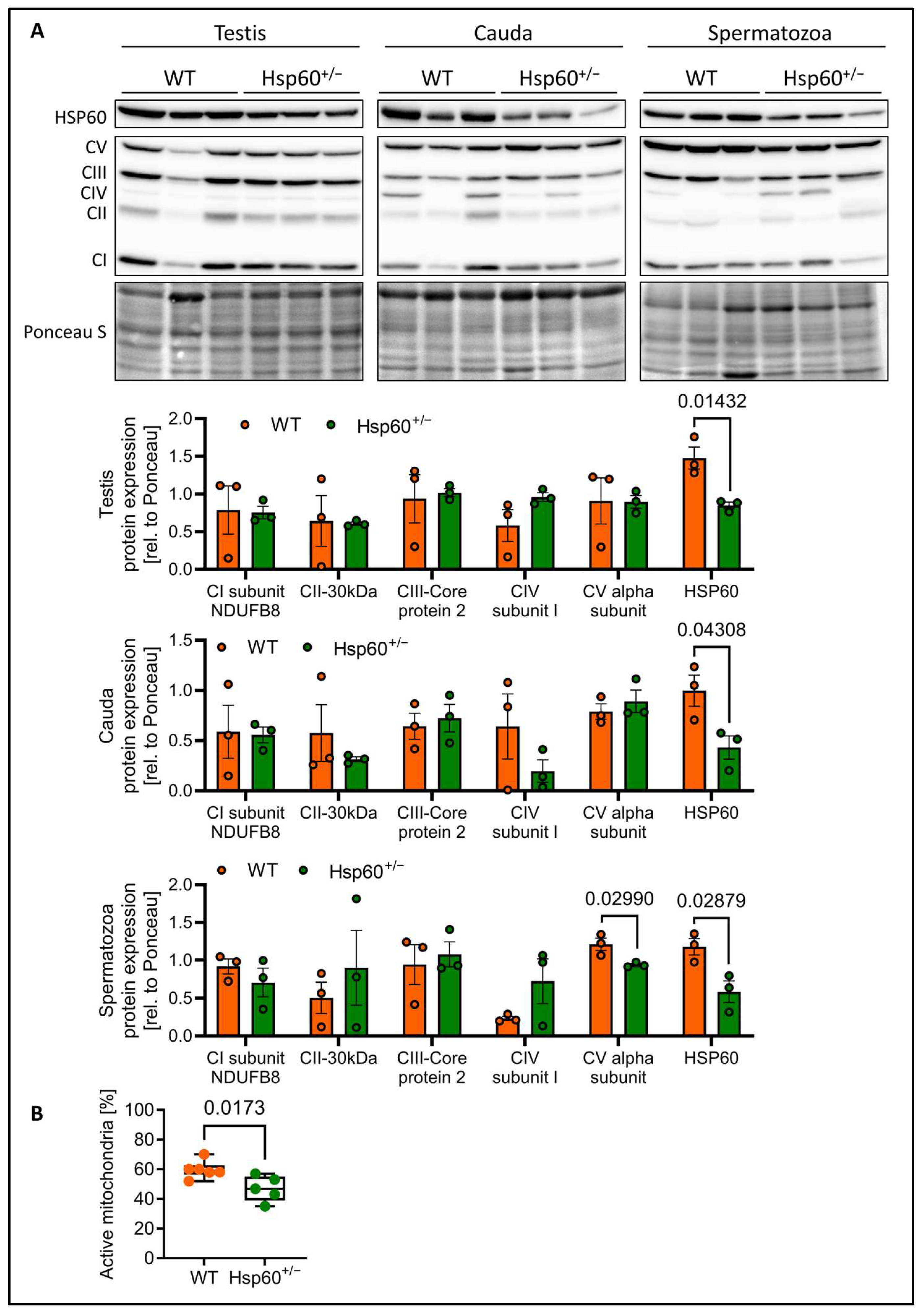
As the speed of spermatozoa is dependent on mitochondrial activity, we performed immunofluorescence (IF) staining of spermatozoa smears for HSP60 and MitoTracker Orange. This dye incorporates into mitochondria dependent on mitochondrial membrane potential. Here, the IF analysis showed the accumulation of HSP60 in the mitochondria-containing midpiece as expected. Furthermore, the analysis of spermatozoa from Hsp60
+/− mice seems to indicate an overall reduction in mitochondrial activity, as determined by the intensity of the MitoTracker Orange signal (
Supplementary Figure S1C,D). Mitochondrial energy output is dependent on oxidative phosphorylation using the complexes of the electron transport chain. Interestingly, while testis, cauda epididymis and spermatozoa all show the expected reduction in HSP60 protein by 50%, only spermatozoa display a significant reduction specifically in a subunit of complex V (CV), the ATP synthase by 23% (
Figure 4A). This suggests impaired energy generation in spermatozoa of Hsp60
+/− mice. This raised the question of to what degree and at what stage HSP60 is still expressed in spermatogenesis. As both HSP60 WT and the null allele carrying sperm cells stem from the same diploid cell, they should possess equal HSP60 protein load to begin with. A recent study [
18] provides single-cell RNA sequencing data, where they differentiate 17 distinct cell clusters, based on known marker genes. Looking at the supplementary raw sequencing data, we see that they find an expression signal for Hspd1 (the gene coding for HSP60) in adult spermatids at the beginning stages of meiosis, but also, critically, in haploid cells in spermiogenesis stages 4–5. Next, in trying to assess whether this constitutes a relevant time-frame during which HSP60 protein levels could be affected in HSP60 null spermatids, we analyzed general turnover for HSP60 in cells. For lack of a sperm cell culture model, we opted to use mouse embryonic fibroblasts and treated them with the protein synthesis inhibitor Cycloheximide (CHX). We observed a striking reduction in HSP60 protein levels even after 6 h and decreasing steadily until 36 h. The protein beta-actin, known to have low turnover, does not decrease during that timeframe (
Supplementary Figure S1E). This shows that HSP60 undergoes rapid turnover, which could suggest that in haploid cells lacking the HSP60 gene, HSP60 levels could drop relatively quickly and therefore a mitochondrial dysfunction could occur, even if the cells originally had the same HSP60 protein load.
Leading on from this, we analyzed the percentage of spermatozoa with active mitochondria via flow cytometry. Strikingly, we detected a mean 22% reduction in sperm cells with active mitochondria in spermatozoa from Hsp60
+/− males (
Figure 4B).
Thus, Hsp60 heterozygous males seem to have fewer active mitochondria in their gametes and slower rapid spermatozoa motion. Both seem to be associated with the reduction in the expected average offspring genotype ratio in males, offering an explanation for high expression levels of HSP60 to facilitate proper genotype distribution.
4. Discussion
The proper transmission of the genetic information to the next generation is crucial for the fitness of the individual offspring as well as the population fitness. Furthermore, alterations of a balanced sex ratio in a population can alter behavioral traits and impact populations fitness [
19]. Thus, it is imperative for an animal to invest large amounts of energy to ensure optimal male and female germ cell quality.
It had been shown that the offspring of Hsp60 heterozygous mice are born viable and show no obvious signs of an altered development [
3,
14]. However, we find a genotype difference in the offspring, where Hsp60
+/− mice produce less Hsp60
+/− offspring than expected by mendelian ratio (
Figure 1B). These differences between previous studies and our own observations in offspring characteristics can be explained by a difference in genetic background. Our mice are on a C57BL/6N background, which differs from the J background in several aspects, including a difference in antioxidative capacity by lacking the NNT gene [
20] or several behavioral [
19,
21] and metabolic traits [
22,
23] Additionally, C57BL/6N mice exhibit increased insulin-degrading enzyme (IDE) levels compared to the J background [
24]. It has been reported that reduced IDE levels impact testes and sperm quality [
25], suggesting that genetic differences between the J and N background in combination with the Hsp60 mutation might affect male fertility characteristics. More research is needed to address this hypothesis in detail. Interestingly, the genotype distribution does not seem to be driven mainly by embryonic lethality, as we observe similar average litter sizes of Hsp60
+/−/WT and WT/WT breedings (
Figure 1E).
On a molecular level, we show that reduced HSP60 expression decreases mitochondrial ATP synthase expression and function in spermatozoa, confirming the already well-known effect of decreased mammalian HSP60 levels on mitochondrial function [
2,
9,
14,
26]. As such, it is possible that diploid sperm progenitors from Hsp60
+/− males with dysfunctional mitochondria could confer those to all haploid spermatozoa, explaining why we observe a generalized lower mitochondrial function across the Hsp60
+/− samples. The ATP synthase complex is a potential biomarker for male fertility [
27]. Our data provide new insights into the upstream regulators of this important complex that affects fertility. The percentage of spermatozoa with active mitochondria has been shown to be a predictor for fertility in domestic animals [
6,
28] and can explain the reproductive disadvantage of Hsp60
+/− spermatozoa. Reduced mitochondrial activity and diminished sperm motility—especially in the population of rapid spermatozoa—can be indicative of decreased fertilization potential, supporting a mechanistic link between HSP60 expression and male reproductive success. Indeed, VCL has been shown to be a predictor of healthy sperm and shows strong correlation to fertilization [
29] and has been shown to be closely associated with reproductive success in mammals [
30,
31,
32,
33]. Based on our finding about the expression of HSP60 during spermiogenesis and its rapid turnover (
Supplementary Figure S1E), there is a possibility that spermatids lacking de novo HSP60 synthesis do not develop as rapid or efficient as WT spermatids. If true, our observed effects could be explained by an overall lower proportion of HSP60 null spermatozoa in samples from Hsp60
+/− males, leading to ultimately lower HSP60, mitochondrial function and speed.
Previous reports on this mouse model indicated that more males were born. [
3]. These data indicate that HSP60 expression is crucial for a balanced sex ratio, that allows for a dynamic and adaptable mammalian population [
17]. In our study, we observe a difference in the sex ratio, similar to the aforementioned study. However, as opposed to the above-mentioned reports, this was independent of the parental genotypes, although this effect did not quite reach significance in male Hsp60
+/− breedings (
Figure 1E). Subdividing the data further, according to the genotype of the offspring, suggests that the reduction in female offspring could be attributed towards mitochondrial dysfunction, as it is present only in pups that either develop, or have pre-existing mitochondrial dysfunction. For example, breeding WT females with Hsp60
+/− males, seems to drive a reduction in female offspring, specifically within pups with the Hsp60
+/− genotype. Interestingly, breeding WT males with Hsp60
+/− females, shows the expected reduction in Hsp60
+/− genotype in general, but additionally, a large decrease in female pups with the WT genotype. Our interpretation of this effect is that (i) when the genotype is transmitted via the male lineage, mitochondrial dysfunction develops after fertilization and could result in a selective disadvantage of the XX embryos. Another effect is that (ii) female Hsp60
+/− mice transmit their dysfunctional mitochondria to the oocyte and thereby to the fertilized cell, which is why this selective disadvantage appears in the WT genotypes. Furthermore, this seems to occur relatively early during development, as when female HSP60 mice have WT offspring, the reduction in female pups is present, yet the mitochondria may regenerate functionality post-fertilization.
However, the exact reason for this shift in offspring sex is still unclear. It raises the interesting question of whether a mitochondrial dysfunction in oocytes presents a selective disadvantage for a XX genotype post-fertilization. We propose further pre- and post-implantation studies, to assess at which stage female embryos are lost.
HSP60 regulates yolk sac cell survival [
33] and is expressed in the female reproductive system, for instance on the surface of bovine oviduct epithelial cells which interact with spermatozoa [
11,
34], providing a potential explanation as to why the non-mendelian genotype distribution was also found when breeding with female Hsp60
+/− mice. The lower mitochondrial activity does associate with decreased motion velocity specifically in the population of rapid spermatozoa from Hsp60
+/− males, arguing for a selective disadvantage of mice with decreased Hsp60
+/− levels in spermatozoa. The population of rapid spermatozoa is the one with the highest chance to fertilize because the mean and maximum spermatozoa swimming speed are crucial for male fertility [
35]. To facilitate constant high expression of HSP60 in mammals, the promoter of Hsp60 is devoid of a TATA Box, reveals housekeeping characteristics and exhibits high gene regulation conservation across mammalian species [
36].