Improvement in Sperm Recovery Rate and Total Motile Sperm Count Using α-Chymotrypsin in Highly Viscous Semen Sample Without Adversely Affecting Assisted Reproductive Technology Outcomes
Abstract
1. Introduction
2. Materials and Methods
3. Results
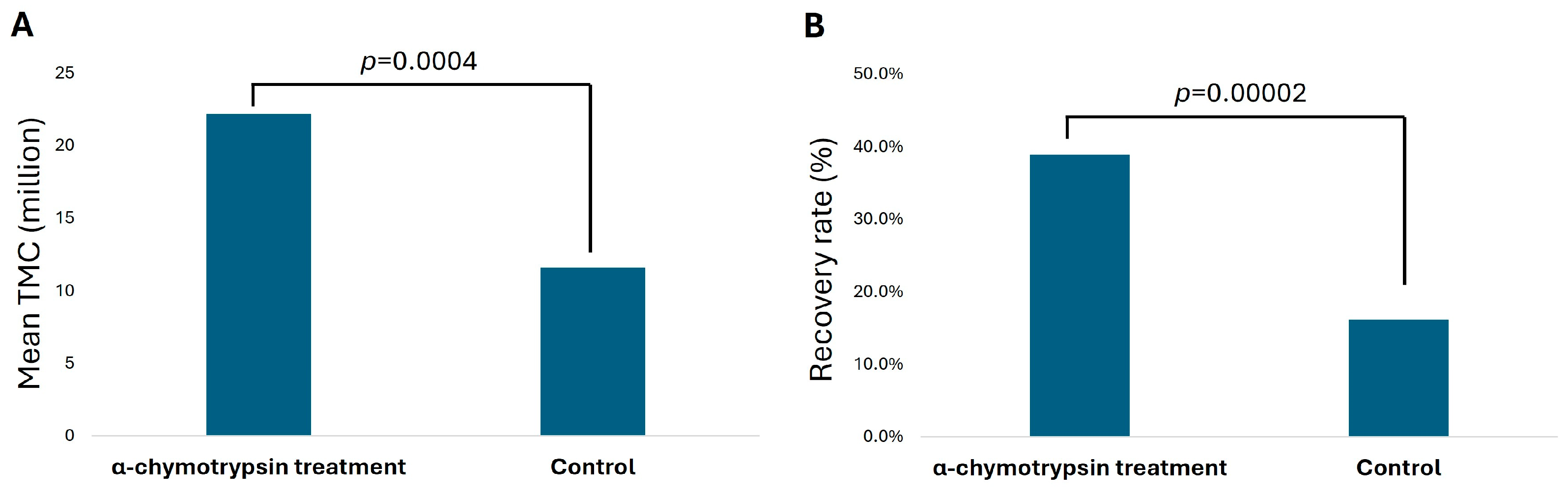
3.1. Paired Analysis for Total Motile Sperm Count (TMC) and Recovery Rate in α-Chymotrypsin Treatment
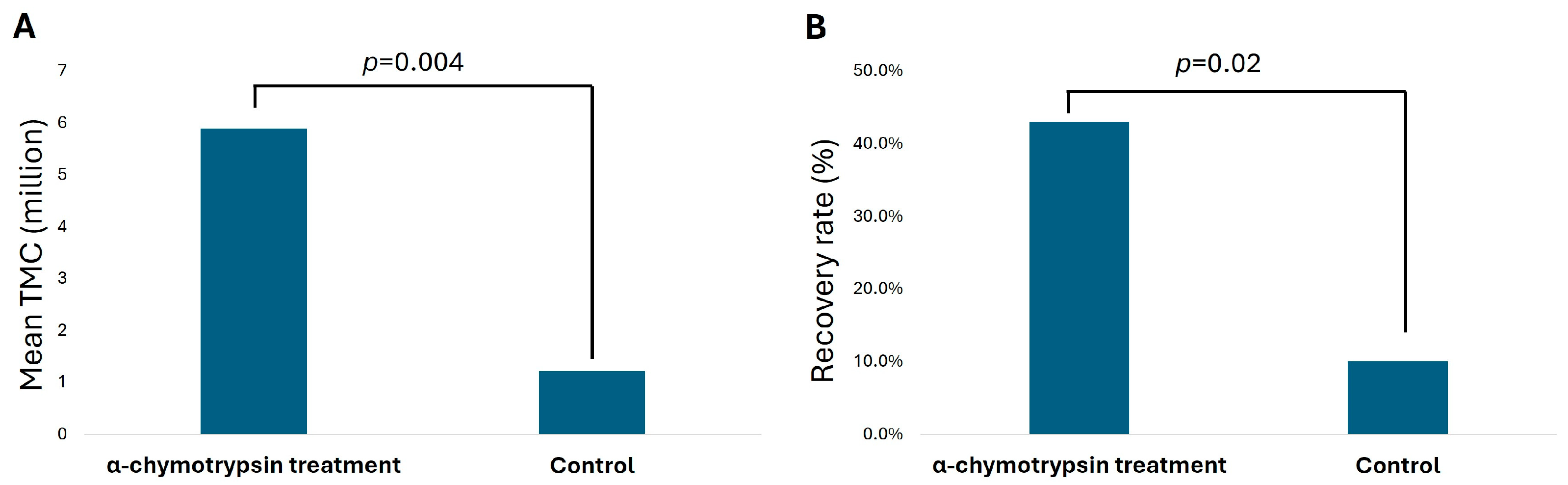
3.2. TMC and Average Recovery Rate in Patients with Severely Low Sperm Count
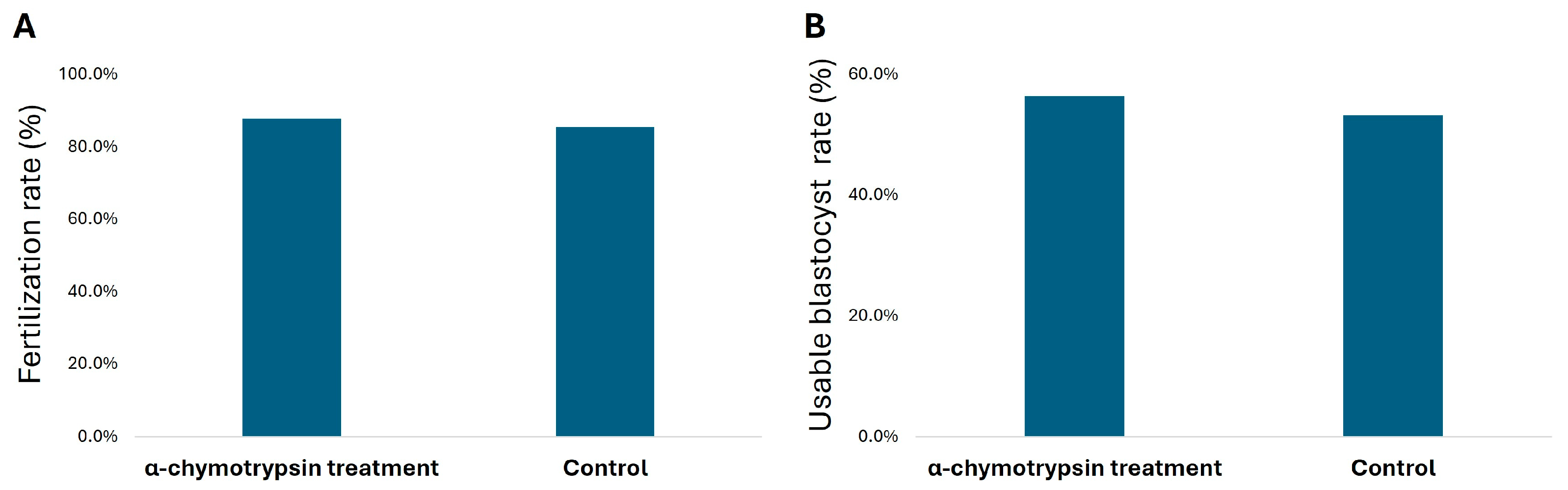
3.3. Fertilization and Blastocyst Rate in IVF Cycle of α-Chymotrypsin Treated Semen
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Sigman, M.; Collura, B.; de Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J. Urol. 2021, 205, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.-S. Male infertility. Nat. Rev. Dis. Prim. 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; de Lamirande, E.; Gukturk, A.; San Gabriel, M.C.; Nazemian, Z.; Burjaq, H.; Casper, R.F.; Zini, A. Seminal hyperviscosity is not associated with semenogelin degradation or sperm deoxyribonucleic acid damage: A prospective study of infertile couples. Fertil. Steril. 2014, 101, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Gagnon, C. Sperm motility inhibitor from human seminal plasma: Association with semen coagulum. Hum. Reprod. 1995, 10, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E. Semenogelin, the Main Protein of the Human Semen Coagulum, Regulates Sperm Function. Semin. Thromb. Hemost. 2007, 33, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A. Semen hyperviscosity causes consequences and cures. Front. Biosci. 2013, E5, E610. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sechler, S.; Mason, A.; Amadio, A.; Flyckt, R.; Kim, S.T. Predictors for success of intrauterine insemination: A retrospective analysis. Fertil. Steril. 2021, 116, e19. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Sharma, R.; Samanta, L. Treatment of semen samples with α-chymotrypsin alters the expression pattern of sperm functional proteins—A pilot study. Andrology 2018, 6, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Burjaq, H.; Gotlieb, L.; Casper, R.F. Seminal hyperviscosity is associated with poor outcome of in vitro fertilization and embryo transfer: A prospective study. Fertil. Steril. 2008, 90, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Socias, T.; Cortez, J.; Llanos, M.N. Evidences for the presence of chymotrypsin-like activity in human spermatozoa with a role in the acrosome reaction. Mol. Reprod. Dev. 1994, 38, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, A.; Bakjaji, F.; Königsberger, S.; John, J.; Färber, C.; Schmidt, E.; Breitenbach-Koller, H.; Allam, J.-P.; Verguts, J.; Sänger, N. Effect of mild α-chymotrypsin treatment of highly viscous semen samples on fertilization rates. Transl. Androl. Urol. 2021, 10, 448–454. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; pp. 31–32. [Google Scholar]
- Shelley, D.L.; Hernandez-Nieto, C.; Gounko, D.; Lee, J.A.; Bar-Chama, N.; Roth, R.M.; Briton-Jones, C.; Copperman, A.B.; Slifkin, R.E. A retrospective analysis of chymotrypsin use for IVF sperm preparation and its effects on fertilization, blastulation and ploidy. Fertil. Steril. 2022, 118, e144. [Google Scholar] [CrossRef]
- Starosta, A.; Gordon, C.E.; Hornstein, M.D. Predictive factors for intrauterine insemination outcomes: A review. Fertil. Res. Pract. 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyar, A.; Khalil, M.; Wong, M.; Chung, R.; Coyne, K.; Findley, J.; Weinerman, R.; Flyckt, R.; Sofaly, K.P.; Kim, S.T. Improvement in Sperm Recovery Rate and Total Motile Sperm Count Using α-Chymotrypsin in Highly Viscous Semen Sample Without Adversely Affecting Assisted Reproductive Technology Outcomes. Reprod. Med. 2025, 6, 17. https://doi.org/10.3390/reprodmed6030017
Ayyar A, Khalil M, Wong M, Chung R, Coyne K, Findley J, Weinerman R, Flyckt R, Sofaly KP, Kim ST. Improvement in Sperm Recovery Rate and Total Motile Sperm Count Using α-Chymotrypsin in Highly Viscous Semen Sample Without Adversely Affecting Assisted Reproductive Technology Outcomes. Reproductive Medicine. 2025; 6(3):17. https://doi.org/10.3390/reprodmed6030017
Chicago/Turabian StyleAyyar, Archana, Marian Khalil, Maggie Wong, Rebecca Chung, Kathryn Coyne, Joseph Findley, Rachel Weinerman, Rebecca Flyckt, Katelyn Perroz Sofaly, and Sung Tae Kim. 2025. "Improvement in Sperm Recovery Rate and Total Motile Sperm Count Using α-Chymotrypsin in Highly Viscous Semen Sample Without Adversely Affecting Assisted Reproductive Technology Outcomes" Reproductive Medicine 6, no. 3: 17. https://doi.org/10.3390/reprodmed6030017
APA StyleAyyar, A., Khalil, M., Wong, M., Chung, R., Coyne, K., Findley, J., Weinerman, R., Flyckt, R., Sofaly, K. P., & Kim, S. T. (2025). Improvement in Sperm Recovery Rate and Total Motile Sperm Count Using α-Chymotrypsin in Highly Viscous Semen Sample Without Adversely Affecting Assisted Reproductive Technology Outcomes. Reproductive Medicine, 6(3), 17. https://doi.org/10.3390/reprodmed6030017






