Abstract
Pregnancies complicated by type 1 diabetes (TID) are associated with an increased risk of obstetric and neonatal adverse outcomes. Optimal glycemic control prior to and through pregnancy is crucial to reduce complications. The use of diabetes technology is rapidly increasing. The aim of the study was to investigate the use and effects of diabetes technology in pregnant women with type 1 diabetes. A retrospective cohort study was conducted; 84 women were included in the analysis and were divided into subgroups according to their glucose monitoring method and insulin delivery method. HbA1c values declined during pregnancy in all subgroups with no significant difference between the subgroups. A difference was, however, found in birth weight z-scores. Women using a sensor and an insulin pump had larger babies compared to women without these treatment modalities. The results of the study indicate that diabetes technology, including insulin pumps and/or glucose sensors are not superior to self-monitoring blood glucose measurement and multiple daily injection insulin therapy, which is comforting in the light of the unequal access to health benefits.
1. Introduction
Maternal hyperglycemia during pregnancy has been shown to increase the risk for several adverse pregnancy outcomes including stillbirth, congenital malformations, admission to a neonatal intensive care unit, a low APGAR score and large-for-gestational-age babies [1,2,3,4,5,6]. Despite the fact that modern technology for blood glucose monitoring and insulin management is becoming a more central part of treating diabetes, the frequencies of adverse pregnancy outcomes remain significantly higher in babies born of women with diabetes and the rate of babies being born large for gestational age is increasing [7,8,9]. In addition, the overall incidence of pregnancies complicated by maternal diabetes is increasing significantly, making this a relevant concern for today’s healthcare professionals [3,10,11].
Adverse pregnancy outcomes can have detrimental long-term consequences for the offspring; thus, the increased risk of congenital malformations such as a heart defect makes type 1 diabetes an indication for additional surveillance during pregnancy, i.e., fetal echocardiography [12]. Being born large for gestational age (LGA) has been shown to be an independent predictor of being overweight later in childhood, and being born LGA is associated with high blood pressure in childhood and adolescence and an increased risk for heart disease up to middle age [13,14,15,16,17]. High HbA1c during pregnancy complicated by type 1 diabetes is associated with a higher rate of adverse pregnancy outcomes, suggesting this parameter to be one of the key modifiable risk factors for adverse outcomes in both the short and long term [9].
The use of technology has taken on an ever-increasingly large role in the management of diabetes over the last decades. The methods for monitoring blood glucose have undergone a great development since the 1970s, when the first digital glucometer was launched. Self-monitoring blood glucose modalities have improved greatly since then, and the options for monitoring blood glucose today also include flash glucose monitors (FGMs) and continuous glucose monitors (CGMs), which are different systems of interstitial glucose monitoring. A CGM provides information about the blood glucose level at all times via a continuous Bluetooth connection, while an FGM requires intermittent scanning (or “flash”) of the sensor to download glucose recordings. Both FGMs and CGMs evaluate glycemic targets using similar parameters including time in range (TIR), the coefficient of variation (CV) and glucose management indicator (GMI). Both FGMs and CGMs save the patient from numerous finger pricks during a day, provide warnings of actual or impending hypo- or hyperglycemia and feature details about trends in blood glucose [18]. Several studies have proven that continuous glucose monitoring leads to improved glycemic control as compared to conventional therapy in non-pregnant patients with type 1 diabetes [19,20].
Intensive insulin treatment has developed from consisting of multiple daily injections to now include continuous subcutaneous insulin infusion using a pump [21]. Administering insulin by pump has for a long time been perceived as the best way to mimic physiological blood glucose concentrations and advantages with respect to HbA1c, and the risk of severe hypoglycemia has been proven when compared to multiple daily injections [22]. Hybrid closed-loop systems represent the most recent technological advancement in diabetes care. In non-pregnant populations, closed-loop systems have been suggested to improve glycemic regulation compared with sensor-augmented insulin pumps [23,24].
It is uncertain whether these different advantages also apply in pregnant women with diabetes, as study results are inconsistent so far [25,26,27,28]. Additionally, modern diabetes technology is not equally accessible for every patient in today’s healthcare, making it highly relevant to investigate how many patients are provided with this kind of technology and how it affects the women and their offspring. The aim of this study was to investigate the prevalence and possible role of diabetes technology in pregnant women with type 1 diabetes. We hypothesized that women provided with diabetes technology would have a better glycemic regulation and thus fewer adverse outcomes of their pregnancy.
2. Materials and Methods
We present a retrospective cohort study based on data routinely collected in the clinic. The cohort consisted of women with type 1 diabetes who gave birth between January 2021 and May 2022. The women were affiliated to the outpatient clinic at Department of Obstetrics and Gynecology at Aarhus University Hospital in collaboration with Steno Diabetes Center Aarhus during their pregnancy and followed the local routine care program. Aarhus University Hospital is a tertiary hospital and follows all pregnant women with diabetes in the Central Denmark Region. Exclusion criteria were type 2 diabetes, maturity-onset diabetes of the young (MODY) and type 3c diabetes, stillbirth, termination of pregnancy and twin pregnancy (Figure 1). As the project was categorized as a quality assurance project, ethical approval was not required. Approvals by the Board of directors at Aarhus University Hospital and the head of Department of Obstetrics and Gynecology at Aarhus University Hospitals were obtained, according to the general data protection regulation (GDPR) and the Danish data protection legislation.
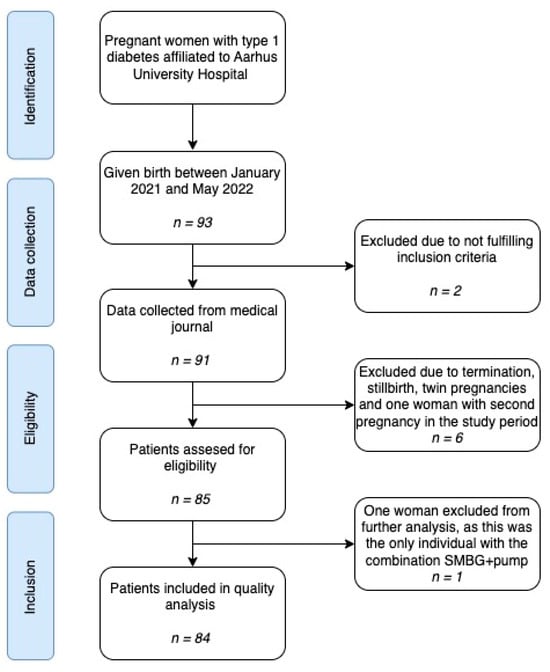
Figure 1.
Flowchart illustrating the study identification, data collection, inclusion and exclusion.
- Exposure
From the women’s medical records we identified method of blood-glucose monitoring, i.e., self-monitoring of blood-glucose (SMBG), flash glucose monitoring (FGM) or continuous glucose monitoring (CGM), and insulin injection modality, i.e., insulin infusion pump or multiple daily injection therapy (MDI). Based on this, women were divided into three groups: women using SMBG and multiple daily injection (MDI); women with sensor-based glucose monitoring (CGM or FGM) and MDI; and women with sensor-based glucose monitoring and insulin pump.
- Outcomes
We collected 3rd trimester total daily insulin dose and HbA1c, based on the last registered value closest to birth. Insulin dose was divided by the weight on the day of the recorded insulin dose. If no weight was available, the weight closest to the insulin dose information within two weeks was used, otherwise we classified these data as missing. The timing of these measurements varied between gestational week 32 and 39 depending on the time of delivery. Delivery mode, i.e., vaginal, emergency caesarean section or elective caesarian section, was grouped into vaginal or caesarean section. Continuous values of gestational weight gain (GWG) were calculated as the difference between pre-gestational weight and last available weight measurement after 200 days of gestation. GWG was categorized as “above recommended” if exceeding the recommendations based on pregestational body mass index (BMI): BMI < 18.5: >18 kg; BMI 18.5–24.9: >15 kg; BMI 25–29.9: >10 kg; BMI ≥ 30: >9 kg [29].
The following data on the offspring were collected: Gestational age at delivery and pre-term delivery defined as birth before 37 weeks of gestation. Birthweight z-score was calculated using a Scandinavian reference population and additionally categorized as large for gestational age if ≥2 SD [30]. APGAR score after 5 min was used, and additionally defined as low if ≤7.
- Co-variates
The following pre-pregnancy data were collected: age, self-reported pre-pregnancy weight and height used to calculate BMI, smoking status, level of education, parity and duration of diabetes. BMI was categorized as normal when below 25 kg/m2, overweight when 25–30 kg/m2 and obese when above 30 kg/m2. Total daily insulin dose and HbA1c, defined as the value closest to pregnancy, was also collected. Insulin dose was reported as units (IU)/kg/day using self-reported pre-gestational weight. Level of education was divided into two different groups: low educational level covering no tertiary education and tertiary education of short duration (maximum of one year), and high educational level covering tertiary education of two years and more. This distribution was chosen to avoid groups being too small.
- Statistical Analysis
Population characteristics are reported as means with standard deviation (SD) for continuous variables and as n (%) for categorical variables.
Mean group estimates with standard error (SE) and mean group differences with 95% confidence intervals (CI) were estimated for continuous outcomes using linear regression. For categorical outcomes, odds ratios (OR) with 95% CI were estimated using logistic regression.
Data analysis was performed using R (version 4.3.0, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A total of 93 pregnant women with T1D were identified and 84 women were included in the analysis (Figure 1). Seventy-two percent of the women were provided with a sensor and 14% of the women used an insulin infusion pump. All pump users used a sensor as well. The mean pre-gestational HbA1c levels in the subgroups SMBG + MDI vs. sensor + MDI vs. sensor + pump varied from 55 to 59 mmol/mol (Table 1). The only difference in the baseline characteristics was the duration of diabetes. The mean duration of diabetes until birth was 11 years in the SMBG + MDI group vs. 15–17 years in the sensor groups, with p = 0.015. The level of education was not different across the subgroups.

Table 1.
Baseline characteristics of participants according to technology status.
3.1. Continuous Outcomes
HbA1c values declined during pregnancy in all subgroups and the mean HbA1c 3rd trimester levels were similar between the subgroups, with 49 in the SMBG + MDI group vs. 47 in the sensor + MDI group vs. 48 in sensor + pump group (Table 2).

Table 2.
Metabolic and obstetric/neonatal continuous health outcomes.
The pre-gestational insulin dosage in the sensor + MDI and sensor + pump groups was similar with mean values of 0.64 and 0.63 IU/kg/day, respectively. In comparison, the mean pre-gestational insulin was 0.74 IU/kg/day in the SMBG + MDI group. The insulin dosage in the 3rd trimester increased as expected compared to the pre-gestational dosage. The total insulin dose in the 3rd trimester was similar between the subgroups, with values of 0.97–1.06 IU/kg/day.
Newborns in the sensor + MDI group had a birthweight z-score of 0.86 [CI 0.18–1.55], higher than babies in the SMBG + MDI group. Likewise, the babies in the sensor + pump group had a birthweight z-score of 1.30 [CI 0.39–2.22], higher than babies in the SMBG + MDI group (Table 2). Women with a sensor and MDI gained on average 4.53 kg [CI 1.63–7.44] more than the women with SMBG and MDI during pregnancy. Additionally, women with a sensor and a pump gained on average 3.99 kg [CI −0.03–8.01] more than the SMBG + MDI group. When comparing the sensor + MDI and sensor + pump groups, no statistically significant difference was found (Table 2).
3.2. Dichotomous Outcomes
Women with a sensor and MDI had a 3.68 [CI 1.27–10.69] higher odds ratio of giving birth to a LGA child compared to women with SMBG and MDI. When comparing the SMBG + MDI group with the sensor + pump group, the odds ratio was 3.05 [CI 0.77–12.14] and 0.83 [CI 0.25–2.78] for women with a sensor and a pump compared to women with a sensor and an MDI (Table 3). In relation to pre-term birth, women with a sensor and MDI had a 1.23 [CI 0.38–4.05] higher odds ratio compared to those with SMBG and MDI; women with a sensor and a pump had a 2.70 [CI 0.63–11.51] higher odds ratio for pre-term birth than women with SMBG and MDI; and women with a sensor and a pump compared to women with a sensor and MDI had a 2.19 [CI 0.63–7.60] higher odds ratio. The odds ratio for birth by C-section was similar with values between 0.88 and 1.04 in the subgroups (Table 3).

Table 3.
Metabolic and obstetric/neonatal dichotomous health outcomes.
The odds ratio for women with a sensor and MDI for having offspring with a low APGAR-5 score was 1.50 [CI 0.15–15.26] higher compared to the women with SMBG and MDI. For women with a sensor and a pump the OR was 3.67 [CI 0.30–44.72] for a low APGAR-5 score compared to the women with SMBG and MDI. When comparing the sensor + pump and sensor + MDI groups, the odds was 1.42 [CI 0.38–5.22] for a low APGAR-5 score in the insulin pump group compared to the women with MDI (Table 3).
4. Discussion
In this retrospective cohort study of pregnant women with type 1 diabetes using different modalities of diabetes technology, analyses of the glycemic control before and during pregnancy showed no difference between the groups. However, women with diabetes technology had significantly larger babies and gained more weight during pregnancy compared to women without the technology.
It is well-established in non-pregnant individuals with type 1 diabetes that continuous glucose monitoring and continuous subcutaneous insulin pump treatment both separately and combined reduces glucose fluctuations and improves glycemic control, thereby possibly reducing diabetes-related complications [18,19,20,22,31]. However, studies involving pregnant women with T1D results are conflicting. The majority of studies have not been able to demonstrate that insulin pump therapy is superior to MDI therapy regarding glycemic control or pregnancy outcomes in women with T1D [32,33,34,35]. In a large multicenter study, women using insulin pumps in pregnancy had lower HbA1c without an increased risk of severe hypoglycemia but no improvement in pregnancy outcomes, and the women using insulin pumps even had offspring larger for gestational age, similar to the findings in the current study [36]. A recent prospective multicenter study discovered that the risk of congenital malformations was not lower in the offspring of women treated with insulin pumps when compared to women using MDI therapy, and thus no advantage of pump treatment was demonstrated despite better glycemic control in early pregnancy [27]. A review of both randomized controlled trials (RCTs) and non-RCTs evaluating continuous subcutaneous insulin infusion and MDI in T1D-complicated pregnancies showed better 1st trimester glycemic control with lower HbA1c level in groups using continuous subcutaneous insulin infusion, but this difference tapered off later in pregnancy. Continuous subcutaneous insulin infusion was found to be associated with lower insulin requirements, higher gestational weight gain and altered risk for offspring being large for gestational age [37].
Hybrid closed-loop insulin therapy has been shown to be promising in the management of T1D during pregnancy as it significantly improved maternal glycemic control during pregnancy in a multicenter controlled trial. The percentage of time that maternal glucose levels were in the target range was higher in the closed-loop group compared to the standard care group and included less time spent in a hyperglycemic state [25]. The study reported no unanticipated safety problems associated with the use of closed-loop-therapy during pregnancy. The observed improvements in glycemic control with hybrid closed-loop therapy suggest a potential reduction in the risks associated with poorly controlled diabetes during pregnancy. The study contributes valuable evidence supporting the efficacy of diabetes technology in improving maternal glycemic control during pregnancies complicated by T1D [25].
An international multicenter RCT from the CONCEPTT study group found that pregnant women using CGM had better glycemic control compared to pregnant women using SMBG, and CGM users spent more time in the target glucose range and less time in the hyperglycemic range. However, the difference in HbA1c between the two groups was small and no between-group differences in caesarean delivery, gestational age or preterm delivery were found. The study only found a decreased proportion of large-for-gestational-age babies (OR 0.51 [CI 0.28–0.90]; p = 0.021) of mothers randomly assigned to CGM [26]. In our study, we found a small and not statistically significant difference in HbA1c between the groups. With these results in mind, it is reasonable to question whether HbA1c in itself is adequate for describing glycemic control. In our study, HbA1c was used because of its widespread clinical use, but during pregnancy it is influenced by gestational changes in red cell turnover, anemia and iron supplementation [38]. HbA1c does not reflect the complexities of glycemic control in women with T1D, who most frequently achieve tight glycemic control under close clinical supervision in the 1st trimester [26,39,40,41]. Nevertheless, in the absence of more detailed data on the glycemic control, e.g., time in range, time in high and low range, and hypoglycemic events, HbA1c values may serve as a proxy for glycemic control.
The reason for the apparent lack of improvement in glycemic control and neonatal outcomes in pregnant women with T1D using technology is unclear, but one reason could be that most women with diabetes are extremely motivated to obtain good glycemic control in pregnancy and put a large effort into their diabetes treatment no matter the treatment modality used. It could also be speculated that women provided with CGM or insulin pumps may represent women with a more complicated case of diabetes, since insulin pump treatment is often initiated in women with inadequate glycemic control, and in this study women with diabetes technology also had a longer history of diabetes. In addition, pregnant women who are relatively well-regulated as judged using HbA1c levels, but who still experience adverse neonatal outcomes, could have a history of prolonged and more severe diabetes [23]. It could be assumed that women using CGM and insulin pumps may be more inclined to have a larger caloric intake and possibly a different diet quality as CGM readings are easier to perform than using a finger prick. Hypothetically, this could to some extent explain the higher GWG in the women with CGM and insulin pumps. Another explanation for the differences in weight gain could be due to differences in physical activity (PA). Data on PA were, however, not available in the study. In summary, the use of diabetes technology cannot be transferred directly from non-pregnant to pregnant women, and it cannot stand alone in the treatment of pregnant women with type 1 diabetes.
In the present study, there was no difference in the educational level between the groups and hence no clear difference in socioeconomic status (SES) was found. SES is often considered as a protective factor regarding the compliance and management of disease, and could have been associated with better glycemic regulation, though this was not the case in this study. A recent multinational cohort study found that a higher socioeconomic status among pregnant women using an insulin pump was not associated with a lower prevalence of congenital malformations when compared to pregnant women using MDI therapy. Thorius et al. suggested an explanation for this was that insulin pump treatment is often initiated in women with inappropriate glycemic control, and thus represents a group of women with more challenging diabetes [27]. In relation to this, it is relevant to consider whether women with a high SES are more likely to be treated with the latest technology as they may advocate more strongly to their caregivers for the use of such resources. Clearly, the impact of SES is an important consideration in today’s healthcare. However, this current study sample is not suitable for the evaluation of this issue as the study group was relatively homogenous and all women were treated within the Danish universal healthcare system. Further studies including more diverse populations are needed to fully evaluate the impact of SES in the treatment of diabetes.
The availability of technology for diabetes treatment is unequal in today’s healthcare. In Denmark, diabetes technology is completely or partially paid for by the hospitals, but in every situation the decision relies on a medical assessment, and balancing resources and demands often leads to a less consistent or widespread use of diabetes technology [42]. In many parts of the world, access to diabetes technology is gained through private health insurance or self-funding and the consequences of this could be relevant to investigate. To our knowledge, no previous studies have examined either medical bias in the distribution of diabetes technology or the consequences of different types of healthcare assistants, e.g., private vs. public. A recent study compared the use of technology across socioeconomic groups in two contrasting funding models. This showed that the use of technology was similar across socioeconomic groups when it was nationally subsidized, whereas user-paid technology resulted in lower use with socioeconomic deprivation, emphasizing the inequality of this funding model [43]. Different healthcare system models are strongly associated with unequal access to diabetes technology, as described in two recent reviews from the U.S. [44,45]. With this in mind, the results of the present study not demonstrating modern technology to be superior to other modalities are somewhat comforting. However, it should be emphasized that this study was made in a Danish setting based on a population with universal healthcare and a generally high SES. Thus, more studies are needed to fully evaluate the association between SES, access to healthcare and technology distribution.
A significant strength of the current study is that it was based on data extracted from medical records of the study population, thus making the information as precise as possible. The number of study participants could be a limitation, as statistically significant differences can be difficult to detect when sample sizes are too small. Furthermore, as mentioned above, the study was performed as a single-center study in a Danish setting and the SES between groups was similar, which can impact the generalizability of the current study. The timeframe of the study setting was chosen to be quite short in order to unify treatment modalities and healthcare, as technology and healthcare offers tend to change rapidly. In line with this, another weakness of the study is that treatment with the hybrid closed-loop system was not included as this treatment modality was very new and had only been used by extremely few pregnant women in the inclusion period. In future studies, hybrid closed-loop systems compared to sensor use and MDI therapy would be of interest, since at least CGM is now available for most women with T1D in developed countries. As mentioned previously, the use of HbA1c as the only parameter for glycemic control can also be a limitation, as it does not provide information about time in range, hyper- or hypoglycemic events, etc., which are parameters considered to represent the glycemic regulation in a more detailed manner.
5. Conclusions
With this relatively small, but detailed, study of 84 pregnant women with type 1 diabetes, we conclude that the type of diabetes technology did not make a difference regarding glycemic control before and during pregnancy. However, higher birthweight z-score was found in women with sensor and insulin pump compared to women without these treatment modalities. Thereby, this study suggests that diabetes technology, including glucose sensors and/or insulin pumps per se are not leading to a better glycemic control during pregnancy. Also, the same number of adverse obstetric outcomes were observed across groups, emphasizing that diabetes technology cannot stand alone in the complex treatment of pregnant women with type 1 diabetes.
Author Contributions
Conceptualization, S.F. and U.K.; data curation, S.Y.G., P.B.G., P.G.O. and J.F.; formal analysis, S.Y.G., P.B.G. and M.L.-M.; investigation, S.Y.G. and P.B.G.; project administration, U.K.; supervision, P.G.O., J.F., S.F., H.D.M. and U.K.; writing—original draft, S.Y.G., P.B.G. and M.L.-M.; writing—review and editing, S.Y.G., P.B.G., M.L.-M., P.G.O., J.F., S.F., H.D.M. and U.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval and informed consent was not required. Access to data was approved by the Board of directors at Aarhus University Hospital and the head of Department of Obstetrics and Gynecology at Aarhus University Hospitals according to the GDPR regulation and the Danish data protection legislation.
Informed Consent Statement
Patient consent was waived as the project was categorized as a quality assurance project and ethical approval or informed consent was not required.
Data Availability Statement
Datasets generated and analyzed during the current study are not publicly available due to the personal character of the data but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest and no disclosures except that the salaries of P.G.O. and U.K. are partly supported by The Novo Nordisk Foundation.
References
- Evers, I.M.; de Valk, H.W.; Visser, G.H. Risk of complications of pregnancy in women with type 1 diabetes: Nationwide prospective study in the Netherlands. BMJ 2004, 328, 915. [Google Scholar] [CrossRef]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Hwee, J.; Shah, B.R.; Booth, G.L.; Bierman, A.S.; Lipscombe, L.L. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: A large, population-based study in Ontario, Canada, 1996–2010. Diabetes Care 2014, 37, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Damm, P.; Moelsted-Pedersen, L.; Ovesen, P.; Westergaard, J.G.; Moeller, M.; Beck-Nielsen, H. Outcomes in type 1 diabetic pregnancies: A nationwide, population-based study. Diabetes Care 2004, 27, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Nilsson, I.A.K.; Gissler, M.; Lavebratt, C. Associations of Maternal Diabetes and Body Mass Index With Offspring Birth Weight and Prematurity. JAMA Pediatr. 2019, 173, 371–378. [Google Scholar] [CrossRef] [PubMed]
- He, L.R.; Yu, L.; Guo, Y. Birth weight and large for gestational age trends in offspring of pregnant women with gestational diabetes mellitus in southern China, 2012–2021. Front. Endocrinol. 2023, 14, 1166533. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, O.; Eitenberger, M.; Bilir, A.; Yeung, A.W.K.; Parvanov, E.D.; MohanaSundaram, A.; Horbańczuk, J.O.; Atanasov, A.G.; Willschke, H. Patent analysis of digital sensors for continuous glucose monitoring. Front. Public Health 2023, 11, 1205903. [Google Scholar] [CrossRef] [PubMed]
- Fazekas-Pongor, V.; Svébis, M.M.; Major, D.; Pártos, K.; Dósa, N.; Mészáros, Á.; Horváth, V.J.; Domján, B.A.; Zsirai, L.; Tabák, A.G. Trend of pregnancy outcomes in type 1 diabetes compared to control women: A register-based analysis in 1996–2018. Front. Endocrinol. 2023, 14, 1232618. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Howgate, C.; O’Keefe, J.; Myers, J.; Morgan, M.; Coleman, M.A.; Jolly, M.; Valabhji, J.; Scott, E.M.; Knighton, P.; et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: A 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 153–164. [Google Scholar] [CrossRef]
- Mackin, S.T.; Nelson, S.M.; Kerssens, J.J.; Wood, R.; Wild, S.; Colhoun, H.M.; Leese, G.P.; Philip, S.; Lindsay, R.S. Diabetes and pregnancy: National trends over a 15 year period. Diabetologia 2018, 61, 1081–1088. [Google Scholar] [CrossRef]
- Fadl, H.E.; Simmons, D. Trends in diabetes in pregnancy in Sweden 1998–2012. BMJ Open Diabetes Res. Care 2016, 4, e000221. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to do a fetal cardiac scan. Arch. Gynecol. Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Kuciene, R.; Dulskiene, V.; Medzioniene, J. Associations between high birth weight, being large for gestational age, and high blood pressure among adolescents: A cross-sectional study. Eur. J. Nutr. 2018, 57, 373–381. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, S.J.; Fu, G.J.; Zhao, Y.; Xie, Y.J.; Zhang, Y.; Wang, Y.X. The associations of high birth weight with blood pressure and hypertension in later life: A systematic review and meta-analysis. Hypertens. Res. 2013, 36, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Janszky, I.; Gissler, M.; Cnattingius, S.; Roos, N.; Miao, M.; Yuan, W.; Li, J.; László, K.D. Preterm Birth, Small for Gestational Age, and Large for Gestational Age and the Risk of Atrial Fibrillation Up to Middle Age. JAMA Pediatr. 2023, 177, 599–607. [Google Scholar] [CrossRef]
- Sparano, S.; Ahrens, W.; De Henauw, S.; Marild, S.; Molnar, D.; Moreno, L.A.; Suling, M.; Tornaritis, M.; Veidebaum, T.; Siani, A.; et al. Being macrosomic at birth is an independent predictor of overweight in children: Results from the IDEFICS study. Matern. Child Health J. 2013, 17, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lee, J.E. Large for Gestational Age and Obesity-Related Comorbidities. J. Obes. Metab. Syndr. 2021, 30, 124–131. [Google Scholar] [CrossRef]
- Hirsch, I.B. Introduction: History of Glucose Monitoring. In Role of Continuous Glucose Monitoring in Diabetes Treatment; American Diabetes Association: Arlington, VA, USA, 2018. [Google Scholar]
- Lind, M.; Polonsky, W.; Hirsch, I.B.; Heise, T.; Bolinder, J.; Dahlqvist, S.; Schwarz, E.; Ólafsdóttir, A.F.; Frid, A.; Wedel, H.; et al. Continuous Glucose Monitoring vs. Conventional Therapy for Glycemic Control in Adults with Type 1 Diabetes Treated with Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA 2017, 317, 379–387. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults with Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef]
- Pickup, J.C.; Keen, H.; Parsons, J.A.; Alberti, K.G. Continuous subcutaneous insulin infusion: An approach to achieving normoglycaemia. Br. Med. J. 1978, 1, 204–207. [Google Scholar] [CrossRef]
- Pala, L.; Dicembrini, I.; Mannucci, E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: An updated meta-analysis of randomized clinical trials. Acta Diabetol. 2019, 56, 973–980. [Google Scholar] [CrossRef]
- Thayer, S.M.; Williams, K.J.; Lawlor, M.L. The role of technology in the care of diabetes mellitus in pregnancy: An expert review. AJOG Glob. Rep. 2023, 3, 100245. [Google Scholar] [CrossRef]
- Lal, R.A.; Basina, M.; Maahs, D.M.; Hood, K.; Buckingham, B.; Wilson, D.M. One Year Clinical Experience of the First Commercial Hybrid Closed-Loop System. Diabetes Care 2019, 42, 2190–2196. [Google Scholar] [CrossRef]
- Lee, T.T.M.; Collett, C.; Bergford, S.; Hartnell, S.; Scott, E.M.; Lindsay, R.S.; Hunt, K.F.; McCance, D.R.; Barnard-Kelly, K.; Rankin, D.; et al. Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 1566–1578. [Google Scholar] [CrossRef]
- Feig, D.S.; Donovan, L.E.; Corcoy, R.; Murphy, K.E.; Amiel, S.A.; Hunt, K.F.; Asztalos, E.; Barrett, J.F.R.; Sanchez, J.J.; de Leiva, A.; et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet 2017, 390, 2347–2359. [Google Scholar] [CrossRef]
- Thorius, I.H.; Husemoen, L.L.N.; Nordsborg, R.B.; Alibegovic, A.C.; Gall, M.A.; Petersen, J.; Mathiesen, E.R. Congenital malformations among offspring of women with type 1 diabetes who use insulin pumps: A prospective cohort study. Diabetologia 2023, 66, 826–836. [Google Scholar] [CrossRef]
- Żurawska-Kliś, M.; Kosiński, M.; Kuchnicka, A.; Rurka, M.; Hałucha, J.; Wójcik, M.; Cypryk, K. Continuous subcutaneous insulin infusion does not correspond with pregnancy outcomes despite better glycemic control as compared to multiple daily injections in type 1 diabetes—Significance of pregnancy planning and prepregnancy HbA1c. Diabetes Res. Clin. Pract. 2021, 172, 108628. [Google Scholar] [CrossRef] [PubMed]
- Danish Health Authority. Sunde Vaner. 2022. Available online: https://www.sst.dk/-/media/Udgivelser/2022/Foerste-1000-dage/Sunde_vaner_8_-udgave-2022.ashx (accessed on 20 December 2023).
- Marsál, K.; Persson, P.H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Shen, Y.; Chen, Y. Better TIR, HbA1c, and less hypoglycemia in closed-loop insulin system in patients with type 1 diabetes: A meta-analysis. BMJ Open Diabetes Res. Care 2022, 10, e002633. [Google Scholar] [CrossRef]
- Abell, S.K.; Suen, M.; Pease, A.; Boyle, J.A.; Soldatos, G.; Regan, J.; Wallace, E.M.; Teede, H.J. Pregnancy Outcomes and Insulin Requirements in Women with Type 1 Diabetes Treated with Continuous Subcutaneous Insulin Infusion and Multiple Daily Injections: Cohort Study. Diabetes Technol. Ther. 2017, 19, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ringholm, L.; Damm, P.; Mathiesen, E.R. Improving pregnancy outcomes in women with diabetes mellitus: Modern management. Nat. Rev. Endocrinol. 2019, 15, 406–416. [Google Scholar] [CrossRef]
- Pratley, R.E.; Kanapka, L.G.; Rickels, M.R.; Ahmann, A.; Aleppo, G.; Beck, R.; Bhargava, A.; Bode, B.W.; Carlson, A.; Chaytor, N.S.; et al. Effect of Continuous Glucose Monitoring on Hypoglycemia in Older Adults with Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2020, 323, 2397–2406. [Google Scholar] [CrossRef]
- Mathiesen, J.M.; Secher, A.L.; Ringholm, L.; Nørgaard, K.; Hommel, E.; Andersen, H.U.; Damm, P.; Mathiesen, E.R. Changes in basal rates and bolus calculator settings in insulin pumps during pregnancy in women with type 1 diabetes. J. Matern. Fetal Neonatal Med. 2014, 27, 724–728. [Google Scholar] [CrossRef]
- Kallas-Koeman, M.M.; Kong, J.M.; Klinke, J.A.; Butalia, S.; Lodha, A.K.; Lim, K.I.; Duan, Q.M.; Donovan, L.E. Insulin pump use in pregnancy is associated with lower HbA1c without increasing the rate of severe hypoglycaemia or diabetic ketoacidosis in women with type 1 diabetes. Diabetologia 2014, 57, 681–689. [Google Scholar] [CrossRef]
- Rys, P.M.; Ludwig-Slomczynska, A.H.; Cyganek, K.; Malecki, M.T. Continuous subcutaneous insulin infusion vs multiple daily injections in pregnant women with type 1 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials and observational studies. Eur. J. Endocrinol. 2018, 178, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Mamet, Y. Red blood cell survival and kinetics during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 93, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.R.; Ekbom, P.; Damm, P.; Glümer, C.; Frandsen, M.M.; Jensen, D.M.; Mathiesen, E.R. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004, 27, 1200–1201. [Google Scholar] [CrossRef]
- Kerssen, A.; Evers, I.M.; de Valk, H.W.; Visser, G.H. Poor glucose control in women with type 1 diabetes mellitus and ‘safe’ hemoglobin A1c values in the first trimester of pregnancy. J. Matern. Fetal Neonatal Med. 2003, 13, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Law, G.R.; Gilthorpe, M.S.; Secher, A.L.; Temple, R.; Bilous, R.; Mathiesen, E.R.; Murphy, H.R.; Scott, E.M. Translating HbA(1c) measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia 2017, 60, 618–624. [Google Scholar] [CrossRef]
- Dansk Endokrinologisk Selskab. Kontinuerlig Glukosemåling (CGM). 2020. Available online: https://endocrinology.dk/nbv/diabetes-melitus/kontinuerlig-glukosemaaling-cgm-og-flash-glukosemaaling-fgm-til-boern-unge-og-voksne/ (accessed on 23 November 2022).
- Lomax, K.E.; Taplin, C.E.; Abraham, M.B.; Smith, G.J.; Haynes, A.; Zomer, E.; Ellis, K.L.; Clapin, H.; Zoungas, S.; Jenkins, A.J.; et al. Socioeconomic status and diabetes technology use in youth with type 1 diabetes: A comparison of two funding models. Front. Endocrinol. 2023, 14, 1178958. [Google Scholar] [CrossRef]
- Agarwal, S.; Simmonds, I.; Myers, A.K. The Use of Diabetes Technology to Address Inequity in Health Outcomes: Limitations and Opportunities. Curr. Diabetes Rep. 2022, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, D.; Bellini, N.J.; Biba, U.; Cai, A.; Close, K.L. Health Care Disparities in Use of Continuous Glucose Monitoring. Diabetes Technol. Ther. 2021, 23, S81–S87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).