Exposure of Early Postnatal Oocytes to Chemotherapy Alters the Potential Ovarian Reserve, According to an Ex Vivo Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
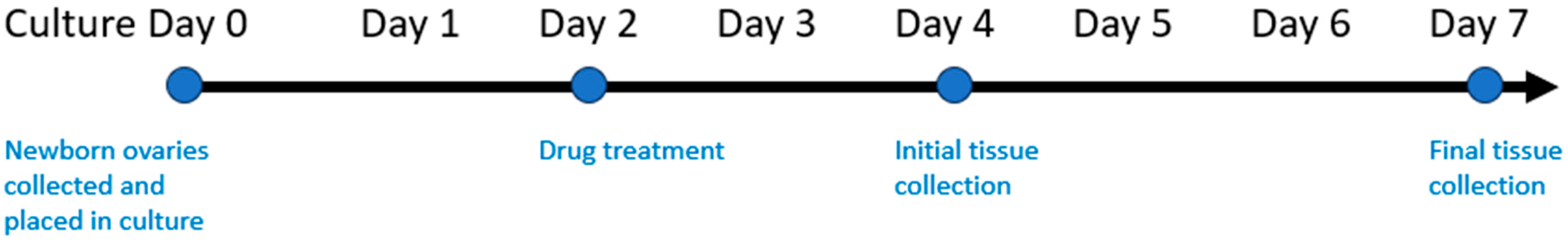
2.2. Chemotherapy Exposure
2.3. Immunofluorescent Staining
2.4. Cell Death Staining
2.5. Statistical Analysis of Data
3. Results
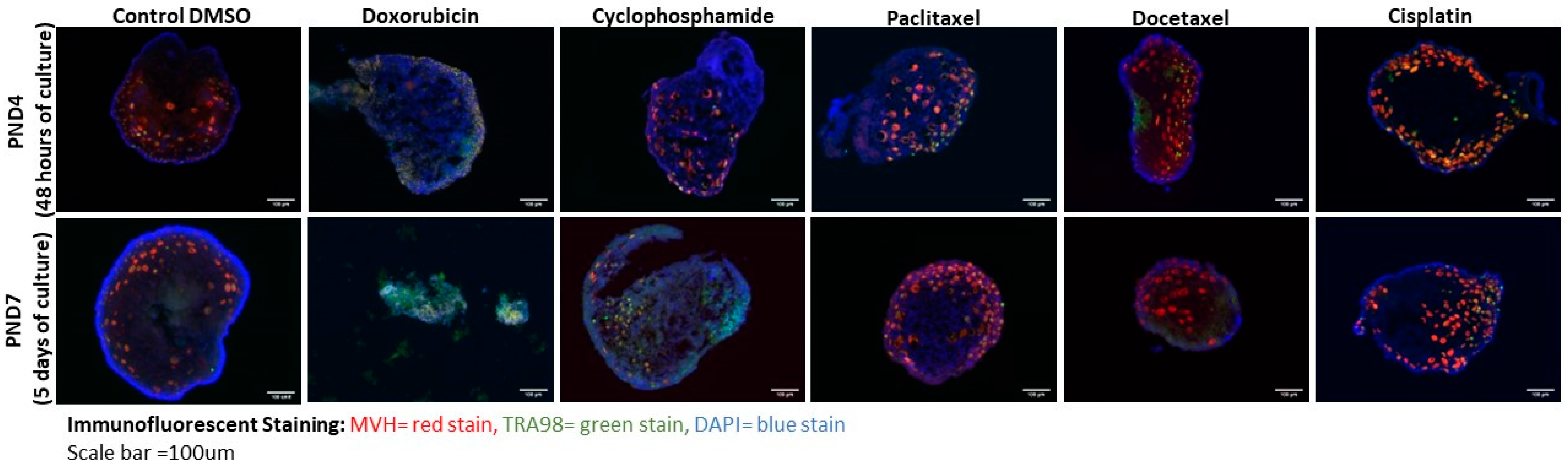
3.1. Doxorubicin and Cyclophosphamide Exposure Result in Dramatic Loss of Oocytes in Culture
3.2. Cisplatin, Docetaxel, and Paclitaxel Exposure Did Not Lead to Oocyte Loss
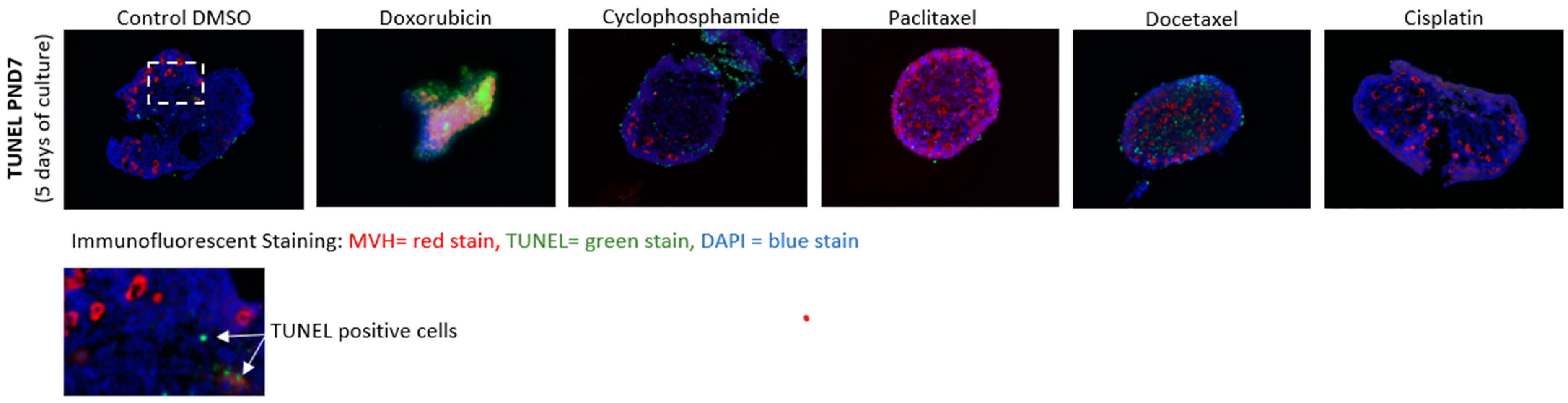
3.3. Cell Death Analysis
4. Discussion
5. Impact on Clinical Practice
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs). Available online: https://seer.cancer.gov/statfacts/html/aya.html (accessed on 4 February 2022).
- Stensheim, H.; Moller, B.; van Dijk, T.; Fossa, S.D. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J. Clin. Oncol. 2009, 27, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A., Jr.; Peccatori, F.A.; Pavlidis, N. Treatment of the pregnant mother with cancer: A systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part I: Solid tumors. Cancer Treat. Rev. 2010, 36, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A., Jr.; Pavlidis, N.; Peccatori, F.A. Treatment of the pregnant mother with cancer: A systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part II: Hematological tumors. Cancer Treat. Rev. 2010, 36, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G.; Pavlidis, N. Cancer and pregnancy: Poena magna, not anymore. Eur. J. Cancer 2006, 42, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Margioula-Siarkou, C.; Petousis, S.; Papanikolaou, A.; Gullo, G.; Margioula-Siarkou, G.; Laganà, A.S.; Dinas, K.; Guyon, F. Neoadjuvant chemotherapy in advanced-stage ovarian cancer—State of the art. Prz. Menopauzalny 2022, 21, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, K.; Heyns, L.; De Smet, F.; Van Eycken, L.; Gziri, M.M.; Van Gemert, W.; Halaska, M.; Vergote, I.; Ottevanger, N.; Amant, F. Cancer during pregnancy: An analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J. Clin. Oncol. 2010, 28, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Cardonick, E.; Iacobucci, A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004, 5, 283–291. [Google Scholar] [CrossRef]
- Cardonick, E.; Usmani, A.; Ghaffar, S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: Results of an international registry. Am. J. Clin. Oncol. 2010, 33, 221–228. [Google Scholar] [CrossRef]
- Cardonick, E.H.; Gringlas, M.B.; Hunter, K.; Greenspan, J. Development of children born to mothers with cancer during pregnancy: Comparing in utero chemotherapy-exposed children with nonexposed controls. Am. J. Obstet. Gynecol. 2015, 212, 658.e1–658.e8. [Google Scholar] [CrossRef]
- Cardonick, E.H.; O’Laughlin, A.E.; So, S.C.; Fleischer, L.T.; Akoto, S. Paclitaxel use in pregnancy: Neonatal follow-up of infants with positive detection of intact paclitaxel and metabolites in meconium at birth. Eur. J. Pediatr. 2022, 181, 1763–1766. [Google Scholar] [CrossRef]
- Avilés, A.; Neri, N. Hematological malignancies and pregnancy: A final report of 84 children who received chemotherapy in utero. Clin. Lymphoma 2001, 2, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Van Calsteren, K.; Halaska, M.J.; Gziri, M.M.; Hui, W.; Lagae, L.; Willemsen, M.A.; Kapusta, L.; Van Calster, B.; Wouters, H.; et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: An observational study. Lancet Oncol. 2012, 13, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Vandenbroucke, T.; Verheecke, M.; Fumagalli, M.; Halaska, M.J.; Boere, I.; Han, S.; Gziri, M.M.; Peccatori, F.; Rob, L.; et al. Pediatric Outcome after Maternal Cancer Diagnosed during Pregnancy. N. Engl. J. Med. 2015, 373, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Han, S.N.; Gziri, M.M.; Dekrem, J.; Van Calsteren, K. Chemotherapy during pregnancy. Curr. Opin. Oncol. 2012, 24, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.M.; Johnson, P.H.; Gordon, N.; Kuerer, H.; Middleton, L.; Ramirez, M.; Yang, W.; Perkins, G.; Hortobagyi, G.N.; Theriault, R.L. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 2006, 107, 1219–1226. [Google Scholar] [CrossRef]
- Burgio, S.; Polizzi, C.; Buzzaccarini, G.; Laganà, A.S.; Gullo, G.; Perricone, G.; Perino, A.; Cucinella, G.; Alesi, M. Psychological variables in medically assisted reproduction: A systematic review. Menopause Rev./Przegląd Menopauzalny 2022, 21, 47–63. [Google Scholar] [CrossRef]
- Gullo, G.; Etrusco, A.; Cucinella, G.; Basile, G.; Fabio, M.; Perino, A.; De Tommasi, O.; Buzzaccarini, G.; Morreale, C.; Marchi, L.; et al. Ovarian tissue cryopreservation and transplantation in menopause: New perspective of therapy in postmenopausal women and the importance of ethical and legal frameworks. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9107–9116. [Google Scholar] [CrossRef]
- Zaami, S.; Stark, M.; Signore, F.; Gullo, G.; Marinelli, E. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur. J. Contracept. Reprod. Health Care 2022, 27, 335–340. [Google Scholar] [CrossRef]
- Calsteren, K.V.; Verbesselt, R.; Devlieger, R.; De Catte, L.; Chai, D.C.; Van Bree, R.; Heyns, L.; Beijnen, J.; Demarsin, S.; de Bruijn, E.; et al. Transplacental transfer of paclitaxel, docetaxel, carboplatin, and trastuzumab in a baboon model. Int. J. Gynecol. Cancer 2010, 20, 1456–1464. [Google Scholar]
- Witt, K.L.; Bishop, J.B. Mutagenicity of anticancer drugs in mammalian germ cells. Mutat. Res. 1996, 355, 209–234. [Google Scholar] [CrossRef]
- Gondos, B.; Bhiraleus, P.; Hobel, C.J. Ultrastructural observations on germ cells in human fetal ovaries. Am. J. Obstet. Gynecol. 1971, 110, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Tingen, C.; Kim, A.; Woodruff, T.K. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol. Hum. Reprod. 2009, 15, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.K.M.; Sheikh, S.; Williams, S.A. In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries. Reproduction 2019, 157, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jefferson, W.N.; Newbold, R.R.; Padilla-Banks, E.; Pepling, M.E. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 2007, 148, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G.; on behalf of the ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), VI160–VI170. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Grive, K.J.; Seymour, K.A.; Mehta, R.; Freiman, R.N. TAF4b promotes mouse primordial follicle assembly and oocyte survival. Dev. Biol. 2014, 392, 42–51. [Google Scholar] [CrossRef]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, G.M.; Nottoli, R. Placental transfer of drugs administered to the mother. Clin. Pharmacokinet. 1995, 28, 235–269. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V.; Perry, M.C. Classical chemotherapy: Mechanisms, toxicities and the therapeutic window. Cancer Biol. Ther. 2003, 2, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, D.B.; Lu, X.; Wei, H.; Zhu, R.; Zhu, S.S.; Jiang, H.; Yang, Z.J. Doxorubicin induces apoptosis in H9c2 cardiomyocytes: Role of overexpressed eukaryotic translation initiation factor 5A. Biol. Pharm. Bull. 2010, 33, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef]
- Grant, C.H.; Gourley, C. Cancer Treatment and the Ovary, Web ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Loibl, S.; Han, S.N.; von Minckwitz, G.; Bontenbal, M.; Ring, A.; Giermek, J.; Fehm, T.; Van Calsteren, K.; Linn, S.C.; Schlehe, B.; et al. Treatment of breast cancer during pregnancy: An observational study. Lancet Oncol. 2012, 13, 887–896. [Google Scholar] [CrossRef]
- Murthy, R.K.; Theriault, R.L.; Barnett, C.M.; Hodge, S.; Ramirez, M.M.; Milbourne, A.; Rimes, S.A.; Hortobagyi, G.N.; Valero, V.; Litton, J.K. Outcomes of children exposed in utero to chemotherapy for breast cancer. Breast Cancer Res. 2014, 16, 500. [Google Scholar] [CrossRef]

| Agent | Clinical Dose | CMID (μg/mL) | CMAX (μg/mL) | Mechanism of Action |
|---|---|---|---|---|
| Cyclophosphamide | 600 mg/m2 | 16.7 | 33.4 | Alkylation of DNA that is not cell-cycle or phase specifics resulting in inhibition of DNA replication and transcription. |
| Doxorubicin | 60 mg/m2 | 1.8 | 3.7 | Inhibit RNA and DNA synthesis. Also, inhibiting topoisomerase II, causing inhibition of DNA repair. |
| Docetaxel | 100 mg/m2 | 2.2 | 4.4 | Disruption in the equilibrium of polymerization and depolymerization of microtubules causing abnormal cellular function and disruption of replication leading to apoptosis. |
| Paclitaxel | 175 mg/m2 | 1.8 | 3.7 | Disruption in the equilibrium of polymerization and depolymerization of microtubules causing abnormal cellular function and disruption of replication leading to apoptosis. |
| Cisplatin | 80 mg/m2 (ovary) 50 mg/m2 (breast) 40 mg/m2 (cervix) | 2.6 3.5 | 4.3 | Platinum binds DNA forming intra-stranded and inter-stranded crosslinks. This inhibits DNA replication and transcription. |
| Condition | Oocyte Density at PND4 | Oocyte Density at PND7 | Adjusted p Value Two-Way ANOVA |
|---|---|---|---|
| (% of Control) | (% of Control) | ||
| Control | 693 oocytes/mm2 | 570 oocytes/mm2 | n/a |
| (1 µL DMSO) | StErr = 62 | StErr = 71 | |
| (--) | (--) | ||
| Doxorubicin | 21 oocytes/mm2 | 23 oocytes/mm2 | <0.0001 |
| Mid dose | StErr = 16 | StErr = 9 | |
| (1.83 µg/ml) | (3%) | (4%) | |
| Doxorubicin | 63 oocytes/mm2 | 27 oocytes/mm2 | <0.0001 |
| Max Dose | StErr = 33 | StErr = 8 | |
| (3.66 µg/ml) | (4%) | (5%) | |
| Cyclophosphamide | 586 oocytes/mm2 | 366 oocytes/mm2 | 0.35 |
| Mid dose | StErr = 120 | StErr = 29 | |
| (16.704 µg/ml) | (85%) | (64%) | |
| Cyclophosphamide | 438 oocytes/mm2 | 282 oocytes/mm2 | 0.0004 |
| Max Dose | StErr = 45 | StErr = 8 | |
| (33.408 µg/ml) | (63%) | (49%) | |
| Paclitaxel | 628 oocytes/mm2 | 796 oocytes/mm2 | 0.7024 |
| Mid dose | StErr = 50 | StErr = 115 | |
| (1.825 µg/ml) | (91%) | (140%) | |
| Paclitaxel | 668 oocytes/mm2 | 718 oocytes/mm2 | 0.8157 |
| Max Dose | StErr = 27 | StErr = 98 | |
| (3.65 µg/ml) | (96%) | (126%) | |
| Docetaxel | 353 oocytes/mm2 | 531 oocytes/mm2 | 0.2794 |
| Mid dose | StErr = 2 | StErr = 96 | |
| (2.21 µg/ml) | (51%) | (93%) | |
| Docetaxel | 538 oocytes/mm2 | 624 oocytes/mm2 | 0.8616 |
| Max Dose | StErr = 173 | StErr = 61 | |
| (4.42 µg/ml) | (78%) | (109%) | |
| Cisplatin: Low dose | 975 oocytes/mm2 | 608 oocytes/mm2 | 0.326 |
| (2.593 µg/ml) | StErr = 120 | StErr = 52 | |
| (51%) | (93%) | ||
| Cisplatin: Mid dose | 663 oocytes/mm2 | 591 oocytes/mm2 | >0.9999 |
| (3.457 µg/ml) | StErr = 29 | StErr = 27 | |
| (96%) | (104%) | ||
| Cisplatin: Max Dose | 736 oocytes/mm2 | 524 oocytes/mm2 | >0.9999 |
| (4.321 µg/ml) | StErr = 151 | StErr = 80 | |
| (106%) | (92%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozcan, M.C.H.; Chaqour, J.; Woodman-Sousa, M.F.; Grive, K.J. Exposure of Early Postnatal Oocytes to Chemotherapy Alters the Potential Ovarian Reserve, According to an Ex Vivo Mouse Model. Reprod. Med. 2023, 4, 248-258. https://doi.org/10.3390/reprodmed4040023
Ozcan MCH, Chaqour J, Woodman-Sousa MF, Grive KJ. Exposure of Early Postnatal Oocytes to Chemotherapy Alters the Potential Ovarian Reserve, According to an Ex Vivo Mouse Model. Reproductive Medicine. 2023; 4(4):248-258. https://doi.org/10.3390/reprodmed4040023
Chicago/Turabian StyleOzcan, Meghan C. H., Julienne Chaqour, Morgan F. Woodman-Sousa, and Kathryn J. Grive. 2023. "Exposure of Early Postnatal Oocytes to Chemotherapy Alters the Potential Ovarian Reserve, According to an Ex Vivo Mouse Model" Reproductive Medicine 4, no. 4: 248-258. https://doi.org/10.3390/reprodmed4040023
APA StyleOzcan, M. C. H., Chaqour, J., Woodman-Sousa, M. F., & Grive, K. J. (2023). Exposure of Early Postnatal Oocytes to Chemotherapy Alters the Potential Ovarian Reserve, According to an Ex Vivo Mouse Model. Reproductive Medicine, 4(4), 248-258. https://doi.org/10.3390/reprodmed4040023






