Abstract
Chromosomal defects are a significant cause of perinatal death and childhood disability, occurring in 3.6–6.0 per 1000 births in unscreened populations. Common chromosomal defects include trisomy 21, 18, and 13, triploidy, and sex chromosome abnormalities. Screening for these defects began in the mid-1960s with the advent of amniocentesis, and various methods have since been developed to improve screening performance. Initial screening was based solely on maternal and gestational age, a method incorporated later into all subsequent screening methods giving an a priori background risk. This a priori background risk, which is further refined by maternal serum biochemistry, results of ultrasound examinations, and most recently, results of non-invasive prenatal testing by cell-free DNA in maternal blood. This paper will describe methods of screening for all chromosomal defects and their performance. Unlike most reviews, this paper covers not only screening tests for Down syndrome, but also screening methods for the other most common and less common chromosomal defects.
1. Introduction
Chromosomal defects are a major cause of perinatal death and childhood disability [1]. Their natural frequency at birth in an unscreened population is between 3.6–6.0 in 1000 births [2,3]. The most common chromosomal abnormality among liveborn infants is Down syndrome, with a reported incidence of 2.1 per 1000 live births [3], followed by trisomy 18 (0.5 in 1000 births) and 13 (0.19 in 1000 births), sex chromosome anomalies, and triploidy [3]. These incidence rates reflect prevalence at the time of birth.
In the mid-1960s, accurate prenatal diagnosis of chromosomal defects by amniocentesis defects became possible [4]. While the procedure is generally considered safe in skilled hands, its invasiveness and the small but real risk of miscarriage led to the development of prenatal screening strategies to assess the likelihood of chromosomal abnormalities before proceeding to diagnostic testing.
Screening tests originally targeted Down syndrome, the most common chromosomal defect. First, it was known that older women had a higher likelihood of carrying a baby with Down syndrome [1,5], and thus the initial strategy was based on risk estimation solely on maternal age [1,6]. Additionally, it was discovered that the risk of many chromosomal defects decreases with gestational age, primarily due to the natural in utero loss of affected fetuses [1,2]. These two screening strategies were later combined to form the background risk, which is currently further refined by newer, more accurate markers of Down syndrome and other chromosomal defects [2].
Initially, in the 1980s, screening by maternal serum biochemistry in the second trimester followed by a genetic scan in the second trimester were utilized to recalculate and refine the population-based risk of Down syndrome and the most common chromosomal defects [1]. In the 1990s, screening moved to the first trimester after it was discovered that most fetuses with major aneuploidies could be identified by increased nuchal translucency [1,7,8]. After nuchal translucency (NT) was identified as a marker of chromosomal defects, laboratories developed first-trimester maternal serum biochemistry screening, resulting in the combined test that integrates both methods [9]. Over the subsequent decade, additional first-trimester ultrasound markers of chromosomal anomalies were identified [1], significantly enhancing the performance of this first-trimester multi-ultrasound-marker combined test. Several methods combining first- and second-trimester screening have been evaluated, including the integrated, contingent, and sequential tests [2,6,10,11]. The integrated test shows strong performance [6], surpassing the basic first-trimester screening combining nuchal translucency with biochemistry. Still, due to the delayed results of integrated tests, the first-trimester combined test by NT biochemistry remained to be the primary screening method in most countries. Additionally, the first-trimester multi-ultrasound markers combined test outperforms the integrated test and other combined models [12,13,14]. In 2011, non-invasive prenatal testing (NIPT) using cell-free DNA (cfDNA) from maternal plasma was introduced [15]. This groundbreaking method detects chromosomal abnormalities with high sensitivity and specificity, approaching the accuracy of diagnostic tests. Numerous validation studies have demonstrated the superior performance of NIPT methods as a screening test for trisomies 21, 18, and 13 in both high-risk and low-risk populations [15,16,17,18,19,20]. Consequently, healthcare systems have begun incorporating NIPT into standard prenatal care. In most countries, NIPT is conducted after the first-trimester combined test. However, in the Netherlands, cfDNA testing has been introduced as a first-tier screening test [19,20]. Also, ACMG (American College of Medical Genetics and Genomics) strongly recommends NIPT over traditional screening methods for all pregnant patients with singleton and twin gestations for fetal trisomies 21, 18, and 13, and strongly recommends NIPT to be offered to screen for fetal sex chromosome aneuploidies [21]. NIPT methods enable screening for sex chromosome abnormalities, microdeletion syndromes, triploidy, molar pregnancies, rare autosomal trisomies, Rh status, single gene disorders, and segmental imbalances [22,23,24]. Nevertheless, reporting on rare autosomal trisomies and structural chromosomal aberrations should be avoided due to their low positive predictive value [19,25]. Most of these findings originate from placental DNA, which is the source of fetal DNA analyzed in NIPT methods. As a result, positive NIPT results for rare autosomal trisomies are often due to placental trisomies, leading to unnecessary invasive testing and increased maternal anxiety [19].
This review aims to describe the strengths and limitations of current screening methods for chromosomal abnormalities, covering not only the common trisomies but also less common chromosomal defects.
The performance of available screening tests for Down syndrome is summarized in Table 1. The graphical visualization of the progressively improving performance of the screening for trisomy 21 is presented in Figure 1.

Table 1.
Prenatal screening tests for Down syndrome.
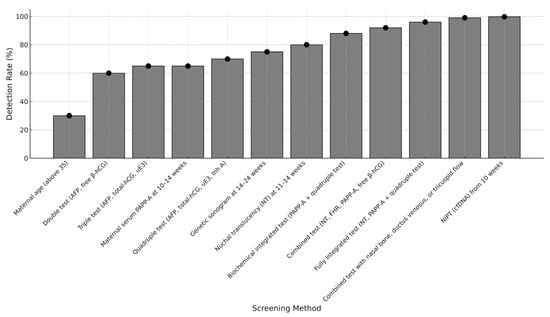
Figure 1.
Chronological improvement in the performance of prenatal screening methods for Down syndrome (comparison of detection rate at 5% false positive rate, only in case of maternal age at 20% false positive rate).
2. Screening Strategies for Chromosomal Defects
2.1. Basic Facts About the Prenatal Screening for Chromosomal Defects
All pregnant women face a potential risk of chromosomal abnormalities in their fetus. To determine an individual risk, background risk (derived from maternal and gestational age) is combined with additional screening factors, including family history, ultrasound findings, and biochemical tests. Each test result refines the background risk by multiplying it with a risk factor, generating a more precise probability of a chromosomal defect, that is representing a newer more accurate background risk for further screening method [26].
A screening test is considered positive when the calculated risk exceeds a predetermined threshold, prompting further counseling and diagnostic testing. Screening test performance is assessed using key metrics:
- False Positive Rate (FPR)—Percentage of unaffected pregnancies incorrectly flagged as high risk.
- Detection Rate (DR)—Percentage of actual cases correctly identified.
- Positive and Negative Predictive Values (PPV/NPV)—The likelihood that a positive or negative result is correct.
Higher-performing screening methods aim to maximize detection rates while minimizing false positives [27,28]. The cut-off for risk assessment is determined based on the desired balance between detection and false positives, or both, ensuring an effective approach to identifying chromosomal abnormalities at term or mid-trimester [13]. In clinical practice, cut-offs are also influenced by national policies, population characteristics, and local resource availability. To compare screening tests performance, the same cut/off for all tests must be used.
Additionally, multiple marker screening methods rely on Multiples of the Median (MoM) values. These standardize biochemical and ultrasound marker levels by comparing in-dividual results to population-based medians. This approach allows for better consistency across different testing programs and laboratories [27].
2.2. Screening by Maternal and Gestational Age
The risk of most chromosomal defects increases with maternal age [26,29,30]. Seminal data by Hook and colleagues also established maternal age-specific risks for chromosomal abnormalities, demonstrating a continuous rise in the incidence of trisomy 21 and other aneuploidies with increasing maternal age [31]. Table 2 displays the maternal age-specific risks of trisomy 21 and of all chromosomal abnormalities at the time of amniocentesis (16–18 weeks of gestation) as described in this study.

Table 2.
Maternal-age-specific risks of trisomy 21 and of all chromosomal abnormalities at the time of amniocentesis (16–18 weeks of gestation) by Hook in 1983.
Similar age-related risks of chromosomal defects were reported in a recent large Danish study [29]. The results of this study align with previous reports, indicating that advanced maternal age (≥35 years) is associated with an increased risk of trisomies 21, 18, 13, and sex chromosome aberrations [29]. However, no age-related associations were found for triploidy or monosomy X, microdeletions, and duplications [20,29].
Fetuses with chromosomal abnormalities have a higher likelihood of in utero death, leading to a decrease in risk as gestation advances [26].
The rate of fetal death between 12 weeks and term is about 30–40% for trisomy 21, 80% for trisomies 18 and 13, 70% for Turner syndrome [1,30,32], and nearly 100% for triploidy [1,26,32,33]. The likelihood of sex chromosome abnormalities other than Turner syndrome remains relatively constant regardless of gestational age [1,32].
In the early 1970s, around 5% of pregnant women were aged 35 or older, accounting for about 30% of fetuses with trisomy 21. Screening based on maternal age (35 years as the high-risk cut-off) resulted in a 5% false positive rate and a 30% detection rate. Currently, around 20% of pregnant women are 35 years or older, and this group accounts for about 50% of fetuses with trisomy 21 [26]. A false positive rate of 20% (thus a 20% rate of invasive testing) combined with a 50% detection rate for trisomy 21 represents very poor test performance, which is markedly less effective than newer screening strategies incorporating multiple biochemical and ultrasound markers. Therefore, maternal and gestational age should not be used to screen for chromosomal defects but rather to establish background risk, which is further refined with newer, more precise markers of chromosomal defects.
2.3. Previous Affected Pregnancy
The risk of trisomies is higher in women who have previously had a fetus or child with a trisomy, beyond what would be expected based on maternal and gestational age alone [26,34,35]. This increased risk applies to both the same type of trisomy (homotrisomy) and different types (heterotrisomy). Interestingly, the risk is particularly elevated in younger women (under 35 years old). This pattern suggests potential underlying factors, such as genetic predispositions, including gonadal mosaicism or an increased susceptibility to meiotic nondisjunction. In cases of gonadal mosaicism, trisomic cells in the ovaries or sperm can raise the likelihood of producing trisomic gametes, leading to a recurrence of the same trisomy. However, gonadal mosaicism does not fully explain the higher risk for heterotrisomy. This may instead be attributed to genetic susceptibility—heritable factors that increase the chance of chromosomal segregation errors during meiosis. Additionally, parental chromosomal abnormalities, such as balanced translocations, inversions, or other rearrangements, may also contribute by leading to the formation of unbalanced gametes and increasing the risk of aneuploid conceptions [36]. Therefore, parental karyotyping is recommended when a pregnancy is affected by aneuploidy, particularly in cases of recurrent pregnancy loss or multiple chromosomally abnormal pregnancies. Although conventional karyotyping detects the majority of balanced rearrangements, rare cryptic abnormalities below the resolution of standard karyotype may require advanced genomic techniques for identification [37].
2.4. Second-Trimester Maternal Serum Biochemistry
In 1984, researchers identified that low maternal serum alpha-fetoprotein (MSAFP) was more common in pregnancies with chromosomal abnormalities, particularly trisomies 21, 18, and sex chromosome disorders [38]. Initially, MSAFP combined with maternal age was the only marker used for Down syndrome screening. By 1988, unconjugated estriol (MSuE3) and human chorionic gonadotropin (MShCG) were introduced, improving detection rate [39]. The double test (AFP + free β-hCG) achieves a 60–65% detection rate at a 5% false positive rate, while the triple test (AFP + free β-hCG + MShCG) reaches a 65–70% detection rate at the same false positive rate [26,40]. The addition of Inhibin A (quadruple test) further increases detection rates to 70–75% at a 5% false positive rate [26,40,41,42]. Using intact hCG instead of free β-hCG slightly reduced detection rates by about 5% [26].
Beyond Down syndrome, several maternal second-trimester biochemistry screening programs involve also screening for trisomy 18 [2,27]. The double test detects 50% of trisomies 18 at a 1% false positive rate, while the triple test improves detection rate to 60% with a 0.3% false positive rate [2]. The triple test also screens for neural tube defects, placental dysfunction, and conditions like Smith–Lemli–Opitz syndrome (SLOS) [27]. Smith–Lemli–Opitz syndrome is an autosomal recessive disorder associated with moderate to severe mental retardation and multisystem congenital anomalies [43]. It is caused by a defect in the cholesterol biosynthetic pathway that results in low cholesterol concentrations and the accumulation of the precursors 7-dehydrocholesterol and 8-dehydrocholesterol in blood and tissues [44]. This finding has enabled the development of a reliable diagnostic test in amniotic fluid [45]. Smith–Lemli–Opitz syndrome is associated with low MSuE3 levels in maternal serum [46], with the triple test detecting 62% of cases at a 0.34% false positive rate at a cut-off level risk of 1:50 [45].
2.5. First-Trimester Screening
2.5.1. Nuchal Translucency
In 1992, it was first described that increased nuchal translucency above 3 mm is associated with an increased risk of chromosomal defects [7]. Soon after, it was found that the higher the nuchal translucency (and maternal age) the higher the risk of chromosomal defects, and that increased nuchal translucency also increases the risk of structural, mainly cardiac, defects [8,47]. For a 5% false positive rate, fetal NT screening identifies 75 to 80% of fetuses with trisomy 21 and other major aneuploidies [48].
Several subsequent studies confirmed that the risk of genetic and structural defects increases with increasing NT measurements [49,50,51,52]. In a recent study, Bardi et al. [53] reported the outcome of 1901 pregnancies with fetal nuchal translucency (NT) above the 95th percentile. Among these fetuses, 43.0% were classified as abnormal, with 23.9% having trisomies 21, 18, and 13, and 5.4% having other chromosomal abnormalities detectable solely by karyotyping. Single-gene disorders or abnormalities identified only through microarray analysis were found in 2.0% of the cases, while isolated structural anomalies were detected in 9.3% of the fetuses. According to these data, increased nuchal translucency should be considered an indication for genetic counseling and diagnostic testing. However, the cut-off size of NT remains controversial. Diagnostic testing is clearly indicated in cases of increased nuchal translucency associated with structural defects. However, the situation is more difficult in cases of isolated increased NT [20]. The majority of authors recommend to discuss an option of diagnostic testing with microarray analysis if in the combined test the NT size is above the 99th percentile (around 3.5 mm across the gestational ages) [20]. A 2015 meta-analysis indicated that abnormal microarray results occurred in 4.0% of cases with isolated increased NT above 3.5 mm and in 7.0% of cases with other malformations. The most common pathogenic CNVs included deletions and duplications on 22q11.2, 10q26.12q26.3, and 12q21q22. Variants of uncertain significance had a pooled prevalence of 1% [54]. However, this metanalysis did not analyze the risk of adverse pregnancy outcomes in cases with increased NT and normal results of a detailed 20-week scan. In 2007, Bilardo et al. [51] reported a 4% adverse pregnancy outcome in cases with normal karyotype and increased NT but normal results at the 20-week scan. Half of these were detectable by ultrasound. Excluding those, the chance of a healthy baby after a normal 20-week scan was 98%. They concluded that if the detailed ultrasound at 20 weeks is normal, a favorable outcome can be confidently expected despite any initially increased NT. Senat et al. [55] reported an 11.1% rate of abnormalities diagnosed, but half of these abnormalities were cardiac malformations that could potentially be detected by specialized fetal echocardiography. In summary, for cases with increased NT and normal karyotype or low-risk results from NIPT or the first-trimester combined test, a favorable outcome is likely when the 20-week scan is normal. However, caution is advised for extended NT (6.5 mm or more) thickened nuchal fold and subtle ultrasound findings at the 20-week scan [52]. Diagnostic testing with karyotyping and microarray analysis should be offered to parents in these cases. If results are normal and NT exceeds 5 mm, RASopathy testing (e.g., for Noonan syndrome and related disorders) using gene panels or whole exome sequencing (WES) is recommended [56,57].
2.5.2. Maternal Serum Biochemistry
In the 1990s, studies showed that in pregnancies affected by trisomy 21, maternal serum levels of free β-hCG were approximately twice as high, while PAPP-A levels were about half compared to euploid pregnancies [1]. When used as individual markers alongside maternal age, detection rates for trisomy 21 range from 42–46% for β-hCG and 48–52% for PAPP-A at a fixed 5% false positive rate for samples collected between 10 and 14 weeks of gestation [2]. Combining both markers with maternal age improves the detection rate to approximately 65% at the same false positive rate [1]. Screening is more effective at 11 weeks than at 13 weeks. Although the distinction between trisomy 21 and unaffected pregnancies based on serum free β-hCG becomes more pronounced as gestation progresses, the diminishing difference in PAPP-A levels has a greater impact, reducing overall screening accuracy at a later stage [58,59].
Beyond trisomy 21, abnormal free β-hCG and PAPP-A levels are also observed in other common chromosomal abnormalities [1,60,61,62,63]. In trisomies 18 and 13, both markers are reduced to around 0.4 MoM [60]. In diandric triploidy, free β-hCG is markedly elevated (above 8 MoM), whereas in digynic triploidy, both free β-hCG and PAPP-A are extremely low (below 0.2 MoM and 0.1 MoM, respectively [2,62]. Turner syndrome is associated with PAPP-A levels below 0.5 MoM, though free β-hCG remains within the normal range [2,63], making maternal serum biochemistry less distinctive for this condition.
A significantly increased risk of atypical chromosomal abnormalities is observed when maternal serum markers show extreme deviations. Specifically, either a marker falling below 0.2 MoM or free β-hCG exceeding 5.0 MoM is linked to a higher likelihood of chromosomal anomalies [64,65]. Peterson’s large-scale study of 193,638 pregnancies identified an inverse relationship between PAPP-A levels and the prevalence of atypical karyotypes. The occurrence of atypical chromosomal abnormalities was 4.2% when PAPP-A was below 0.2 MoM but dropped to just 0.1% for PAPP-A levels of 0.4 MoM or higher. Similarly, the prevalence was 7% in cases with low free β-hCG (<0.2 MoM) and 0.47% when free β-hCG exceeded 5 MoM [64].
2.5.3. First-Trimester Screening by Combined Test (NT-Maternal Serum Biochemistry)
The combined test is a combination of maternal serum biochemistry (PAPP-A and free β-hCG) and the size of NT. This combination provides better performance than screening by the size of fetal NT or maternal serum biochemistry alone. Several prospective interventional studies have demonstrated that for a 5% false positive rate, the first-trimester combined test identifies about 90% of trisomy 21 pregnancies [1]. Apart from trisomy 21, it was found that 90% of the other most common chromosomal anomalies can be identified for an additional 1% false positive rate [2]. Because the screening by fetal NT and maternal serum biochemistry cannot distinguish a clear discriminatory pattern between trisomy 13 and trisomy 18, first a combined trisomy 13/18-risk algorithm was developed [2]. The observation that fetal heart rate is also altered in a number of chromosomal anomalies allowed for the later development of a separate algorithm specifically identifying risk for trisomy 13 and trisomy 18. In screening by NT, fetal heart rate (FHR), free β-hCG, and PAPP-A, using specific algorithms for trisomy 21 and trisomies 18 and 13 at the risk cut-off of 1:100, the estimated detection rate (DR) was 87.0% for trisomy 21 and 91.8% for trisomies 18 and 13, at a false positive rate (FPR) of 2.2% [66].
A large recent study by Santorum et al. [28] investigated the test performance of the combined test in screening for chromosomal defects in 108 112 pregnancies. At a risk cut-off of 1 in 100 for trisomy 21, 18, or 13, at the gestational age of screening (cut-off of 1:150 at term) the observed false positive rate was 4.6% and the detection rate was 92%, 96%, and 93% for trisomies 21, 18, and 13, respectively. At the same risk cut-off, 98% of monosomies X, 97% of triploidies, and 55% of other chromosomal abnormalities were detected. Adding fetal heart rate into the combined test improved the detection rate for trisomy 13 but did not significantly affect the detection rate of other chromosomal abnormalities [28].
Typical markers of the most common aneuploidies in the combined test are displayed in Table 3.

Table 3.
First trimester biochemical and ultrasound marker patterns of the most common aneuploidies.
2.5.4. Additional First-Trimester Ultrasound Markers
In addition to increased nuchal translucency (NT), other first-trimester ultrasound markers associated with trisomy 21 include the absence of the nasal bone, abnormal blood flow in the ductus venosus, and tricuspid regurgitation [1]. These markers are present in approximately 60%, 66%, and 55% of trisomy 21 cases, respectively, while their occurrence in euploid fetuses is much lower (2.5%, 3.0%, and 1.0%) [1,67,68,69,70]. Integrating these markers into first-trimester screening, along with maternal age, fetal NT, and serum free β-hCG and PAPP-A, improves detection rates to 93–96% while lowering the false positive rate to 2.5% [12,67,69,70]. This improvement is effective whether all pregnancies undergo universal assessment or whether a contingent screening approach is used [1]. In the latter method, first-stage screening by maternal age, fetal NT, and serum free β-hCG and PAPP-A identifies high-risk (≥1 in 50) and low-risk (<1 in 1000) pregnancies, while those with an intermediate risk (1 in 51 to 1 in 1000, about 15% of cases) undergo additional ultrasound evaluation. If the adjusted risk after this second-stage screening is ≥1 in 100, the pregnancy is classified as high risk [1,71]. Ghaffari et al. have conducted as the only one a study on the first-trimester combined test, which includes biochemistry with assessment of all four ultrasound markers. The study reported a 100% detection rate at a 3.4% false positive rate [13].
Beyond trisomy 21, absent nasal bone, abnormal ductus venosus blood flow, and tricuspid regurgitation are also associated with trisomies 18 and 13, as well as Turner syndrome. These markers appear in 53%, 58%, and 33% of trisomy 18 cases; 45%, 55%, and 30% of trisomy 13 cases; and in 0%, 75%, and 38% of Turner syndrome cases, respectively [67,69,70].
Apart from the above-described markers, various first-trimester ultrasound anomalies have been linked to trisomy 18, trisomy 13, Turner syndrome, and triploidy [26,72,73]. Trisomy 18 is associated with early-onset fetal growth restriction (FGR), relative bradycardia, and exomphalos [26,74] while trisomy 13 is characterized by tachycardia, FGR, holoprosencephaly, and exomphalos [26,75]. Turner syndrome is characterized by fetal tachycardia and early-onset fetal growth restriction [26,76]. Triploidy features asymmetrical FGR, bradycardia, holoprosencephaly, exomphalos, cerebral cystic posterior fossa anomalies, and molar placental changes [26,77]. A study by Syngelaki et al. [78] examined the prevalence of severe anomalies such as alobar holoprosencephaly, exomphalos, megacystis, and NT ≥ 3.5 mm, identifying them in approximately 1% of pregnancies. Among these cases, over 40% had chromosomal abnormalities, with specific associations: trisomy 13 was most common in holoprosencephaly, trisomy 18 in exomphalos and megacystis, and trisomy 21 in increased NT. Unlike previous findings, the incidence of chromosomal abnormalities in exomphalos cases remained consistent regardless of crown–rump length (CRL), and in megacystis cases, it was similar across different bladder sizes. Similarly, Wagner et al. [72] reported a similar incidence of major defects, identifying them in only 1.3% of euploid fetuses. His study evaluated the effectiveness of first-trimester ultrasound screening for trisomy 18, trisomy 13, triploidy, and Turner syndrome using fetal NT, nasal bone assessment, tricuspid valve blood flow, ductus venosus evaluation, and a detailed anomaly scan at 11–13 weeks of gestation. Major anomalies were detected in 76% of Turner syndrome cases, 83% of trisomy 18 cases, 85% of triploidy cases, and all trisomy 13 cases. He concluded that a detailed anomaly scan at this stage can identify approximately 95% of affected fetuses.
2.6. First-Trimester Screening Followed by Second-Trimester Biochemical Testing
To enhance the accuracy of prenatal screening for chromosomal abnormalities, first- and second-trimester screening methods have been combined, achieving detection rates of 92–96% with a 5% false positive rate [5,10,79].
2.6.1. Integrated Test
The fully integrated test combines first-trimester PAPP-A and NT with the second-trimester quadruple screen, withholding early results to generate one overall risk assessment. In contrast, the serum integrated test uses only PAPP-A and the quadruple screen if NT is not available [1]. At a 5% false positive rate, the detection rate is 88% for serum integrated testing and 96% for the fully integrated approach when performed at 11 weeks [79]. This method has been controversial, as some argue that delaying first-trimester results deprives patients of the opportunity to learn their risk status early [27,79].
2.6.2. Stepwise Sequential Test
This approach involves first-trimester NT, PAPP-A, and free β-hCG screening, with high-risk cases proceeding to chorionic villus sampling (CVS). Intermediate- or low-risk patients undergo second-trimester biochemical screening, and those whose combined risk is high are offered amniocentesis [1,5]. This method detects 95% of trisomy 21 cases with a 5% false positive rate [79].
2.6.3. Independent Sequential Screening
In this method, first-trimester results are reported immediately. Women testing positive may choose CVS, while those testing negative proceed to the quadruple test, which is interpreted separately [79,80]. Platt at al. reported a 98% detection rate for trisomy 21 but with an unacceptably high 17% false positive rate. Similarly, the FASTER study found a 94% detection rate with an 11% false positive rate [27,79]. These findings suggest that this sequential screening approach should be avoided [27].
2.6.4. Contingent Test
The contingent test only conducts second-trimester biochemical screening for women with an intermediate risk after first-trimester NT, serum PAPP-A, and free β-hCG testing [1,5]. Cuckle et al. [81] used data from the SURUSS trial to show that, at a 5% false positive rate, this approach detects 94% of trisomy 21 cases while requiring only 15% of patients to return for additional testing. This method appears to be the most cost-effective compared to other combined screening strategies [5].
2.7. Second-Trimester Ultrasound (Genetic Sonogram)
Most of the chromosomal defects are associated with abnormalities that can be detected by second-trimester ultrasound [82,83]. The genetic sonogram means a more detailed anomaly scan performed in second-trimester fetuses that not only evaluates the fetus for structural malformations but also searches for the sonographic markers of chromosomal defects [84]. These markers are not malformations but rather variants more frequently found in Down syndrome and other chromosomal defects than in euploid fetuses [85]. It was first introduced in 1985 by Benaceraff et al. [86], who reported the association between a thickening of the neck area (nuchal fold) and Down syndrome.
The risk for chromosomal abnormalities rises with the number of identified abnormalities. It is therefore recommended that when a marker is detected, a thorough check is made for the other features of the chromosomal abnormality known to be associated with that marker because the presence of additional defects increases the risk substantially. Conversely, the absence of defects lowers the risk [26,83,87].
If major defects are detected during the second trimester, fetal karyotyping should be offered, even if isolated. Major defects are rare and do not greatly increase invasive testing rates. Karyotyping is also recommended in lethal defects in order to understand the cause and estimate the risk of recurrence [26]. Minor defects or markers are common and usually harmless unless linked to chromosomal abnormalities. Karyotyping for one isolated marker is not usually recommended as it would substantially increase the rate of invasive testing. Instead, an individual risk assessment should be provided by multiplying the background risk (based on maternal age, gestational age, history of previously affected pregnancies, and, where appropriate, the results of previous screening by NT and/or biochemistry in the current pregnancy) by the likelihood ratio of the specific defect/marker [26,83,84,88,89,90].
2.7.1. Down Syndrome
Down syndrome is the most common chromosomal abnormality among liveborn infants. Twenty-five percent of second-trimester fetuses with Down syndrome have major defects recognizable sonographically in the second trimester [91], cardiac defects being the most common, followed by duodenal atresia and ventriculomegaly [89]. In a recent study by Freiman et al., 66% of infants with trisomy 21 displayed cardiovascular defects with septal defects being the most common. This is even more than previously reported by Freeman et al. [89,92], who found cardiac defects in 44% of infants with Down syndrome, with 45% of the defects being atrioventricular septal defects, 35% ventricular septal defects, 8% isolated secundum atrial septal defects, 7% persistent patent ductus arteriosus, and 4% isolated tetralogy of Fallot. Apart from major structural defects, many soft markers have been described in fetuses with Down syndrome. First, in 1985, Benacerraf et al. reported the association between thickening of the neck area (increased nuchal fold thickness) and Down syndrome [86,89,90]. Several other ‘soft’ markers of Down syndrome detectable in the second trimester were subsequently described. The list of soft markers has undergone frequent changes over the years. However, in clinical practice the principal markers that comprise the genetic sonogram have remained unchanged for many years. They include an increased nuchal fold thickness, short femur or humerus, hydronephrosis, echogenic bowel, echogenic intracardiac focus, and any major structural malformation [83,85,93,94]. Using these markers, the genetic sonogram identifies as many as 65–75% of fetuses with Down syndrome, with a false positive rate of 4–15% [84]. In 2013, Agathokleous et al. [87] reviewed the prevalence of well-established markers of Down syndrome in the euploid and and Down syndrome. Fetuses. Moreover, they added nasal bone, aberrant right subclavian artery, and ventriculomegaly to the list of soft markers to be checked during a genetic sonogram. The clinical implications of their meta-analysis are as follows: Firstly, a systematic second-trimester ultrasound examination that demonstrates the absence of all major defects and markers results in a 7.7-fold reduction in the risk for trisomy 21. Secondly, all markers and defects associated with Down syndrome must be checked, and the a priori risk for trisomy 21 should be adjusted using the positive and negative likelihood ratios of each marker. Thirdly, most isolated markers, such as intracardiac echogenic focus, echogenic bowel, mild hydronephrosis, and short femur, have only a minimal effect on modifying the pre-test odds. The markers that significantly alter the risk include the presence of major defects, ventriculomegaly, increased nuchal fold thickness, absent nasal bone, and aberrant right subclavian artery. The soft markers described in this recent meta-analysis, along with their positive, negative, and cumulative likelihood ratios, are presented in Table 4.

Table 4.
Soft markers of trisomy 21 (adapted from Agathokleous et al. 2013 [87]): (LR+: positive likelihood ratio, LR−: negative likelihood ratio, LRc: cumulative likelihood ratio).
Several studies investigated the impact of adding genetic sonography after second-trimester maternal serum biochemistry and first-trimester screening. Krantz et al. [95] used a simulation model to evaluate integrating cumulative likelihood ratios from second-trimester ultrasound markers sequentially or contingently for women who had first-trimester screening. The model demonstrated that adjusting the first-trimester combined risk for Down syndrome using second-trimester ultrasound markers increased detection rates to 94% with a 5.4% false positive rate. Additionally, when a genetic sonogram was offered contingently to women with a risk between 1:300 and 1:2500 after the first-trimester combined test, the incremental detection rate increased by 4.8% and was accompanied by only 0.7% increase in the false positive rate. In the FASTER trial (First- and Second-Trimester Evaluation of Risk), the authors assessed that with a fixed 5% false positive rate, incorporating genetic sonography could increase Down syndrome detection rates from 81% to 90% in the combined test in the first trimester, and from 93% to 98% in the integrated test [96]. However, there are only a few validation studies regarding the potential role of genetic sonography [85]. Their results indicate that genetic sonography has a modest role after the initial first-trimester screening, affecting false positive rates more than detection rates. Mainly, it may help decision-making for women with risk results close to the cut-off threshold [85].
2.7.2. Trisomy 18
Trisomy 18 can be much more easily recognized by genetic sonogram than Down syndrome. Common findings include a strawberry-shaped head, choroid plexus cysts, absent corpus callosum, abnormalities of cisterna magna with Dandy–Walker malformation, facial cleft, micrognathia, nuchal edema, cardiac defects (in 90%), diaphragmatic hernia, esophageal atresia, exomphalos (rather small bowel-containing), single umbilical artery, renal abnormalities, echogenic bowel, myelomeningocele, fetal growth restriction and shortening of the limbs, radial aplasia, clenched hands with overlapping index finger and club or rocker-bottom feet [26,82,89]. The sonographic detection of trisomy 18 improves with gestational age, as one of the major features of trisomy 18 is severe fetal growth restriction, which is often associated with polyhydramnios [83,89]. In experienced hands, the sensitivity of sonographic detection of fetuses with trisomy 18 has been reported to be 95–100% [52,97,98]. The most commonly reported abnormalities in fetuses with trisomy 18 are cardiac defects and choroid plexus cysts [97,98]. Cardiac defects are present in approximately 80–84% of fetuses with trisomy 18 [97,98] and karyotyping is strongly recommended due to their significant association with various chromosomal anomalies. Conversely, choroid plexus cysts are also prevalent in euploid fetuses, and as an isolated finding, they do not markedly increase the risk of trisomy 18 [83,89,99].
2.7.3. Trisomy 13
In trisomy 13, the most frequent associated anomalies are major central nervous system anomalies (alobar holoprosencephaly, agenesis of the corpus callosum, and neural tube defects) with midline facial defects (mid-line facial clefts, hypotelorism, cyclopia, and proboscis) and cardiac defects (more than 90% of cases). Other anomalies include postaxial polydactyly, omphalocele, single umbilical artery, polycystic kidneys (30% of cases), postaxial polydactyly, and club or rocker-bottom feet [26,82,83,89]. The sonographic detection of fetuses with trisomy 13 in the second trimester approaches 90–100% [89,100,101].
2.7.4. Triploidy
Fetuses with triploidy can be classified into those with diandric triploidy (type I) and digynic triploidy (type II). Diandric triploidy, resulting from either a single sperm undergoing reduplication or two sperms fertilizing a haploid ovum, is more common (80%). Less than 20% of triploidies are caused by a double maternal contribution when the ovum fails to complete meiotic division before fertilization [102].
Triploidy is often miscarried or detected in the first trimester, though rare cases persist into the second or third trimester [28,89]. A hallmark of triploidy is severe, early-onset, asymmetrical growth restriction, where the head size remains near normal while the rest of the body is markedly undergrown [51,77,82,102]. This pattern is seen in about 70% of cases and is typically accompanied by oligohydramnios and a small placenta when the extra chromosome set is maternal [102,103]. In contrast, when the additional chromosomes are paternal, the placenta tends to be large and hydropic, resembling partial moles [103]. Besides growth restriction, triploid fetuses frequently exhibit multiple structural defects [102]. Jauniax et al. [102] reported anomalies in 93% of cases. Common sonographic findings include ventriculomegaly, posterior fossa anomalies such as Dandy–Walker malformation, agenesis of the corpus callosum, various cardiac and facial defects (including micrognathia), echogenic bowel, renal malformations, increased nuchal fold thickness, neural tube defects, clubbed feet, and hand abnormalities like syndactyly between the third and fourth digits [89,102,104].
2.7.5. Turner Syndrome and Other Sex Chromosome Aneuploidies
Sex chromosome aneuploidies as a group are the most common chromosomal disorders, affecting up to 1 in 400 newborns [105], with Turner syndrome being the most common. Apart from Turner syndrome, the majority of sex chromosome aneuploidies are not detected in the second-trimester scan [106]. As for Turner syndrome, the majority of affected fetuses are detected in the first-trimester scan [28]. In the second trimester, Turner syndrome is associated with nuchal cystic hygromas, generalized edema, brachycephaly and cardiac defects (mainly left-sided), and renal defects [82,107,108].
2.7.6. DiGeorge Syndrome (Microdeletion 22q11.2)
DiGeorge syndrome is the most common microdeletion disorder in humans, with an estimated incidence between 1:1000 and 1:6000 [109], though the actual prevalence might be higher due to underdiagnosis [23]. The syndrome is associated with a variety of serious clinical issues, including congenital heart defects, developmental delays, learning difficulties, immune deficiencies, palate abnormalities, hypocalcemia, and psychiatric disorders [23,110]. Although the symptoms can range from severe to mild or even be absent, the genetic alteration is typically fully penetrant [23,110].
In the second trimester, many fetuses with DiGeorge syndrome are typically identified due to their association with cardiac conotruncal defects (tetralogy of Fallot, truncus arteriosus, interrupted aortic arch, right aortic arch, double aortic arch, absent pulmonary valve syndrome) [111,112]. Chaoui et al. [112] reported that 13% of conotruncal anomalies were associated with DiGeorge syndrome. Additionally, they examined whether ultrasound assessment of an absent or hypoplastic fetal thymus would help identify fetuses at high risk for the 22q11.2 microdeletion. In their study, 90% (9 out of 10) of cases with del.22q11.2 were recognized through thymus evaluation. The specificity was 98.5%. In 2011, to simplify the ultrasound evaluation of the fetal thymic size and to estimate normal ranges, Chaoui et al. [113] introduced the thymic–thoracic (TT) ratio. This TT ratio is reliable and easy to obtain during fetal echocardiography in the three-vessel view. In normal fetuses (both with and without cardiac defects), the TT ratio did not show any statistically significant change during gestation, with a mean value of 0.44. However, nearly all fetuses with del.22q11 exhibited a significantly low TT ratio of less than 0.3. It was concluded that fetuses with both cardiac defects and a low TT ratio can be considered at high risk for having the microdeletion del.22q11 [113].
Further abnormalities described in del.22q11.2 fetuses include an enlarged cavum septum pellucidi [114], and fetal facial abnormalities such as micrognathia, cleft palate, bulbous nose, and abnormal ears [111]. However, these features are less specific and less common compared to conotruncal cardiac defects with thymic hypoplasia/aplasia.
2.7.7. Smith–Lemli–Opitz (SLO) Syndrome
Smith–Lemli–Opitz or RSH syndrome is an autosomal recessive disorder characterized by multiple congenital malformations and intellectual disability, attributed to a deficiency of 7-dehydrocholesterol reductase in the cholesterol biosynthesis pathway [115]. In the second trimester, it is associated with low levels of uE3 in maternal plasma. According to Palomaki et al. [46], with a risk cut-off level of 1:50, 62% of SLOS pregnancies can be detected at a false positive rate of 0.34%. However, approximately 1 in 90 screen-positive pregnancies will be affected. Therefore, following a positive result from maternal serum biochemistry, a detailed ultrasound examination should be conducted to further refine the risk of this syndrome. Goldenberg et al. [115] conducted a retrospective review of second-trimester ultrasound findings in 30 SLO fetuses. They observed that fetal growth restriction was the most frequent sonographic feature (20/30). In over half of the cases, this was accompanied by at least one other anomaly, including nuchal edema, renal, cardiac, cerebral malformations, genital anomalies, or polydactyly. In five out of 30 cases, isolated nuchal edema (3/30), and isolated cardiac (1/30) or renal malformations (1/30) were the only detectable anomalies. Overall, ultrasound results were abnormal in 83% of cases, but early detection of multiple malformations was uncommon (3/30, 10%). They suggested to re-consider a more systematic sterol analysis when dealing with fetal growth restriction, especially when associated anomalies are detected [115]. Similar associated abnormalities have also been reported by other authors [96,116,117].
2.7.8. Rare Chromosomal Abnormalities (RCA)
The European Surveillance of Congenital Anomalies database indicates that the prevalence of RCA is around 0.07 per 1000 births with a prenatal ultrasound detection rate averaging 65% (range 5–92%) [118]. The EUROSCAN study reported similar rates, ranging from 31% to 75%, depending on the type of chromosomal abnormality [106].
Common ultrasound findings in rare chromosomal abnormalities include cardiac defects, nuchal edema, cerebral and renal defects, and limb abnormalities. These structural issues are linked to fetal growth restriction, increasing the risk of chromosomal abnormalities [82,106]. Generally, if a second-trimester scan reveals several abnormal findings, including fetal growth restriction, structural defects and soft markers, karyotyping should be offered, as the risk of all (including less common) chromosomal defects is markedly increased.
2.8. Non-Invasive Prenatal Cell-Free DNA Testing (NIPT)
2.8.1. The Principle of NIPT
In 1998 Lo et al. demonstrated that fetal DNA concentrations in maternal plasma could be measured [119]. It is generally assumed that apoptosis of villous cytotrophoblast cells, a process occurring in all pregnant women, leads to the release of cell-free DNA (cfDNA) into the circulation [120]. Cell-free DNA can be detected from 4 weeks gestation [121] and unlike fetal cells, following delivery, fetal cell-free DNA is undetectable after two hours, having a half-life of just 16 min [122].
Initial clinical uses of fetal cell-free DNA (cfDNA) were for fetal sex determination and RhD genotyping [123]. However, further developments in technology led to the use of the test (known as non-invasive prenatal testing or NIPT) for screening for fetal aneuploidies, which can be performed from 9 weeks of pregnancy. Compared to the traditional first-trimester combined test, NIPT achieves a significantly improved positive predictive value, from around 3–4% for first-trimester combined test [15] up to around 90–95% for NIPT for trisomy 21 [124]. Moreover, it has a high sensitivity for all common trisomies [125].
While NIPT’s lower false positive rates offer clinical benefits, biological challenges can result in discordant results. CfDNA originates from the placenta, not directly from the fetus. Usually, placental and fetal DNA are identical, but differences (e.g., confined placental or fetal mosaicism) can lead to false positives or negatives via processes like trisomy rescue or non-disjunction [126]. In addition to affecting test accuracy, confined placental mosaicism has also been associated with adverse pregnancy outcomes such as being small for gestational age (SGA), fetal growth restriction (FGR), and hypertensive disorders, which may guide further clinical monitoring [127,128] Additionally, the blood sample taken for NIPT contains cfDNA from the mother herself, which may reflect mosaicism or other chromosome variations in her. About 1 in 1000 women has cancer during pregnancy [129], and this can interfere with the NIPT results. Also, a vanished twin may have been present. A vanished twin’s DNA can persist for 15 weeks or more after the demise of the twin [130] and since the demise may have been related to chromosomal anomalies, this can account for some discordant NIPT results. Finally, triploidy can be difficult to detect by many NIPT technologies [131].
The choice of condition to screen for and the technology used affect NIPT results. Since NIPT became widely used, its applications have expanded from detecting common aneuploidies to include sex chromosome aneuploidies, microdeletions [132], copy number variants [19,133], and some single gene disorders [134,135]. It must be kept in mind that all these technologies assess cell-free DNA from the placenta, not the fetus, which can lead to discordant results for several reasons including confined placental mosaicism. Therefore, NIPT is a screening test, not a diagnostic one. High-risk results need diagnostic confirmation, and for low-risk results, the patient should be aware that there is still a small chance of a fetus being affected by one of the screened conditions [136].
2.8.2. NIPT Methodologies
Although there are various different approaches to obtaining data for NIPT analysis, from microarrays to rolling ball analysis to Multiple Parallel Shotgun Sequencing (MPSS) and single nucleotide polymorphism sequencing (SNPs) [137], they can be divided into two main categories in terms of the way in which the data lead to an assessment of the chance of anomaly—the counting approaches and the SNP approach.
In the counting approach, all the cell-free DNA fragments from all sources are analyzed together according to which chromosome they originated from. There is a reference chromosome that is expected to be normal when used for comparison. The observed fragments from this reference are compared to those from each chromosome or region of interest. There is an expected ratio of reference chromosome fragments to chromosome of interest fragments. If the expected ratio of reference chromosome fragments to those of the chromosome of interest is abnormal, this can indicate changes due to fetal material. However, anomalies like an abnormal reference chromosome, vanished twin material, or maternal chromosome issues may lead to discordant NIPT results.
With the SNP approach, the test is sequencing areas of the cell-free DNA that are frequent sites for single nucleotide polymorphisms—the normal variations which distinguish one DNA profile from another. At each point where there is a SNP difference between the maternal and fetal DNA profile, an allele ratio can be assessed. This gives an assessment of whether, for each chromosome or area of interest, the fetus is likely to have disomy or trisomy (or monosomy in the case of the X chromosome). This approach allows a distinction to be made between different DNA profiles, and so it helps to assess whether an anomaly might originate from the maternal DNA, or whether there is an additional unexpected DNA haplotype present which could reflect the presence of vanished twin material or a triploidy. This helps to identify a number of situations that can lead to discordant results for NIPT. For twin pregnancies, it allows an assessment of whether the twins are monozygotic or dizygotic. This has an impact on the calculation of the risk of trisomy 21, as the prior risk for dizygotic twins is greater than for monozygotic twins [138].
A further factor in the assessment of fetal genetic conditions through NIPT is whether a test is targeted or untargeted, and if untargeted, what range of results are reported. A targeted test will focus on specific chromosomes or regions of interest (typically selected microdeletions). This means that a narrower range of conditions is being assessed but it can also be easier to achieve a greater depth of sequencing at the regions that are assessed, which can assist with detecting small changes such as those that occur with microdeletions [133]. An untargeted test will look at areas of DNA scattered across all the chromosomes. This can generate results for trisomies of chromosomes other than 21, 13, and 18 or the sex chromosomes. It can also assess copy number variants, which are typically large areas of cell-free DNA that are duplicated or deleted. Often these are areas of 10 Mb or greater, which is larger than most microdeletions, which typically have a span of 1–3 Mb [19].
Demko et al. [18] in their meta-analysis evaluating the performance of NIPT methods for trisomies 21, 18, and 13 and monosomy X reported a higher detection rate and lower false positive rate for SNP than other methods, significantly so for MPSS. The performance of the different methods in the 84 clinical experience studies was consistent with validation studies. He concluded that all NIPT methods are highly effective for fetal aneuploidy screening, with performance differences across methodologies.
2.8.3. Performance of NIPT for the Common Conditions Screened
Although the performances of different approaches to NIPT may vary from one to the other and depending on the background risk of the population being screened, a meta-analysis demonstrated sensitivities in singleton pregnancies of over 99% for trisomy 21, with a false positive of 0.04%. For trisomy 18 the sensitivity was around 98% with a false positive rate of 0.04% and for trisomy 13 the sensitivity was 99% with a false positive rate of 0.04% [17]. Given these very low residual risks after a negative cfDNA result, soft markers for autosomal trisomies, such as echogenic intracardiac focus or mild ventriculomegaly, should not be considered significant. This is the reason why, according to the ISUOG Practice Guidelines, these second-trimester soft markers should not be reported after a negative cfDNA screening result [139].
For twin pregnancies, the sensitivity for trisomy 21 was 98% with a 0.05% false positive rate, and for trisomy 18 the sensitivity was around 89% with a false positive rate of 0.03%. Sensitivity for trisomy 13 has been more challenging to establish for twin pregnancies due to the small numbers of affected samples for analysis but the false positive rate was calculated at 0.19% [140].
A further aneuploidy that may be screened for with NIPT is monosomy X (Turner’s syndrome), though it has a higher false positive rate due to factors like maternal loss of the X chromosome with age, maternal mosaicism, and placental mosaicism. Positive predictive values range from 20–50% [141,142]. Sensitivity is hard to determine without genetic testing on all babies in a study, but one such study found an 83% sensitivity [142,143].
Fetal sex determination by NIPT is generally highly accurate. Mackie et al. [144] in a systematic review reported a sensitivity of 98.9% and a specificity of 99.6%. Fetal sex determination by NIPT can be affected by factors such as the unrecognized presence of vanished twin cfDNA. Detecting vanished twin cfDNA through the SNP method can help reduce the likelihood of incorrect fetal sex determinations. It is noteworthy that discrepancies in fetal sex between NIPT and prenatal ultrasound might be due to a disorder of sexual development. To investigate this, one may consider repeating the NIPT, excluding the possibility of a sample swap, repeating the ultrasound scan, verifying the laboratory results, and considering invasive prenatal testing as appropriate [145].
2.8.4. NIPT No-Calls and Complex Results
Not all NIPT samples result in high or low risk. Some may return no-call due to low fetal fraction or insufficient sample quality or be uninformative or atypical depending on the lab. One of the most common reasons for a no-call is a low fetal fraction. Fetal fraction is the proportion of cfDNA which is of fetoplacental rather than maternal origin. It has been highlighted by various professional societies as an important quality metric for NIPT [125,146].
Some NIPT tests have a threshold fetal fraction below which they do not return a result; while others do not measure fetal fraction or do not have a threshold. However, failure to measure the fetal fraction, or using a method that does not accurately distinguish between maternal and fetal fraction can lead to incorrect NIPT results, including normal female results in non-pregnant women [147]. Various measurement methods exist, such as measuring cfDNA fragment lengths; fetoplacental fragments are generally shorter than maternal ones [148]. However, there is a significant overlap in fragment length between fetal and maternal fragments, and the true value may vary from that measured, with concomitant risk of under- or over-estimation [148]. The SNP method looks at the paternal contribution by analyzing the allele ratios at points where the mother and fetus have different nucleotides [149,150]. This method has been demonstrated to have increased accuracy [150]. For dizygotic twin pregnancies, it has been shown that the fetal fraction may vary significantly between twins [151]. The fetal fragment length approach does not differentiate a fetal fraction for each twin but rather estimates the total fetal fraction. The use of SNPs for fetal fraction measurement allows individual fetal fraction assessment, reducing false negatives due to insufficient fetal fraction in one twin [151]. Several different factors can contribute to a low fetal fraction including high maternal BMI, early gestational age, maternal heparin therapy, and aneuploidy (particularly trisomy 18 and 13 and triploidy) [125,146].
In the case of a no-call due to low fetal fraction, or another sample-specific quality issue, a redraw will resolve the issue in around 75% of cases and give a result [15] although this percentage of successful redraws will be lower in patients with a high BMI [152]. It is important to note that no-call results have a higher aneuploidy risk, with one study showing a 2.7% risk compared to 0.4% for the whole cohort [15]. Delaying definitive testing to repeat the sample could impact the reproductive options available to patients, especially those who have NIPT at later gestations. This should be considered when evaluating options after a no-call.
Some results may be uninterpretable, or atypical (there are variations in the terminology used by different laboratories) where the test algorithm cannot clearly call a sample as either normal or a typical aneuploidy. This excludes samples failing due to low fetal fraction or other quality issues. Depending on the test methodology, this type of result could be because of a normal variation, or a finding outside of the scope of the test such as mosaicism or a copy number variant in the fetus or placenta, or in the mother. For example, multiple copy number variants across the chromosomes screened have been found to be associated with an increased risk of maternal malignancy [153]. Unusual or multiple aneuploidies have also been associated with an increased risk of maternal cancer, and ACOG recommends that patients with such a result are referred for genetic counselling and maternal–fetal medicine consultation [125]. If the results are uninterpretable, a redraw is unlikely to resolve the issue. Therefore, other forms of investigation should be considered. Test failures and complex or unclear results can differ among NIPT tests due to varying technologies and lab reporting specifics. Clinicians should consult with the testing lab if unsure about potential causes, and plan further investigations accordingly.
2.8.5. Expanded Testing with NIPT
Moreover, NIPT tests also allow testing for microdeletions, rare autosomal trisomies (RATs), and untargeted genome-wide copy number variants.
There are many different microdeletion syndromes recognized in humans and collectively they occur frequently [154]. The most common is 22q11 microdeletion syndrome, with a frequency of up to 1 in 1000 during pregnancy [155]. Many NIPT tests now screen for 22q11, but their sensitivity and positive predictive value vary. Recently, Dar et al. [156] in a SMART study validated an updated version of SNP-based NIPT screening for 22q11 in 20,887 pregnancies, finding a sensitivity of 83.3%, specificity of 99.95%, positive predictive value of 52.6%, and negative predictive value of 99.99%. The false positive rate was 0.05%. Currently, the American College of Medical Genetics (ACMG) recommends that all pregnant patients are offered screening for 22q11 deletion syndrome [21]. NIPT tests may also include a range of other microdeletions, but screening for these is not currently recommended by professional societies such as the American College of Medical Genetics and the American College of Gynecology and Obstetrics. It is important to note that, in general, the lower the prevalence of a condition the lower the positive predictive value of a screening test will be. For conditions with a prevalence of 1 in 50,000 or lower (as is the case with many microdeletions) the positive predictive value of an NIPT screen may be less than 1%.
Untargeted NIPT tests sometimes offer screening for Rare Autosomal Trisomies (RATs). RATs are often confined to the placenta as confirmed in a large study in the Netherlands. This study showed a positive predictive value of just 6% for RATS [19], and thus their reporting should be avoided [19,25].
Untargeted genome-wide copy number variants represent large deletions and duplications across all chromosomes. The lack of validated performance data for these has led to them not being recommended by professional societies including the American College of Medical Genetics and the International Society for Prenatal Diagnosis.
NIPT is currently being used in some areas to screen for fetal RhD status in women who are Rhesus negative and in recent years, NIPT tests are also offering screening for some single gene disorders [157]. If using these approaches in the context of known family history, it is important to communicate with the providing laboratory regarding the mutation that is known in the family so that the laboratory can state their anticipated likelihood of detecting the mutation if present. Patients should be aware that this is only a screening, and that for definitive diagnosis a diagnostic test would be necessary. A few laboratories are offering a different type of approach that they call ‘NIPD’ or non-invasive prenatal diagnosis for couples with known risks of single gene disorders, sometimes specifically designed for couples using advanced techniques, but such tests are beyond the scope of this discussion.
2.8.6. The Future of NIPT
Low fetal fraction has been identified as having an association with adverse pregnancy outcomes and complications including hypertensive disorders in pregnancy, gestational diabetes, and a fetus being small for gestational age or growth restricted [158]. Work is underway to investigate how it could be used alongside other markers in the prediction of pre-eclampsia along with other potential complications of pregnancy [159].
With increasing options for therapies available for several previously untreatable genetic disorders, as well as emerging developments in the field of fetal therapies [160], prenatal identification of single gene disorders is likely to be of growing interest. As sequencing capabilities advance, an increasing range of conditions will become available for screening. However, it is important to assess the clinical value of early knowledge of the conditions alongside the capacity of a test to perform well for each condition as shown by published validation data.
3. Conclusions
To conclude, the current best option for prenatal screening of chromosomal defects is the combination of the first-trimester combined test (NT-biochemistry) with an anomaly scan and cell-free DNA (cfDNA) non-invasive prenatal testing (NIPT), followed by a detailed anomaly scan in the second trimester. This screening strategy allows detection of the majority of common chromosomal defects and approximately half of less common chromosomal defects in the first trimester, as well as many of the remaining, predominantly less common, chromosomal defects in the second trimester.
NIPT cfDNA testing can be implemented as a first-tier test for the entire population, followed by the first-trimester combined test, or after the first-trimester combined test using a contingent strategy. Due to the economic costs associated with NIPT, the contingent strategy has been adopted by most countries. However, it is anticipated that the cost of cfDNA testing will decrease sufficiently in the near future to enable routine screening. Nevertheless, NIPT cannot replace ultrasound examinations in either the first or second trimester.
Importantly, when a negative NIPT result is obtained, soft markers for trisomies (such as echogenic intracardiac focus or mild ventriculomegaly) should not be considered significant, as emphasized by the ISUOG guidelines. However, major structural abnormalities must still be carefully evaluated by ultrasound, regardless of NIPT results.
NIPT cfDNA testing represents the most effective screening method for Down syndrome and is also efficient in identifying trisomy 18, trisomy 13, Turner syndrome, sex chromosome abnormalities, and also 22q11.2 deletion (DiGeorge Syndrome) and triploidy using SNP methods. However, ultrasound examination remains essential for identifying less common chromosomal defects and structural abnormalities. Additionally, the measurement of nuchal translucency in the first trimester provides critical information regarding pregnancy prognosis and the risk of defects and complications.
In the near future, improvement and further development of NIPT cfDNA methods can be expected. These methods will potentially enable screening for microdeletion syndromes, molar pregnancies, rare autosomal trisomies, Rh status, single gene disorders, and segmental imbalances. Some NIPT result types have been shown to be associated with an increased risk of maternal morbidity including neoplasms, and further work is underway to better understand how to use this information in future.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Nicolaides, K.H. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K. Aneuploidy screening in the first trimester. Am. J. Med. Genet. C Semin. Med. Genet. 2007, 145, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Loane, M.; Garne, E. The prevalence of congenital anomalies in Europe. Adv. Exp. Med. Biol. 2010, 686, 349–364. [Google Scholar] [PubMed]
- Jindal, A.; Sharma, M.; Karena, Z.V.; Chaudhary, C. Amniocentesis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Anderson, C.L.; Brown, C.E. Fetal chromosomal abnormalities: Antenatal screening and diagnosis. Am. Fam. Physician 2009, 79, 117–123. [Google Scholar]
- Alldred, S.K.; Takwoingi, Y.; Guo, B.; Pennant, M.; Deeks, J.J.; Neilson, J.P.; Alfirevic, Z. First and second trimester serum tests with and without first trimester ultrasound tests for Down’s syndrome screening. Cochrane Database Syst. Rev. 2017, 3, CD012599. [Google Scholar]
- Nicolaides, K.H.; Azar, G.; Byrne, D.; Mansur, C.; Marks, K. Fetal nuchal translucency: Ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ 1992, 304, 867–869. [Google Scholar] [CrossRef]
- Pandya, P.P.; Snijders, R.J.; Johnson, S.P.; De Lourdes Brizot, M.; Nicolaides, K.H. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. Br. J. Obstet. Gynaecol. 1995, 102, 957–962. [Google Scholar] [CrossRef]
- Spencer, K.; Souter, V.; Tul, N.; Snijders, R.; Nicolaides, K.H. A screening program for trisomy 21 at 10–14 weeks using fetal nuchal translucency, maternal serum free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. 1999, 13, 231–237. [Google Scholar] [CrossRef]
- Wald, N.J.; Rodeck, C.; Hackshaw, A.K.; Rudnicka, A. SURUSS in perspective. Semin. Perinatol. 2005, 29, 225–235. [Google Scholar] [CrossRef]
- Cuckle, H.S.; Malone, F.D.; Wright, D.; Porter, T.F.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Berkowitz, R.L.; Ferreira, J.C.; Dugoff, L.; et al. Contingent screening for Down syndrome—results from the FaSTER trial. Prenat. Diagn. 2008, 28, 89–94. [Google Scholar] [CrossRef]
- Abele, H.; Wagner, P.; Sonek, J.; Hoopmann, M.; Brucker, S.; Artunc-Ulkumen, B.; Kagan, K.O. First trimester ultrasound screening for Down syndrome based on maternal age, fetal nuchal translucency and different combinations of the additional markers nasal bone, tricuspid and ductus venosus flow. Prenat. Diagn. 2015, 35, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.R.; Tahmasebpour, A.R.; Jamal, A.; Hantoushzadeh, S.; Eslamian, L.; Marsoosi, V.; Fattahi, F.; Rajaei, M.; Niroomanesh, S.; Borna, S.; et al. First-trimester screening for chromosomal abnormalities by integrated application of nuchal translucency, nasal bone, tricuspid regurgitation and ductus venosus flow combined with maternal serum free beta-hCG and PAPP-A: A 5-year prospective study. Ultrasound Obstet. Gynecol. 2012, 39, 528–534. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Cheng, P.J.; Shaw, S.W.; Hsu, J.J.; Chen, R.C.; Tseng, Y.J.; Chu, W.C. Extended first-trimester screening using multiple sonographic markers and maternal serum biochemistry: A five-year prospective study. Fetal Diagn. Ther. 2014, 35, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.E.; Jacobsson, B.; Swamy, G.K.; Laurent, L.C.; Ranzini, A.C.; Brar, H.; Tomlinson, M.W.; Pereira, L.; Spitz, J.L.; Hollemon, D.; et al. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015, 372, 1589–1597. [Google Scholar] [CrossRef]
- Iwarsson, E.; Jacobsson, B.; Dagerhamn, J.; Davidson, T.; Bernabe, E.; Heibert Arnlind, M. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population—A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2017, 96, 7–18. [Google Scholar] [CrossRef]
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef]
- Demko, Z.; Prigmore, B.; Benn, P. A Critical Evaluation of Validation and Clinical Experience Studies in Non-Invasive Prenatal Testing for Trisomies 21, 18, and 13 and Monosomy X. J. Clin. Med. 2022, 11, 4760. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, K.R.M.; Sistermans, E.A.; Macville, M.V.E.; Stevens, S.J.C.; Bax, C.J.; Bekker, M.N.; Bilardo, C.M.; Boon, E.M.J.; Boter, M.; Diderich, K.E.M.; et al. TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am. J. Hum. Genet. 2019, 105, 1091–1101. [Google Scholar] [CrossRef]
- Kagan, K.O.; Sonek, J.; Kozlowski, P. Antenatal screening for chromosomal abnormalities. Arch. Gynecol. Obstet. 2022, 305, 825–835. [Google Scholar] [CrossRef]
- Dungan, J.S.; Klugman, S.; Darilek, S.; Malinowski, J.; Akkari, Y.M.N.; Monaghan, K.G.; Erwin, A.; Best, R.G. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023, 25, 100336. [Google Scholar] [CrossRef]
- Benn, P.; Cuckle, H. Overview of Noninvasive Prenatal Testing (NIPT) for the Detection of Fetal Chromosome Abnormalities; Differences in Laboratory Methods and Scope of Testing. Clin. Obstet. Gynecol. 2023, 66, 536–556. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.J. Reproductive genetic screening for information: Evolving paradigms? J. Perinat. Med. 2021, 49, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Gilstrop Thompson, M.; Xu, W.; Moore, B.; Wang, T.; Sun, N.; Pewar, H.; Avent, N.D.; Vernaza, A.; Acosta, F.; Saben, J.L.; et al. Clinical Validation of a Prenatal Cell-Free DNA Screening Test for Fetal RHD in a Large U.S. Cohort. Obstet. Gynecol. 2025, 145, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.C.; Barrie, E.S.; Malinowski, J.; Jenkins, G.P.; McClain, M.R.; LaGrave, D.; Leung, M.L.; Practice, A.P.; Guidelines, C. Systematic evidence-based review: The application of noninvasive prenatal screening using cell-free DNA in general-risk pregnancies. Genet. Med. 2022, 24, 1992. [Google Scholar] [CrossRef]
- Nicolaides, K.H. Screening for chromosomal defects. Ultrasound Obstet. Gynecol. 2003, 21, 313–321. [Google Scholar] [CrossRef]
- Chitayat, D.; Langlois, S.; Douglas Wilson, R.; Sogc Genetics, C.; Ccmg Prenatal Diagnosis, C. Prenatal screening for fetal aneuploidy in singleton pregnancies. J. Obstet. Gynaecol. Can. 2011, 33, 736–750. [Google Scholar] [CrossRef]
- Santorum, M.; Wright, D.; Syngelaki, A.; Karagioti, N.; Nicolaides, K.H. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet. Gynecol. 2017, 49, 714–720. [Google Scholar] [CrossRef]
- Elmerdahl Frederiksen, L.; Olgaard, S.M.; Roos, L.; Petersen, O.B.; Rode, L.; Hartwig, T.; Ekelund, C.K.; Danish Central Cytogenetics Registry Study, G.; Vogel, I. Maternal age and the risk of fetal aneuploidy: A nationwide cohort study of more than 500 000 singleton pregnancies in Denmark from 2008 to 2017. Acta Obstet. Gynecol. Scand. 2024, 103, 351–359. [Google Scholar] [CrossRef]
- Snijders, R.J.; Sundberg, K.; Holzgreve, W.; Henry, G.; Nicolaides, K.H. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet. Gynecol. 1999, 13, 167–170. [Google Scholar] [CrossRef]
- Hook, E.B.; Cross, P.K.; Schreinemachers, D.M. Chromosomal abnormality rates at amniocentesis and in live-born infants. JAMA 1983, 249, 2034–2038. [Google Scholar] [CrossRef]
- Snijders, R.J.; Sebire, N.J.; Nicolaides, K.H. Maternal age and gestational age-specific risk for chromosomal defects. Fetal Diagn. Ther. 1995, 10, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Wald, N.J.; Watt, H.C. Fetal loss in Down syndrome pregnancies. Prenat. Diagn. 1999, 19, 142–145. [Google Scholar] [CrossRef]
- Warburton, D.; Dallaire, L.; Thangavelu, M.; Ross, L.; Levin, B.; Kline, J. Trisomy recurrence: A reconsideration based on North American data. Am. J. Hum. Genet. 2004, 75, 376–385. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.; Halliday, J.; Chan, A.; Bower, C.; Morris, J.K. Recurrence risks for trisomies 13, 18, and 21. Am. J. Med. Genet. A 2009, 149, 2716–2722. [Google Scholar] [CrossRef]
- van Zutven, L.; Mijalkovic, J.; van Veghel-Plandsoen, M.; Goense, M.; Polak, M.; Knapen, M.; de Weerd, S.; Joosten, M.; Diderich, K.E.M.; Hoefsloot, L.H.; et al. What proportion of couples with a history of recurrent pregnancy loss and with a balanced rearrangement in one parent can potentially be identified through cell-free DNA genotyping? Mol. Cytogenet. 2023, 16, 26. [Google Scholar] [CrossRef]
- Xie, M.; Xue, J.; Zhang, Y.; Zhou, Y.; Yu, Q.; Li, H.; Li, Q. Combination of trio-based whole exome sequencing and optical genome mapping reveals a cryptic balanced translocation that causes unbalanced chromosomal rearrangements in a family with multiple anomalies. Front. Genet. 2023, 14, 1248544. [Google Scholar] [CrossRef]
- Merkatz, I.R.; Nitowsky, H.M.; Macri, J.N.; Johnson, W.E. An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. Am. J. Obstet. Gynecol. 1984, 148, 886–894. [Google Scholar] [CrossRef]
- Wald, N.J.; Cuckle, H.S.; Densem, J.W.; Nanchahal, K.; Royston, P.; Chard, T.; Haddow, J.E.; Knight, G.J.; Palomaki, G.E.; Canick, J.A. Maternal serum screening for Down’s syndrome in early pregnancy. BMJ 1988, 297, 883–887. [Google Scholar] [CrossRef]
- Wald, N.J.; Huttly, W.J.; Hackshaw, A.K. Antenatal screening for Down’s syndrome with the quadruple test. Lancet 2003, 361, 835–836. [Google Scholar] [CrossRef]
- Wald, N.J.; Densem, J.W.; George, L.; Muttukrishna, S.; Knight, P.G. Prenatal screening for Down’s syndrome using inhibin-A as a serum marker. Prenat. Diagn. 1996, 16, 143–153. [Google Scholar] [CrossRef]
- Cuckle, H. Biochemical screening for Down syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 92, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D. RSH/Smith-Lemli-Opitz syndrome: A multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab. 2000, 71, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Jira, P.E.; Waterham, H.R.; Wanders, R.J.; Smeitink, J.A.; Sengers, R.C.; Wevers, R.A. Smith-Lemli-Opitz syndrome and the DHCR7 gene. Ann. Hum. Genet. 2003, 67, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, G.E.; Bradley, L.A.; Knight, G.J.; Craig, W.Y.; Haddow, J.E. Assigning risk for Smith-Lemli-Opitz syndrome as part of 2nd trimester screening for Down’s syndrome. J. Med. Screen. 2002, 9, 43–44. [Google Scholar] [CrossRef]
- Bradley, L.A.; Palomaki, G.E.; Knight, G.J.; Haddow, J.E.; Opitz, J.M.; Irons, M.; Kelley, R.I.; Tint, G.S. Levels of unconjugated estriol and other maternal serum markers in pregnancies with Smith-Lemli-Opitz (RSH) syndrome fetuses. Am. J. Med. Genet. 1999, 82, 355–358. [Google Scholar] [CrossRef]
- Pandya, P.P.; Kondylios, A.; Hilbert, L.; Snijders, R.J.; Nicolaides, K.H. Chromosomal defects and outcome in 1015 fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 1995, 5, 15–19. [Google Scholar] [CrossRef]
- Nicolaides, K.H. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am. J. Obstet. Gynecol. 2004, 191, 45–67. [Google Scholar] [CrossRef]
- Kagan, K.O.; Avgidou, K.; Molina, F.S.; Gajewska, K.; Nicolaides, K.H. Relation between increased fetal nuchal translucency thickness and chromosomal defects. Obstet. Gynecol. 2006, 107, 6–10. [Google Scholar] [CrossRef]
- Souka, A.P.; Krampl, E.; Bakalis, S.; Heath, V.; Nicolaides, K.H. Outcome of pregnancy in chromosomally normal fetuses with increased nuchal translucency in the first trimester. Ultrasound Obstet. Gynecol. 2001, 18, 9–17. [Google Scholar] [CrossRef]
- Bilardo, C.M.; Muller, M.A.; Pajkrt, E.; Clur, S.A.; van Zalen, M.M.; Bijlsma, E.K. Increased nuchal translucency thickness and normal karyotype: Time for parental reassurance. Ultrasound Obstet. Gynecol. 2007, 30, 11–18. [Google Scholar] [CrossRef]
- Bekker, M.N. A normal 20-week scan of a euploid fetus with a history of first-trimester increased nuchal translucency: Caution or reassurance? Ultrasound Obstet. Gynecol. 2007, 30, 8–10. [Google Scholar] [CrossRef]
- Bardi, F.; Bosschieter, P.; Verheij, J.; Go, A.; Haak, M.; Bekker, M.; Sikkel, E.; Coumans, A.; Pajkrt, E.; Bilardo, C. Is there still a role for nuchal translucency measurement in the changing paradigm of first trimester screening? Prenat. Diagn. 2020, 40, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.; Jansen, F.A.; Blumenfeld, Y.J.; Fisher, A.; Odibo, A.O.; Haak, M.C.; Borrell, A. Genomic microarray in fetuses with increased nuchal translucency and normal karyotype: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 46, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Senat, M.V.; Bussieres, L.; Couderc, S.; Roume, J.; Rozenberg, P.; Bouyer, J.; Ville, Y. Long-term outcome of children born after a first-trimester measurement of nuchal translucency at the 99th percentile or greater with normal karyotype: A prospective study. Am. J. Obstet. Gynecol. 2007, 196, 53.e1–53.e6. [Google Scholar] [CrossRef][Green Version]
- Stuurman, K.E.; Joosten, M.; van der Burgt, I.; Elting, M.; Yntema, H.G.; Meijers-Heijboer, H.; Rinne, T. Prenatal ultrasound findings of rasopathies in a cohort of 424 fetuses: Update on genetic testing in the NGS era. J. Med. Genet. 2019, 56, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.; McGillivray, G.; Meagher, S.; Hui, L. Increased nuchal translucency after low-risk noninvasive prenatal testing: What should we tell prospective parents? Prenat. Diagn. 2021, 41, 1305–1315. [Google Scholar] [CrossRef]
- Wright, D.; Spencer, K.; Kagan, K.K.; Torring, N.; Petersen, O.B.; Christou, A.; Kallikas, J.; Nicolaides, K.H. First-trimester combined screening for trisomy 21 at 7–14 weeks’ gestation. Ultrasound Obstet. Gynecol. 2010, 36, 404–411. [Google Scholar] [CrossRef]
- Kagan, K.O.; Wright, D.; Baker, A.; Sahota, D.; Nicolaides, K.H. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. 2008, 31, 618–624. [Google Scholar] [CrossRef]
- Spencer, K.; Nicolaides, K.H. A first trimester trisomy 13/trisomy 18 risk algorithm combining fetal nuchal translucency thickness, maternal serum free beta-hCG and PAPP-A. Prenat. Diagn. 2002, 22, 877–879. [Google Scholar] [CrossRef]
- Spencer, K.; Ong, C.; Skentou, H.; Liao, A.W.; Nicolaides, K.H. Screening for trisomy 13 by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10–14 weeks of gestation. Prenat. Diagn. 2000, 20, 411–416. [Google Scholar] [CrossRef]
- Spencer, K.; Liao, A.W.; Skentou, H.; Cicero, S.; Nicolaides, K.H. Screening for triploidy by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10–14 weeks of gestation. Prenat. Diagn. 2000, 20, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Tul, N.; Nicolaides, K.H. Maternal serum free beta-hCG and PAPP-A in fetal sex chromosome defects in the first trimester. Prenat. Diagn. 2000, 20, 390–394. [Google Scholar] [CrossRef]
- Petersen, O.B.; Vogel, I.; Ekelund, C.; Hyett, J.; Tabor, A.; Danish Fetal Medicine Study, G.; Danish Clinical Genetics Study, G. Potential diagnostic consequences of applying non-invasive prenatal testing: Population-based study from a country with existing first-trimester screening. Ultrasound Obstet. Gynecol. 2014, 43, 265–271. [Google Scholar] [CrossRef]
- Wijngaard, R.; Casals, E.; Mercade, I.; Laguna, J.; Madrigal, I.; Badenas, C.; Borrell, A.; Rodriguez-Revenga, L. Significance of Low Maternal Serum Beta-hCG Levels in the Assessment of the Risk of Atypical Chromosomal Abnormalities. Fetal Diagn. Ther. 2021, 48, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Syngelaki, A.; Bradbury, I.; Akolekar, R.; Nicolaides, K.H. First-trimester screening for trisomies 21, 18 and 13 by ultrasound and biochemical testing. Fetal Diagn. Ther. 2014, 35, 118–126. [Google Scholar] [CrossRef]
- Kagan, K.O.; Cicero, S.; Staboulidou, I.; Wright, D.; Nicolaides, K.H. Fetal nasal bone in screening for trisomies 21, 18 and 13 and Turner syndrome at 11–13 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 259–264. [Google Scholar] [CrossRef]
- Cicero, S.; Avgidou, K.; Rembouskos, G.; Kagan, K.O.; Nicolaides, K.H. Nasal bone in first-trimester screening for trisomy 21. Am. J. Obstet. Gynecol. 2006, 195, 109–114. [Google Scholar] [CrossRef]
- Kagan, K.O.; Valencia, C.; Livanos, P.; Wright, D.; Nicolaides, K.H. Tricuspid regurgitation in screening for trisomies 21, 18 and 13 and Turner syndrome at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 18–22. [Google Scholar] [CrossRef]
- Maiz, N.; Valencia, C.; Kagan, K.O.; Wright, D.; Nicolaides, K.H. Ductus venosus Doppler in screening for trisomies 21, 18 and 13 and Turner syndrome at 11–13 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 512–517. [Google Scholar] [CrossRef]
- Kagan, K.O.; Staboulidou, I.; Cruz, J.; Wright, D.; Nicolaides, K.H. Two-stage first-trimester screening for trisomy 21 by ultrasound assessment and biochemical testing. Ultrasound Obstet. Gynecol. 2010, 36, 542–547. [Google Scholar] [CrossRef]
- Wagner, P.; Sonek, J.; Hoopmann, M.; Abele, H.; Kagan, K.O. First-trimester screening for trisomies 18 and 13, triploidy and Turner syndrome by detailed early anomaly scan. Ultrasound Obstet. Gynecol. 2016, 48, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Staboulidou, I.; Syngelaki, A.; Cruz, J.; Nicolaides, K.H. The 11-13-week scan: Diagnosis and outcome of holoprosencephaly, exomphalos and megacystis. Ultrasound Obstet. Gynecol. 2010, 36, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sherod, C.; Sebire, N.J.; Soares, W.; Snijders, R.J.; Nicolaides, K.H. Prenatal diagnosis of trisomy 18 at the 10-14-week ultrasound scan. Ultrasound Obstet. Gynecol. 1997, 10, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.J.; Sebire, N.J.; Nayar, R.; Souka, A.; Nicolaides, K.H. Increased nuchal translucency in trisomy 13 fetuses at 10-14 weeks of gestation. Am. J. Med. Genet. 1999, 86, 205–207. [Google Scholar] [CrossRef]
- Sebire, N.J.; Snijders, R.J.; Brown, R.; Southall, T.; Nicolaides, K.H. Detection of sex chromosome abnormalities by nuchal translucency screening at 10-14 weeks. Prenat. Diagn. 1998, 18, 581–584. [Google Scholar] [CrossRef]
- Jauniaux, E.; Brown, R.; Snijders, R.J.; Noble, P.; Nicolaides, K.H. Early prenatal diagnosis of triploidy. Am. J. Obstet. Gynecol. 1997, 176, 550–554. [Google Scholar] [CrossRef]
- Syngelaki, A.; Guerra, L.; Ceccacci, I.; Efeturk, T.; Nicolaides, K.H. Impact of holoprosencephaly, exomphalos, megacystis and increased nuchal translucency on first-trimester screening for chromosomal abnormalities. Ultrasound Obstet. Gynecol. 2017, 50, 45–48. [Google Scholar] [CrossRef]
- Malone, F.D.; Canick, J.A.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Bukowski, R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N. Engl. J. Med. 2005, 353, 2001–2011. [Google Scholar] [CrossRef]
- Smith, M.; Visootsak, J. Noninvasive screening tools for Down syndrome: A review. Int. J. Womens Health 2013, 5, 125–131. [Google Scholar]
- Cuckle, H.; Benn, P.; Wright, D. Down syndrome screening in the first and/or second trimester: Model predicted performance using meta-analysis parameters. Semin. Perinatol. 2005, 29, 252–257. [Google Scholar] [CrossRef]
- Nicolaides, K.; Shawwa, L.; Brizot, M.; Snijders, R. Ultrasonographically detectable markers of fetal chromosomal defects. Ultrasound Obstet. Gynecol. 1993, 3, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, D.A.; Souter, V.L. Sonographic markers of fetal trisomies: Second trimester. J. Ultrasound Med. 2001, 20, 655–674. [Google Scholar] [CrossRef]
- Benacerraf, B.R. The role of the second trimester genetic sonogram in screening for fetal Down syndrome. Semin. Perinatol. 2005, 29, 386–394. [Google Scholar] [CrossRef]
- Odibo, A.O.; Ghidini, A. Role of the second-trimester ‘genetic sonogram’ for Down syndrome screen in the era of first-trimester screening and noninvasive prenatal testing. Prenat. Diagn. 2014, 34, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Benacerraf, B.R.; Barss, V.A.; Laboda, L.A. A sonographic sign for the detection in the second trimester of the fetus with Down’s syndrome. Am. J. Obstet. Gynecol. 1985, 151, 1078–1079. [Google Scholar] [CrossRef]
- Agathokleous, M.; Chaveeva, P.; Poon, L.C.; Kosinski, P.; Nicolaides, K.H. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstet. Gynecol. 2013, 41, 247–261. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Hosmer, W.; Feldstein, V.A.; Deeks, J.J.; Goldberg, J.D. Second-trimester ultrasound to detect fetuses with Down syndrome: A meta-analysis. JAMA 2001, 285, 1044–1055. [Google Scholar] [CrossRef]
- Shipp, T.D.; Benacerraf, B.R. Second trimester ultrasound screening for chromosomal abnormalities. Prenat. Diagn. 2002, 22, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Benacerraf, B.R. The history of the second-trimester sonographic markers for detecting fetal Down syndrome, and their current role in obstetric practice. Prenat. Diagn. 2010, 30, 644–652. [Google Scholar] [CrossRef]
- Bromley, B.; Lieberman, E.; Shipp, T.D.; Benacerraf, B.R. The genetic sonogram: A method of risk assessment for Down syndrome in the second trimester. J. Ultrasound Med. 2002, 21, 1087–1096. [Google Scholar] [CrossRef]
- Freeman, S.B.; Taft, L.F.; Dooley, K.J.; Allran, K.; Sherman, S.L.; Hassold, T.J.; Khoury, M.J.; Saker, D.M. Population-based study of congenital heart defects in Down syndrome. Am. J. Med. Genet. 1998, 80, 213–217. [Google Scholar] [CrossRef]
- Nyberg, D.A.; Souter, V.L.; El-Bastawissi, A.; Young, S.; Luthhardt, F.; Luthy, D.A. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J. Ultrasound Med. 2001, 20, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, F.M.; Malone, F.D.; Lambert-Messerlian, G.; Cuckle, H.S.; Porter, T.F.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Berkowitz, R.L.; Klugman, S.; et al. First- and second-trimester screening: Detection of aneuploidies other than Down syndrome. Obstet. Gynecol. 2007, 110, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Krantz, D.A.; Hallahan, T.W.; Macri, V.J.; Macri, J.N. Genetic sonography after first-trimester Down syndrome screening. Ultrasound Obstet. Gynecol. 2007, 29, 666–670. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Malone, F.D.; Nyberg, D.A.; Porter, T.F.; Cuckle, H.S.; Fuchs, K.; Sullivan, L.; Comstock, C.H.; Saade, G.R.; Eddleman, K.; et al. Role of second-trimester genetic sonography after Down syndrome screening. Obstet. Gynecol. 2009, 114, 1189–1196. [Google Scholar] [CrossRef]
- Yeo, L.; Guzman, E.R.; Day-Salvatore, D.; Walters, C.; Chavez, D.; Vintzileos, A.M. Prenatal detection of fetal trisomy 18 through abnormal sonographic features. J. Ultrasound Med. 2003, 22, 581–590. [Google Scholar] [CrossRef]
- DeVore, G.R. Second trimester ultrasonography may identify 77 to 97% of fetuses with trisomy 18. J. Ultrasound Med. 2000, 19, 565–576. [Google Scholar] [CrossRef]
- Snijders, R.J.; Shawa, L.; Nicolaides, K.H. Fetal choroid plexus cysts and trisomy 18: Assessment of risk based on ultrasound findings and maternal age. Prenat. Diagn. 1994, 14, 1119–1127. [Google Scholar] [CrossRef]
- Benacerraf, B.R.; Miller, W.A.; Frigoletto, F.D., Jr. Sonographic detection of fetuses with trisomies 13 and 18: Accuracy and limitations. Am. J. Obstet. Gynecol. 1988, 158, 404–409. [Google Scholar] [CrossRef]
- Lehman, C.D.; Nyberg, D.A.; Winter, T.C., 3rd; Kapur, R.P.; Resta, R.G.; Luthy, D.A. Trisomy 13 syndrome: Prenatal US findings in a review of 33 cases. Radiology 1995, 194, 217–222. [Google Scholar] [CrossRef]
- Jauniaux, E.; Brown, R.; Rodeck, C.; Nicolaides, K.H. Prenatal diagnosis of triploidy during the second trimester of pregnancy. Obstet. Gynecol. 1996, 88, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Lugthart, M.A.; Horenblas, J.; Kleinrouweler, E.C.; Engels, M.; Knegt, A.C.; Huijsdens, K.; van Leeuwen, E.; Pajkrt, E. Prenatal sonographic features can accurately determine parental origin in triploid pregnancies. Prenat. Diagn. 2020, 40, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Mittal, T.K.; Vujanic, G.M.; Morrissey, B.M.; Jones, A. Triploidy: Antenatal sonographic features with post-mortem correlation. Prenat. Diagn. 1998, 18, 1253–1262. [Google Scholar] [CrossRef]
- Berglund, A.; Stochholm, K.; Gravholt, C.H. The epidemiology of sex chromosome abnormalities. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 202–215. [Google Scholar] [CrossRef]
- De Vigan, C.; Baena, N.; Cariati, E.; Clementi, M.; Stoll, C.; Group, E.W. Contribution of ultrasonographic examination to the prenatal detection of chromosomal abnormalities in 19 centres across Europe. Ann. Genet. 2001, 44, 209–217. [Google Scholar] [CrossRef]
- Reimers, R.; High, F.; Kremen, J.; Wilkins-Haug, L. Prenatal diagnosis of sex chromosome aneuploidy-What do we tell the prospective parents? Prenat. Diagn. 2023, 43, 250–260. [Google Scholar] [CrossRef]
- Papp, C.; Beke, A.; Mezei, G.; Szigeti, Z.; Ban, Z.; Papp, Z. Prenatal diagnosis of Turner syndrome: Report on 69 cases. J. Ultrasound Med. 2006, 25, 711–717. [Google Scholar] [CrossRef]
- Maisenbacher, M.K.; Merrion, K.; Pettersen, B.; Young, M.; Paik, K.; Iyengar, S.; Kareht, S.; Sigurjonsson, S.; Demko, Z.P.; Martin, K.A. Incidence of the 22q11.2 deletion in a large cohort of miscarriage samples. Mol. Cytogenet. 2017, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- McDonald-McGinn, D.M.; Hain, H.S.; Emanuel, B.S.; Zackai, E.H. 22q11.2 Deletion Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Goldmuntz, E.; Bassett, A.S.; Boot, E.; Marino, B.; Moldenhauer, J.S.; Oskarsdottir, S.; Putotto, C.; Rychik, J.; Schindewolf, E.; McDonald-McGinn, D.M.; et al. Prenatal cardiac findings and 22q11.2 deletion syndrome: Fetal detection and evaluation. Prenat. Diagn. 2024, 44, 804–814. [Google Scholar] [CrossRef]
- Chaoui, R.; Kalache, K.D.; Heling, K.S.; Tennstedt, C.; Bommer, C.; Korner, H. Absent or hypoplastic thymus on ultrasound: A marker for deletion 22q11.2 in fetal cardiac defects. Ultrasound Obstet. Gynecol. 2002, 20, 546–552. [Google Scholar] [CrossRef]
- Chaoui, R.; Heling, K.S.; Lopez, A.S.; Thiel, G.; Karl, K. The thymic-thoracic ratio in fetal heart defects: A simple way to identify fetuses at high risk for microdeletion 22q11. Ultrasound Obstet. Gynecol. 2011, 37, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, R.; Heling, K.S.; Zhao, Y.; Sinkovskaya, E.; Abuhamad, A.; Karl, K. Dilated cavum septi pellucidi in fetuses with microdeletion 22q11. Prenat. Diagn. 2016, 36, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.; Wolf, C.; Chevy, F.; Benachi, A.; Dumez, Y.; Munnich, A.; Cormier-Daire, V. Antenatal manifestations of Smith-Lemli-Opitz (RSH) syndrome: A retrospective survey of 30 cases. Am. J. Med. Genet. A 2004, 124, 423–426. [Google Scholar] [CrossRef]
- Lin, A.E.; Ardinger, H.H.; Ardinger, R.H., Jr.; Cunniff, C.; Kelley, R.I. Cardiovascular malformations in Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 1997, 68, 270–278. [Google Scholar] [CrossRef]
- Schoner, K.; Witsch-Baumgartner, M.; Behunova, J.; Petrovic, R.; Bald, R.; Kircher, S.G.; Ramaswamy, A.; Kluge, B.; Meyer-Wittkopf, M.; Schmitz, R.; et al. Smith-Lemli-Opitz syndrome—Fetal phenotypes with special reference to the syndrome-specific internal malformation pattern. Birth Defects Res. 2020, 112, 175–185. [Google Scholar] [CrossRef]
- Wellesley, D.; Dolk, H.; Boyd, P.A.; Greenlees, R.; Haeusler, M.; Nelen, V.; Garne, E.; Khoshnood, B.; Doray, B.; Rissmann, A.; et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur. J. Hum. Genet. 2012, 20, 521–526. [Google Scholar] [CrossRef]
- Lo, Y.M.; Tein, M.S.; Lau, T.K.; Haines, C.J.; Leung, T.N.; Poon, P.M.; Wainscoat, J.S.; Johnson, P.J.; Chang, A.M.; Hjelm, N.M. Quantitative analysis of fetal DNA in maternal plasma and serum: Implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 1998, 62, 768–775. [Google Scholar] [CrossRef]
- Bianchi, D.W. Circulating fetal DNA: Its origin and diagnostic potential—A review. Placenta 2004, 25 (Suppl. A), S93–S101. [Google Scholar] [CrossRef]
- Miltoft, C.B.; Rode, L.; Bundgaard, J.R.; Johansen, P.; Tabor, A. Cell-Free Fetal DNA in the Early and Late First Trimester. Fetal Diagn. Ther. 2020, 47, 228–236. [Google Scholar] [CrossRef]
- Lo, Y.M.; Zhang, J.; Leung, T.N.; Lau, T.K.; Chang, A.M.; Hjelm, N.M. Rapid clearance of fetal DNA from maternal plasma. Am. J. Hum. Genet. 1999, 64, 218–224. [Google Scholar] [CrossRef]
- Everett, T.R.; Chitty, L.S. Cell-free fetal DNA: The new tool in fetal medicine. Ultrasound Obstet. Gynecol. 2015, 45, 499–507. [Google Scholar] [CrossRef] [PubMed]
- DiNonno, W.; Demko, Z.; Martin, K.; Billings, P.; Egbert, M.; Zneimer, S.; Keen-Kim, D.; Benn, P. Quality Assurance of Non-Invasive Prenatal Screening (NIPS) for Fetal Aneuploidy Using Positive Predictive Values as Outcome Measures. J. Clin. Med. 2019, 8, 1311. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet. Gynecol. 2020, 136, e48–e69. [Google Scholar] [CrossRef] [PubMed]
- Raymond, Y.; Fernando, S.; Menezes, M.; Mol, B.W.; McLennan, A.; da Silva Costa, F.; Hardy, T.; Rolnik, D.L. Placental, maternal, fetal, and technical origins of false-positive cell-free DNA screening results. Am. J. Obstet. Gynecol. 2024, 230, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.R.; Ferreira, J.; Benn, P.; Izzi, C.; Verdi, F.; Vercellotti, E.; Dalpiaz, C.; D’Ajello, P.; Filippi, E.; Volpe, N.; et al. Outcomes in pregnancies with a confined placental mosaicism and implications for prenatal screening using cell-free DNA. Genet. Med. 2020, 22, 309–316. [Google Scholar] [CrossRef]
- Spinillo, S.L.; Farina, A.; Sotiriadis, A.; Pozzoni, M.; Giglio, S.; Papale, M.; Candiani, M.; Cavoretto, P.I. Pregnancy outcome of confined placental mosaicism: Meta-analysis of cohort studies. Am. J. Obstet. Gynecol. 2022, 227, 714–727.e1. [Google Scholar] [CrossRef]
- Smith, L.H.; Danielsen, B.; Allen, M.E.; Cress, R. Cancer associated with obstetric delivery: Results of linkage with the California cancer registry. Am. J. Obstet. Gynecol. 2003, 189, 1128–1135. [Google Scholar] [CrossRef]
- Niles, K.M.; Murji, A.; Chitayat, D. Prolonged duration of persistent cell-free fetal DNA from vanishing twin. Ultrasound Obstet. Gynecol. 2018, 52, 547–548. [Google Scholar] [CrossRef]
- Kantor, V.; Jelsema, R.; Xu, W.; DiNonno, W.; Young, K.; Demko, Z.; Benn, P. Non-invasive prenatal screening for fetal triploidy using single nucleotide polymorphism-based testing: Differential diagnosis and clinical management in cases showing an extra haplotype. Prenat. Diagn. 2022, 42, 994–999. [Google Scholar] [CrossRef]
- Soster, E.; Dyr, B.; Rafalko, J.; Almasri, E.; Cacheris, P. Positive cfDNA screening results for 22q11.2 deletion syndrome-Clinical and laboratory considerations. Front. Genet. 2023, 14, 1146669. [Google Scholar] [CrossRef]
- Zaninovic, L.; Baskovic, M.; Jezek, D.; Katusic Bojanac, A. Validity and Utility of Non-Invasive Prenatal Testing for Copy Number Variations and Microdeletions: A Systematic Review. J. Clin. Med. 2022, 11, 3350. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; Paternoster, B.; Povarnitsyn, N.; Scotchman, E.; Chitty, L.; Chandler, N. Non-invasive prenatal diagnosis (NIPD): Current and emerging technologies. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 3–26. [Google Scholar] [CrossRef]
- Mohan, P.; Lemoine, J.; Trotter, C.; Rakova, I.; Billings, P.; Peacock, S.; Kao, C.Y.; Wang, Y.; Xia, F.; Eng, C.M.; et al. Clinical experience with non-invasive prenatal screening for single-gene disorders. Ultrasound Obstet. Gynecol. 2022, 59, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.R.; Hardisty, E.; Dotters-Katz, S.K.; Vora, N.L.; Kuller, J.A. Cell-Free DNA Screening: Complexities and Challenges of Clinical Implementation. Obstet. Gynecol. Surv. 2016, 71, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Benn, P.; Cuckle, H.; Pergament, E. Non-invasive prenatal testing for aneuploidy: Current status and future prospects. Ultrasound Obstet. Gynecol. 2013, 42, 15–33. [Google Scholar] [CrossRef]
- Boyle, B.; Morris, J.K.; McConkey, R.; Garne, E.; Loane, M.; Addor, M.C.; Gatt, M.; Haeusler, M.; Latos-Bielenska, A.; Lelong, N.; et al. Prevalence and risk of Down syndrome in monozygotic and dizygotic multiple pregnancies in Europe: Implications for prenatal screening. BJOG 2014, 121, 809–819; discussion 820. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Audibert, F.; Kagan, K.O.; Paladini, D.; Yeo, G.; Raine-Fenning, N.; Committee, I.C.S. ISUOG updated consensus statement on the impact of cfDNA aneuploidy testing on screening policies and prenatal ultrasound practice. Ultrasound Obstet. Gynecol. 2017, 49, 815–816. [Google Scholar] [CrossRef]
- Gil, M.M.; Galeva, S.; Jani, J.; Konstantinidou, L.; Akolekar, R.; Plana, M.N.; Nicolaides, K.H. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: Update of The Fetal Medicine Foundation results and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 53, 734–742. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Wang, W.; Dong, Y.; Zhang, M.; Wang, X.; Yin, C. Cell-free DNA screening for sex chromosome aneuploidies by non-invasive prenatal testing in maternal plasma. Mol. Cytogenet. 2020, 13, 10. [Google Scholar] [CrossRef]
- Martin, K.A.; Samango-Sprouse, C.A.; Kantor, V.; Dhamankar, R.; Valenti, E.; Trefogli, M.T.; Balosbalos, I.; Lagrave, D.; Lyons, D.; Kao, C.; et al. Detection of maternal X chromosome abnormalities using single nucleotide polymorphism-based noninvasive prenatal testing. Am. J. Obstet. Gynecol. MFM 2020, 2, 100152. [Google Scholar] [CrossRef]
- Christiaens, L.; Chitty, L.S.; Langlois, S. Current controversies in prenatal diagnosis: Expanded NIPT that includes conditions other than trisomies 13, 18, and 21 should be offered. Prenat. Diagn. 2021, 41, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Mackie, F.L.; Hemming, K.; Allen, S.; Morris, R.K.; Kilby, M.D. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: A systematic review and bivariate meta-analysis. BJOG 2017, 124, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Dhamankar, R.; DiNonno, W.; Martin, K.A.; Demko, Z.P.; Gomez-Lobo, V. Fetal Sex Results of Noninvasive Prenatal Testing and Differences With Ultrasonography. Obstet. Gynecol. 2020, 135, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Ellis, K.; Mayen, D.; Pertile, M.D.; Reimers, R.; Sun, L.; Vermeesch, J.; Vora, N.L.; Chitty, L.S. Position statement from the International Society for Prenatal Diagnosis on the use of non-invasive prenatal testing for the detection of fetal chromosomal conditions in singleton pregnancies. Prenat. Diagn. 2023, 43, 814–828. [Google Scholar] [CrossRef]
- Takoudes, T.; Hamar, B. Performance of non-invasive prenatal testing when fetal cell-free DNA is absent. Ultrasound Obstet. Gynecol. 2015, 45, 112. [Google Scholar] [CrossRef]
- Persson, F.; Prensky, L. Variability of “Reported Fetal Fraction” in Noninvasive Prenatal Screening (NIPS). Clin. Chem. 2021, 67, 863–866. [Google Scholar] [CrossRef]
- Benn, P.; Zhang, J.; Lyons, D.; Xu, W.; Leonard, S.; Demko, Z. Accuracy of fetal fraction measurements in a single-nucleotide polymorphism-based noninvasive prenatal test. Prenat. Diagn. 2024, 44, 1218–1224. [Google Scholar] [CrossRef]
- Becking, E.C.; Linthorst, J.; Patton, S.; Gutowska-Ding, W.; Goodall, R.; Khawaja, F.; Morgan, F.; Deans, Z.; Chitty, L.S.; Bekker, M.N.; et al. Variability in Fetal Fraction Estimation: Comparing Fetal Fractions Reported by Noninvasive Prenatal Testing Providers Globally. Clin. Chem. 2023, 69, 160–167. [Google Scholar] [CrossRef]
- Hedriana, H.; Martin, K.; Saltzman, D.; Billings, P.; Demko, Z.; Benn, P. Cell-free DNA fetal fraction in twin gestations in single-nucleotide polymorphism-based noninvasive prenatal screening. Prenat. Diagn. 2020, 40, 179–184. [Google Scholar] [CrossRef]
- Ryan, A.; Hunkapiller, N.; Banjevic, M.; Vankayalapati, N.; Fong, N.; Jinnett, K.N.; Demko, Z.; Zimmermann, B.; Sigurjonsson, S.; Gross, S.J.; et al. Validation of an Enhanced Version of a Single-Nucleotide Polymorphism-Based Noninvasive Prenatal Test for Detection of Fetal Aneuploidies. Fetal Diagn. Ther. 2016, 40, 219–223. [Google Scholar] [CrossRef]
- Goldring, G.; Trotter, C.; Meltzer, J.T.; Souter, V.; Pais, L.; DiNonno, W.; Xu, W.; Weitzel, J.N.; Vora, N.L. Maternal Malignancy After Atypical Findings on Single-Nucleotide Polymorphism-Based Prenatal Cell-Free DNA Screening. Obstet. Gynecol. 2023, 141, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Babiarz, J.E.; Levy, B.; Stosic, M.; Zimmermann, B.; Sigurjonsson, S.; Wayham, N.; Ryan, A.; Banjevic, M.; Lacroute, P.; et al. Expanding the scope of noninvasive prenatal testing: Detection of fetal microdeletion syndromes. Am. J. Obstet. Gynecol. 2015, 212, 332.e1–332.e9. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.R.; Molina Gomes, D.; Ferreira, J.C.; Dupont, C.; Alesi, V.; Gouas, L.; Horelli-Kuitunen, N.; Choy, K.W.; Garcia-Herrero, S.; de la Vega, A.G.; et al. Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenat. Diagn. 2015, 35, 801–809. [Google Scholar] [CrossRef]
- Dar, P.; Jacobsson, B.; Clifton, R.; Egbert, M.; Malone, F.; Wapner, R.J.; Roman, A.S.; Khalil, A.; Faro, R.; Madankumar, R.; et al. Cell-free DNA screening for prenatal detection of 22q11.2 deletion syndrome. Am. J. Obstet. Gynecol. 2022, 227, 79.e1–79.e11. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Saucier, J.B.; Feng, Y.; Jiang, Y.; Sinson, J.; McCombs, A.K.; Schmitt, E.S.; Peacock, S.; Chen, S.; et al. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. Nat. Med. 2019, 25, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Sapantzoglou, I.; Giourga, M.; Pergialiotis, V.; Mantzioros, R.; Daskalaki, M.A.; Papageorgiou, D.; Antsaklis, P.; Theodora, M.; Thomakos, N.; Daskalakis, G. Low fetal fraction and adverse pregnancy outcomes- systematic review of the literature and metanalysis. Arch. Gynecol. Obstet. 2024, 310, 1343–1354. [Google Scholar] [CrossRef]
- Khalil, A.; Bellesia, G.; Norton, M.E.; Jacobsson, B.; Haeri, S.; Egbert, M.; Malone, F.D.; Wapner, R.J.; Roman, A.; Faro, R.; et al. The role of cell-free DNA biomarkers and patient data in the early prediction of preeclampsia: An artificial intelligence model. Am. J. Obstet. Gynecol. 2024, 231, 554.e1–554.e18. [Google Scholar] [CrossRef]
- Mattar, C.N.Z.; Chan, J.K.Y.; Choolani, M. Gene modification therapies for hereditary diseases in the fetus. Prenat. Diagn. 2023, 43, 674–686. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).