Human Maternal-Fetal Interface Cellular Models to Assess Antiviral Drug Toxicity during Pregnancy
Abstract
1. Introduction
2. Viral Infections in Pregnancy
3. Systemic Exclusion of Pregnant Women from Antiviral Therapeutic Trials
4. Human Maternal-Fetal Interface Models to Screen Safe Antiviral Drugs
4.1. Cellular Models Representing Human Placenta
4.1.1. Placental Cell Lines
4.1.2. Isolated Primary Trophoblasts
4.1.3. Trophoblastic Stem-Cell (TSC)-Derived Trophoblast Models
4.1.4. Placental Organoid and Engineered 3D Models
4.1.5. Placental Explants
4.2. Cellular Models Representing the Embryo and Fetal Organ Development
4.2.1. Cellular Models of Early Embryonic Development
4.2.2. Cellular Models of Organ Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, W.; Hu, X.; Cao, B. Viral Infections During Pregnancy: The Big Challenge Threatening Maternal and Fetal Health. Matern.-Fetal Med. 2022, 4, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, B.J.; Carey, J.C. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr. Women’s Health Rep. 2002, 2, 253–258. [Google Scholar]
- FDA. S5(R3) Detection of Toxicity to Reproduction for Human Pharmaceuticals. Available online: https://www.fda.gov/media/108894/download (accessed on 29 November 2022).
- Carter, A.M. Animal models of human pregnancy and placentation: Alternatives to the mouse. Reproduction 2020, 160, R129–R143. [Google Scholar] [CrossRef]
- Radermacher, P.; Haouzi, P. A mouse is not a rat is not a man: Species-specific metabolic responses to sepsis—A nail in the coffin of murine models for critical care research? Intensive Care Med. Exp. 2013, 1, 7. [Google Scholar] [CrossRef]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef]
- Davis, N.L.; King, C.C.; Kourtis, A.P. Cytomegalovirus infection in pregnancy. Birth Defects Res. 2017, 109, 336–346. [Google Scholar] [CrossRef]
- Bouthry, E.; Picone, O.; Hamdi, G.; Grangeot-Keros, L.; Ayoubi, J.M.; Vauloup-Fellous, C. Rubella and pregnancy: Diagnosis, management and outcomes. Prenat. Diagn. 2014, 34, 1246–1253. [Google Scholar] [CrossRef]
- Nanthakumar, M.P.; Sood, A.; Ahmed, M.; Gupta, J. Varicella Zoster in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 283–287. [Google Scholar] [CrossRef]
- Jaan, A.; Rajnik, M. TORCH Complex. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gupta, S.; Gupta, N. Short-term pregnancy outcomes in patients chikungunya infection: An observational study. J. Fam. Med. Prim Care 2019, 8, 985–987. [Google Scholar] [CrossRef]
- Hammad, W.A.B.; Konje, J.C. Herpes simplex virus infection in pregnancy—An update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 38–45. [Google Scholar] [CrossRef]
- Stein, S.J.; Greenspoon, J.S. Rubeola during pregnancy. Obstet. Gynecol. 1991, 78, 925–929. [Google Scholar]
- Giorgio, E.; De Oronzo, M.A.; Iozza, I.; Di Natale, A.; Cianci, S.; Garofalo, G.; Giacobbe, A.M.; Politi, S. Parvovirus B19 during pregnancy: A review. J. Prenat. Med. 2010, 4, 63–66. [Google Scholar]
- Siston, A.M.; Rasmussen, S.A.; Honein, M.A.; Fry, A.M.; Seib, K.; Callaghan, W.M.; Louie, J.; Doyle, T.J.; Crockett, M.; Lynfield, R.; et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010, 303, 1517–1525. [Google Scholar] [CrossRef]
- CDC. Maternal and Infant Outcomes among Severely Ill Pregnant and Postpartum Women with 2009 Pandemic Influenza A (H1N1)—United States, April 2009–August 2010. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6035a2.htm (accessed on 29 November 2022).
- Hause, A.M.; Avadhanula, V.; Maccato, M.L.; Pinell, P.M.; Bond, N.; Santarcangelo, P.; Ferlic-Stark, L.; Munoz, F.M.; Piedra, P.A. A Cross-sectional Surveillance Study of the Frequency and Etiology of Acute Respiratory Illness Among Pregnant Women. J. Infect. Dis. 2018, 218, 528–535. [Google Scholar] [CrossRef]
- Pilorgé, L.; Chartier, M.; Méritet, J.F.; Cervantes, M.; Tsatsaris, V.; Launay, O.; Rozenberg, F.; Krivine, A. Rhinoviruses as an underestimated cause of influenza-like illness in pregnancy during the 2009-2010 influenza pandemic. J. Med. Virol. 2013, 85, 1473–1477. [Google Scholar] [CrossRef]
- Philpott, E.K.; Englund, J.A.; Katz, J.; Tielsch, J.; Khatry, S.; LeClerq, S.C.; Shrestha, L.; Kuypers, J.; Magaret, A.S.; Steinhoff, M.C.; et al. Febrile Rhinovirus Illness During Pregnancy Is Associated With Low Birth Weight in Nepal. Open Forum Infect. Dis. 2017, 4, ofx073. [Google Scholar] [CrossRef]
- Lenahan, J.L.; Englund, J.A.; Katz, J.; Kuypers, J.; Wald, A.; Magaret, A.; Tielsch, J.M.; Khatry, S.K.; LeClerq, S.C.; Shrestha, L.; et al. Human Metapneumovirus and Other Respiratory Viral Infections during Pregnancy and Birth, Nepal. Emerg. Infect. Dis. 2017, 23, 1341–1349. [Google Scholar] [CrossRef]
- Hajra, A.; Bandyopadhyay, D.; Heise, L.R.; Bhadra, R.; Ball, S.; Hajra, S.K. Zika and pregnancy: A comprehensive review. Am. J. Reprod. Immunol. 2017, 77, e12607. [Google Scholar] [CrossRef]
- Messinger, C.J.; Lipsitch, M.; Bateman, B.T.; He, M.; Huybrechts, K.F.; MacDonald, S.; Mogun, H.; Mott, K.; Hernández-Díaz, S. Association Between Congenital Cytomegalovirus and the Prevalence at Birth of Microcephaly in the United States. JAMA Pediatr. 2020, 174, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., 3rd; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Elkafrawi, D.; Sisti, G.; Mercado, F.; Rodriguez, B.; Joseph, J.; Jones, C.; Schiattarella, A.; Upadhyay, R. Hispanic race is a risk factor for COVID-19 during pregnancy: Data from an urban New York City hospital. J. Obstet. Gynaecol. 2022, 42, 1054–1057. [Google Scholar] [CrossRef]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Characteristics and Outcomes of Pregnant Women Admitted to Hospital With Confirmed SARS-CoV-2 Infection in the UK: National Population-based Cohort Study. Obstet. Anesth. Dig. 2021, 41, 22–23. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Baergen, R.N.; Heller, D.S. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, e381–e382. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Liu-Jarin, X.; Hamele-Bena, D.; Cimic, A.; Mourad, M.; Debelenko, L.; Chen, X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: Histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020, 77, 994–999. [Google Scholar] [CrossRef]
- Glynn, S.M.; Yang, Y.J.; Thomas, C.; Friedlander, R.L.; Cagino, K.A.; Matthews, K.C.; Riley, L.E.; Baergen, R.N.; Prabhu, M. SARS-CoV-2 and Placental Pathology: Malperfusion Patterns Are Dependent on Timing of Infection During Pregnancy. Am. J. Surg. Pathol. 2022, 46, 51–57. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency From COVID-19 Causes Stillbirth and Neonatal Death From Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Watkins, J.C.; Torous, V.F.; Roberts, D.J. Defining Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Placentitis. Arch. Pathol. Lab. Med. 2021, 145, 1341–1349. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Murphy, E.A.; Rand, S.; Sukhu, A.; Singh, S.; Schatz-Siemers, N.; Matrai, C.; Tu, J.; Salvatore, C.M.; Prabhu, M.; et al. Placental pathology, neonatal birthweight and Apgar score in acute and distant SARS-CoV-2 infection. J. Clin. Transl. Res. 2022, 8, 351–359. [Google Scholar] [CrossRef]
- Malha, L.; August, P. Safety of Antihypertensive Medications in Pregnancy: Living With Uncertainty. J. Am. Heart Assoc. 2019, 8, e013495. [Google Scholar] [CrossRef]
- Guo, L.; Ma, J.; Tang, J.; Hu, D.; Zhang, W.; Zhao, X. Comparative Efficacy and Safety of Metformin, Glyburide, and Insulin in Treating Gestational Diabetes Mellitus: A Meta-Analysis. J. Diabetes Res. 2019, 2019, 9804708. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Rios, P.; Cogo, E.; Straus, S.E.; Finkelstein, Y.; Kealey, R.; Reynen, E.; Soobiah, C.; Thavorn, K.; Hutton, B.; et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: A systematic review and network meta-analysis. BMJ Open 2017, 7, e017248. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Ergaz, Z. Antidepressants, Antipsychotics, and Mood Stabilizers in Pregnancy: What Do We Know and How Should We Treat Pregnant Women with Depression. Birth Defects Res. 2017, 109, 933–956. [Google Scholar] [CrossRef]
- Budi, D.S.; Pratama, N.R.; Wafa, I.A.; Putra, M.; Wardhana, M.P.; Wungu, C.D.K. Remdesivir for pregnancy: A systematic review of antiviral therapy for COVID-19. Heliyon 2022, 8, e08835. [Google Scholar] [CrossRef]
- Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Hartge, P.; Hatch, E.E.; Herbst, A.L.; et al. Adverse Health Outcomes in Women Exposed In Utero to Diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304–1314. [Google Scholar] [CrossRef]
- Melnick, S.; Cole, P.; Anderson, D.; Herbst, A. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. An update. N. Engl. J. Med. 1987, 316, 514–516. [Google Scholar] [CrossRef]
- Robboy, S.J.; Noller, K.L.; O‘Brien, P.; Kaufman, R.H.; Townsend, D.; Barnes, A.B.; Gundersen, J.; Lawrence, W.D.; Bergstrahl, E.; McGorray, S.; et al. Increased incidence of cervical and vaginal dysplasia in 3,980 diethylstilbestrol-exposed young women. Experience of the National Collaborative Diethylstilbestrol Adenosis Project. JAMA 1984, 252, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Behr, S.C.; Courtier, J.L.; Qayyum, A. Imaging of Müllerian Duct Anomalies. RadioGraphics 2012, 32, E233–E250. [Google Scholar] [CrossRef] [PubMed]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.G. Thalidomide and congenital abnormalities. Lancet 1961, 278, 1358. [Google Scholar] [CrossRef]
- Smithells, R.W.; Newman, C.G. Recognition of thalidomide defects. J. Med. Genet. 1992, 29, 716–723. [Google Scholar] [CrossRef]
- Ren, Z.; Bremer, A.A.; Pawlyk, A.C. Drug development research in pregnant and lactating women. Am. J. Obstet. Gynecol. 2021, 225, 33–42. [Google Scholar] [CrossRef]
- Byrne, J.J.; Saucedo, A.M.; Spong, C.Y. Task force on research specific to pregnant and lactating women. Semin. Perinatol. 2020, 44, 151226. [Google Scholar] [CrossRef]
- Louchet, M.; Sibiude, J.; Peytavin, G.; Picone, O.; Tréluyer, J.M.; Mandelbrot, L. Placental transfer and safety in pregnancy of medications under investigation to treat coronavirus disease 2019. Am. J. Obstet. Gynecol. MFM 2020, 2, 100159. [Google Scholar] [CrossRef]
- Donner, B.; Niranjan, V.; Hoffmann, G. Safety of oseltamivir in pregnancy: A review of preclinical and clinical data. Drug Saf. 2010, 33, 631–642. [Google Scholar] [CrossRef]
- Cowdell, I.; Beck, K.; Portwood, C.; Sexton, H.; Kumarendran, M.; Brandon, Z.; Kirtley, S.; Hemelaar, J. Adverse perinatal outcomes associated with protease inhibitor-based antiretroviral therapy in pregnant women living with HIV: A systematic review and meta-analysis. eClinicalMedicine 2022, 46, 101368. [Google Scholar] [CrossRef]
- FDA, U.S. S5(R3) Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/s5r3-detection-reproductive-and-developmental-toxicity-human-pharmaceuticals (accessed on 29 November 2022).
- Kim, J.H.; Scialli, A.R. Thalidomide: The Tragedy of Birth Defects and the Effective Treatment of Disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar] [CrossRef]
- Tantibanchachai, C.J.Y. Studies of Thalidomide’s Effects on Rodent Embryos from 1962–2008. Available online: https://embryo.asu.edu/pages/studies-thalidomides-effects-rodent-embryos-1962-2008 (accessed on 29 November 2022).
- Blumenthal, K.G.; Shenoy, E.S. Penicillin Allergy in Pregnancy. JAMA 2020, 323, 1216. [Google Scholar] [CrossRef]
- Green, R.H. The association of viral activation with penicillin toxicity in guinea pigs and hamsters. Yale J. Biol. Med. 1974, 47, 166–181. [Google Scholar]
- Anderson, G.D. Pregnancy-induced changes in pharmacokinetics: A mechanistic-based approach. Clin. Pharmacokinet. 2005, 44, 989–1008. [Google Scholar] [CrossRef]
- Rosenkrantz, J.L.; Gaffney, J.E.; Roberts, V.H.J.; Carbone, L.; Chavez, S.L. Transcriptomic analysis of primate placentas and novel rhesus trophoblast cell lines informs investigations of human placentation. BMC Biol. 2021, 19, 127. [Google Scholar] [CrossRef]
- Soares, M.J.; Varberg, K.M.; Iqbal, K. Hemochorial placentation: Development, function, and adaptations. Biol. Reprod. 2018, 99, 196–211. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Ganapathy, V. Drugs of abuse and human placenta. Life Sci. 2011, 88, 926–930. [Google Scholar] [CrossRef]
- Kaufmann, P.; Stark, J.; Stegner, H.E. The villous stroma of the human placenta. Cell Tissue Res. 1977, 177, 105–121. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Ilic, D.; Kapidzic, M.; Genbacev, O. Isolation of human placental fibroblasts. Curr. Protoc. Stem Cell Biol. 2008, 5, 1C-6. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Fu, B.; Tian, Z.; Wei, H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell. Mol. Immunol. 2014, 11, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Salvany-Celades, M.; van der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Bougleux Gomes, H.A.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep. 2019, 27, 2537–2547.e5. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, R.A.; Gey, G.O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968, 28, 1231–1236. [Google Scholar]
- Azizkhan, J.C.; Speeg, K.V., Jr.; Stromberg, K.; Goode, D. Stimulation of Human Chorionic Gonadotropin by JAr Line Choriocarcinoma after Inhibition of DNA Synthesis1. Cancer Res. 1979, 39, 1952–1959. [Google Scholar]
- Frank, H.G.; Gunawan, B.; Ebeling-Stark, I.; Schulten, H.J.; Funayama, H.; Cremer, U.; Huppertz, B.; Gaus, G.; Kaufmann, P.; Füzesi, L. Cytogenetic and DNA-fingerprint characterization of choriocarcinoma cell lines and a trophoblast/choriocarcinoma cell hybrid. Cancer Genet. Cytogenet. 2000, 116, 16–22. [Google Scholar] [CrossRef]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Alvero, A.B.; Aldo, P.B.; Ma, Y.; Guller, S.; Romero, R.; Mor, G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 2009, 30, 939–948. [Google Scholar] [CrossRef]
- Hiden, U.; Wadsack, C.; Prutsch, N.; Gauster, M.; Weiss, U.; Frank, H.-G.; Schmitz, U.; Fast-Hirsch, C.; Hengstschläger, M.; Pötgens, A.; et al. The first trimester human trophoblast cell line ACH-3P: A novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations—TNF-α stimulates MMP15 expression. BMC Dev. Biol. 2007, 7, 137. [Google Scholar] [CrossRef]
- Yagel, S.; Casper, R.F.; Powell, W.; Parhar, R.S.; Lala, P.K. Characterization of pure human first-trimester cytotrophoblast cells in long-term culture: Growth pattern, markers, and hormone production. Am. J. Obstet. Gynecol. 1989, 160, 938–945. [Google Scholar] [CrossRef]
- Chou, J.Y. Human placental cells transformed by tsA mutants of simian virus 40: A model system for the study of placental functions. Proc. Natl. Acad. Sci. USA 1978, 75, 1409–1413. [Google Scholar] [CrossRef]
- Brown, K.; Heller, D.S.; Zamudio, S.; Illsley, N.P. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta 2011, 32, 1041–1049. [Google Scholar] [CrossRef]
- Rothbauer, M.; Patel, N.; Gondola, H.; Siwetz, M.; Huppertz, B.; Ertl, P. A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci. Rep. 2017, 7, 5892. [Google Scholar] [CrossRef]
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 2020, 26, 501–513. [Google Scholar] [CrossRef]
- Muthuraj, P.G.; Pattnaik, A.; Sahoo, P.K.; Islam, M.T.; Pattnaik, A.K.; Byrareddy, S.N.; Hanson, C.; Anderson Berry, A.; Kachman, S.D.; Natarajan, S.K. Palmitoleate Protects against Zika Virus-Induced Placental Trophoblast Apoptosis. Biomedicines 2021, 9, 643. [Google Scholar] [CrossRef]
- Chiu, C.F.; Chu, L.W.; Liao, I.C.; Simanjuntak, Y.; Lin, Y.L.; Juan, C.C.; Ping, Y.H. The Mechanism of the Zika Virus Crossing the Placental Barrier and the Blood-Brain Barrier. Front. Microbiol. 2020, 11, 214. [Google Scholar] [CrossRef]
- Pattnaik, A.; Palermo, N.; Sahoo, B.R.; Yuan, Z.; Hu, D.; Annamalai, A.S.; Vu, H.L.X.; Correas, I.; Prathipati, P.K.; Destache, C.J.; et al. Discovery of a non-nucleoside RNA polymerase inhibitor for blocking Zika virus replication through in silico screening. Antivir. Res. 2018, 151, 78–86. [Google Scholar] [CrossRef]
- Shah, M.; Bourner, L.; Ali, S.; Al-Enazy, S.; Rytting, E. Cytotoxicity of Endocytosis and Efflux Inhibitors in the BeWo Cell Line. J. Pharm. Res. Int. 2017, 17, JPRI.34606. [Google Scholar] [CrossRef]
- Olivier, E.; Wakx, A.; Fouyet, S.; Dutot, M.; Rat, P. JEG-3 placental cells in toxicology studies: A promising tool to reveal pregnancy disorders. Anat. Cell Biol. 2021, 54, 83–92. [Google Scholar] [CrossRef]
- Nabekura, T.; Ishikawa, S.; Tanase, M.; Okumura, T.; Kawasaki, T. Antidepressants induce toxicity in human placental BeWo cells. Curr. Res. Toxicol. 2022, 3, 100073. [Google Scholar] [CrossRef]
- Zuo, Y.; Lu, Y.; Xu, Q.; Sun, D.; Liang, X.; Li, X.; Li, Y. Inhibitory effect of dihydromyricetin on the proliferation of JAR cells and its mechanism of action. Oncol. Lett. 2020, 20, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Poaty, H.; Coullin, P.; Peko, J.F.; Dessen, P.; Diatta, A.L.; Valent, A.; Leguern, E.; Prévot, S.; Gombé-Mbalawa, C.; Candelier, J.-J.; et al. Genome-Wide High-Resolution aCGH Analysis of Gestational Choriocarcinomas. PLoS ONE 2012, 7, e29426. [Google Scholar] [CrossRef] [PubMed]
- Serjilus, A.; Alcendor, D.J. Unique method for human villous trophoblasts isolation from placental tissue explants. Clin. Obstet. Gynecol. Reprod. Med. 2020, 6, 319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Schust, D.J. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod. Biol. Endocrinol. 2015, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Borbely, A.U.; Sandri, S.; Fernandes, I.R.; Prado, K.M.; Cardoso, E.C.; Correa-Silva, S.; Albuquerque, R.; Knöfler, M.; Beltrão-Braga, P.; Campa, A.; et al. The term basal plate of the human placenta as a source of functional extravillous trophoblast cells. Reprod. Biol. Endocrinol. 2014, 12, 7. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef]
- Aagaard, K.M.; Lahon, A.; Suter, M.A.; Arya, R.P.; Seferovic, M.D.; Vogt, M.B.; Hu, M.; Stossi, F.; Mancini, M.A.; Harris, R.A.; et al. Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci. Rep. 2017, 7, 41389. [Google Scholar] [CrossRef]
- Tan, L.; Lacko, L.A.; Zhou, T.; Tomoiaga, D.; Hurtado, R.; Zhang, T.; Sevilla, A.; Zhong, A.; Mason, C.E.; Noggle, S.; et al. Pre- and peri-implantation Zika virus infection impairs fetal development by targeting trophectoderm cells. Nat. Commun. 2019, 10, 4155. [Google Scholar] [CrossRef]
- Xu, R.H.; Chen, X.; Li, D.S.; Li, R.; Addicks, G.C.; Glennon, C.; Zwaka, T.P.; Thomson, J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002, 20, 1261–1264. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1587–E1596. [Google Scholar] [CrossRef]
- Zhou, J.; Choi, S.; Liu, H.; Zhang, J.; Tian, Y.; Edlow, A.G.; Ezashi, T.; Roberts, R.M.; Ma, W.; Schust, D.J. Is SARS-CoV-2 Infection a Risk Factor for Early Pregnancy Loss? ACE2 and TMPRSS2 Coexpression and Persistent Replicative Infection in Primitive Trophoblast. J. Infect. Dis. 2021, 224, S660–S669. [Google Scholar] [CrossRef]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 2018, 22, 50–63.e6. [Google Scholar] [CrossRef]
- Bai, T.; Peng, C.Y.; Aneas, I.; Sakabe, N.; Requena, D.F.; Billstrand, C.; Nobrega, M.; Ober, C.; Parast, M.; Kessler, J.A. Establishment of human induced trophoblast stem-like cells from term villous cytotrophoblasts. Stem Cell Res. 2021, 56, 102507. [Google Scholar] [CrossRef]
- Aghajanova, L.; Shen, S.; Rojas, A.M.; Fisher, S.J.; Irwin, J.C.; Giudice, L.C. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol. Reprod. 2012, 86, 1–21. [Google Scholar] [CrossRef]
- Li, Y.; Moretto-Zita, M.; Soncin, F.; Wakeland, A.; Wolfe, L.; Leon-Garcia, S.; Pandian, R.; Pizzo, D.; Cui, L.; Nazor, K.; et al. BMP4-directed trophoblast differentiation of human embryonic stem cells is mediated through a ΔNp63+ cytotrophoblast stem cell state. Development 2013, 140, 3965–3976. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef]
- Karvas, R.M.; Khan, S.A.; Verma, S.; Yin, Y.; Kulkarni, D.; Dong, C.; Park, K.M.; Chew, B.; Sane, E.; Fischer, L.A.; et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell 2022, 29, 810–825.e8. [Google Scholar] [CrossRef]
- Cui, K.; Chen, T.; Zhu, Y.; Shi, Y.; Guo, Y.; Qin, J. Engineering placenta-like organoids containing endogenous vascular cells from human-induced pluripotent stem cells. Bioeng. Transl. Med. 2022, e10390. [Google Scholar] [CrossRef]
- LaMarca, H.L.; Ott, C.M.; Höner Zu Bentrup, K.; Leblanc, C.L.; Pierson, D.L.; Nelson, A.B.; Scandurro, A.B.; Whitley, G.S.; Nickerson, C.A.; Morris, C.A. Three-dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta 2005, 26, 709–720. [Google Scholar] [CrossRef]
- Ma, T.; Yang, S.-T.; Kniss, D.A. Development of an in Vitro Human Placenta Model by the Cultivation of Human Trophoblasts in a Fiber-Based Bioreactor System. Tissue Eng. 1999, 5, 91–102. [Google Scholar] [CrossRef]
- Shojaei, S.; Ali, M.S.; Suresh, M.; Upreti, T.; Mogourian, V.; Helewa, M.; Labouta, H.I. Dynamic placenta-on-a-chip model for fetal risk assessment of nanoparticles intended to treat pregnancy-associated diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166131. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, C.L.; Fourcaudot, A.B.; Hong, S.J.; Mustoe, T.A.; Hale, R.G.; Leung, K.P. In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell Tissue Res. 2014, 358, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Inohaya, A.; Yasuda, E.; Mogami, H.; Chigusa, Y.; Kawasaki, K.; Kawamura, Y.; Ueda, Y.; Takai, H.; Mandai, M.; et al. Three-dimensional human placenta-like bud synthesized from induced pluripotent stem cells. Sci. Rep. 2021, 11, 14167. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J.; et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Semmes, E.C.; Ovies, C.; Megli, C.; Permar, S.; Gilner, J.B.; Coyne, C.B. Innate immune signaling in trophoblast and decidua organoids defines differential antiviral defenses at the maternal-fetal interface. Elife 2022, 11, e79794. [Google Scholar] [CrossRef]
- Gabrielli, L.; Losi, L.; Varani, S.; Lazzarotto, T.; Eusebi, V.; Landini, M.P. Complete replication of human cytomegalovirus in explants of first trimester human placenta. J. Med. Virol. 2001, 64, 499–504. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Harris, E.; Pereira, L. Zika Virus Replicates in Proliferating Cells in Explants From First-Trimester Human Placentas, Potential Sites for Dissemination of Infection. J. Infect. Dis. 2018, 217, 1202–1213. [Google Scholar] [CrossRef]
- Argueta, L.B.; Lacko, L.A.; Bram, Y.; Tada, T.; Carrau, L.; Rendeiro, A.F.; Zhang, T.; Uhl, S.; Lubor, B.C.; Chandar, V.; et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience 2022, 25, 104223. [Google Scholar] [CrossRef]
- Eliesen, G.A.M.; van Hove, H.; Meijer, M.H.; van den Broek, P.H.H.; Pertijs, J.; Roeleveld, N.; van Drongelen, J.; Russel, F.G.M.; Greupink, R. Toxicity of anticancer drugs in human placental tissue explants and trophoblast cell lines. Arch. Toxicol. 2021, 95, 557–571. [Google Scholar] [CrossRef]
- Kenis, I.; Tartakover-Matalon, S.; Cherepnin, N.; Drucker, L.; Fishman, A.; Pomeranz, M.; Lishner, M. Simvastatin has deleterious effects on human first trimester placental explants. Hum. Reprod. 2005, 20, 2866–2872. [Google Scholar] [CrossRef]
- Quenby, S.; Mountfield, S.; Cartwright, J.E.; Whitley, G.S.; Vince, G. Effects of low-molecular-weight and unfractionated heparin on trophoblast function. Obstet. Gynecol. 2004, 104, 354–361. [Google Scholar] [CrossRef]
- Schneider, H.; Panigel, M.; Dancis, J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am. J. Obstet. Gynecol. 1972, 114, 822–828. [Google Scholar] [CrossRef]
- Gavard, L.; Beghin, D.; Forestier, F.; Cayre, Y.; Peytavin, G.; Mandelbrot, L.; Farinotti, R.; Gil, S. Contribution and limit of the model of perfused cotyledon to the study of placental transfer of drugs. Example of a protease inhibitor of HIV: Nelfinavir. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 157–160. [Google Scholar] [CrossRef]
- Berveiller, P.; Mir, O.; Vinot, C.; Bonati, C.; Duchene, P.; Giraud, C.; Gil, S.; Treluyer, J.M. Transplacental transfer of oseltamivir and its metabolite using the human perfused placental cotyledon model. Am. J. Obstet. Gynecol. 2012, 206, e91–e96. [Google Scholar] [CrossRef]
- Nanovskaya, T.N.; Patrikeeva, S.; Zhan, Y.; Hankins, G.D.; Ahmed, M.S. Transplacental transfer of oseltamivir carboxylate. J. Matern. Fetal Neonatal Med. 2012, 25, 2312–2315. [Google Scholar] [CrossRef][Green Version]
- Orendi, K.; Kivity, V.; Sammar, M.; Grimpel, Y.; Gonen, R.; Meiri, H.; Lubzens, E.; Huppertz, B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 2011, 32 (Suppl. 1), S49–S54. [Google Scholar] [CrossRef]
- Huppertz, B.; Kivity, V.; Sammar, M.; Grimpel, Y.; Leepaz, N.; Orendi, K.; Pekarski, I.; Meiri, H.; Gonen, R.; Lubzens, E. Cryogenic and low temperature preservation of human placental villous explants—A new way to explore drugs in pregnancy disorders. Placenta 2011, 32 (Suppl. 1), S65–S76. [Google Scholar] [CrossRef]
- Brandy, R.C.; Schleiss, M.R.; Witte, D.P.; Siddiqi, T.A.; Fame, P.T. Placental transfer of ganciclovir in a woman with acquired immunodeficiency syndrome and cytomegalovirus disease. Pediatr. Infect. Dis. J. 2002, 21, 796–797. [Google Scholar] [CrossRef]
- van den Brink, S.C.; van Oudenaarden, A. 3D gastruloids: A novel frontier in stem cell-based in vitro modeling of mammalian gastrulation. Trends Cell Biol. 2021, 31, 747–759. [Google Scholar] [CrossRef]
- el Azhar, Y.; Sonnen, K.F. Development in a Dish—In Vitro Models of Mammalian Embryonic Development. Front. Cell Dev. Biol. 2021, 9, 655993. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Babiarz, J.; Kolaja, K.; Chiao, E. A High-Throughput Screen for Teratogens Using Human Pluripotent Stem Cells. Toxicol. Sci. 2013, 137, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.T.; Hopperstad, K.; Deisenroth, C. The DevTox Germ Layer Reporter Platform: An Assay Adaptation of the Human Pluripotent Stem Cell Test. Toxics 2022, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Brickman, J.M.; Serup, P. Properties of embryoid bodies. WIREs Dev. Biol. 2017, 6, e259. [Google Scholar] [CrossRef] [PubMed]
- Jaklin, M.; Zhang, J.D.; Schäfer, N.; Clemann, N.; Barrow, P.; Küng, E.; Sach-Peltason, L.; McGinnis, C.; Leist, M.; Kustermann, S. Optimization of the TeraTox Assay for Preclinical Teratogenicity Assessment. Toxicol. Sci. 2022, 188, 17–33. [Google Scholar] [CrossRef]
- Marikawa, Y.; Chen, H.-R.; Menor, M.; Deng, Y.; Alarcon, V.B. Exposure-based assessment of chemical teratogenicity using morphogenetic aggregates of human embryonic stem cells. Reprod. Toxicol. 2020, 91, 74–91. [Google Scholar] [CrossRef]
- Kagawa, H.; Javali, A.; Khoei, H.H.; Sommer, T.M.; Sestini, G.; Novatchkova, M.; Scholte op Reimer, Y.; Castel, G.; Bruneau, A.; Maenhoudt, N.; et al. Human blastoids model blastocyst development and implantation. Nature 2022, 601, 600–605. [Google Scholar] [CrossRef]
- Yanagida, A.; Spindlow, D.; Nichols, J.; Dattani, A.; Smith, A.; Guo, G. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 2021, 28, 1016–1022.e4. [Google Scholar] [CrossRef]
- Yu, L.; Wei, Y.; Duan, J.; Schmitz, D.A.; Sakurai, M.; Wang, L.; Wang, K.; Zhao, S.; Hon, G.C.; Wu, J. Blastocyst-like structures generated from human pluripotent stem cells. Nature 2021, 591, 620–626. [Google Scholar] [CrossRef]
- Fan, Y.; Min, Z.; Alsolami, S.; Ma, Z.; Zhang, E.; Chen, W.; Zhong, K.; Pei, W.; Kang, X.; Zhang, P.; et al. Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discov. 2021, 7, 81. [Google Scholar] [CrossRef]
- Sozen, B.; Jorgensen, V.; Weatherbee, B.A.T.; Chen, S.; Zhu, M.; Zernicka-Goetz, M. Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat. Commun. 2021, 12, 5550. [Google Scholar] [CrossRef]
- Liu, X.; Tan, J.P.; Schröder, J.; Aberkane, A.; Ouyang, J.F.; Mohenska, M.; Lim, S.M.; Sun, Y.B.Y.; Chen, J.; Sun, G.; et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 2021, 591, 627–632. [Google Scholar] [CrossRef]
- Zhao, C.; Reyes, A.P.; Schell, J.P.; Weltner, J.; Ortega, N.M.; Zheng, Y.; Björklund, Å.K.; Rossant, J.; Fu, J.; Petropoulos, S.; et al. Reprogrammed blastoids contain amnion-like cells but not trophectoderm. bioRxiv 2021. [Google Scholar] [CrossRef]
- Marikawa, Y.; Alarcon, V.B. Remdesivir impairs mouse preimplantation embryo development at therapeutic concentrations. Reprod. Toxicol. 2022, 111, 135–147. [Google Scholar] [CrossRef]
- Xing, J.; Toh, Y.-C.; Xu, S.; Yu, H. A method for human teratogen detection by geometrically confined cell differentiation and migration. Sci. Rep. 2015, 5, 10038. [Google Scholar] [CrossRef]
- Xing, J.; Cao, Y.; Yu, Y.; Li, H.; Song, Z.; Yu, H. In Vitro Micropatterned Human Pluripotent Stem Cell Test (µP-hPST) for Morphometric-Based Teratogen Screening. Sci. Rep. 2017, 7, 8491. [Google Scholar] [CrossRef]
- Fu, J.; Warmflash, A.; Lutolf, M.P. Stem-cell-based embryo models for fundamental research and translation. Nat. Mater. 2021, 20, 132–144. [Google Scholar] [CrossRef]
- Veenvliet, J.V.; Herrmann, B.G. Modeling mammalian trunk development in a dish. Dev. Biol. 2021, 474, 5–15. [Google Scholar] [CrossRef]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schröder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Martinez Arias, A. An in vitro model of early anteroposterior organization during human development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef]
- Mantziou, V.; Baillie-Benson, P.; Jaklin, M.; Kustermann, S.; Arias, A.M.; Moris, N. In vitro teratogenicity testing using a 3D, embryo-like gastruloid system. Reprod. Toxicol. 2021, 105, 72–90. [Google Scholar] [CrossRef]
- Moris, N.; Alev, C.; Pera, M.; Martinez Arias, A. Biomedical and societal impacts of in vitro embryo models of mammalian development. Stem Cell Rep. 2021, 16, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Yao, R.; Zusinaite, E.; Lello, L.S.; Wang, S.; Jo, E.; Yang, J.; Ravlo, E.; Wang, W.; Lysvand, H.; et al. Synergistic Interferon-Alpha-Based Combinations for Treatment of SARS-CoV-2 and Other Viral Infections. Viruses 2021, 13, 2489. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, C.Q.; de Melo, G.R.; de Freitas, C.S.; Rocha, N.; Hoelz, L.V.B.; Miranda, M.; Fintelman-Rodrigues, N.; Marttorelli, A.; Ferreira, A.C.; Barbosa-Lima, G.; et al. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci. Rep. 2017, 7, 40920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Tan, L.; Cederquist, G.Y.; Fan, Y.; Hartley, B.J.; Mukherjee, S.; Tomishima, M.; Brennand, K.J.; Zhang, Q.; Schwartz, R.E.; et al. High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain. Cell Stem Cell 2017, 21, 274–283.e5. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.-D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.-P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-Organization of Polarized Cerebellar Tissue in 3D Culture of Human Pluripotent Stem Cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 2015, 6, 8896. [Google Scholar] [CrossRef]
- Xie, T.; Brown, L.E.; Pak, C.; Sun, Y. Self-organized anteroposterior regionalization of early midbrain and hindbrain using micropatterned human embryonic stem cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.-K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 2020, 27, 937–950.e9. [Google Scholar] [CrossRef]
- Ramani, A.; Müller, L.; Ostermann, P.N.; Gabriel, E.; Abida-Islam, P.; Müller-Schiffmann, A.; Mariappan, A.; Goureau, O.; Gruell, H.; Walker, A.; et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020, 39, e106230. [Google Scholar] [CrossRef]
- Brown, R.M.; Rana, P.S.J.B.; Jaeger, H.K.; O’Dowd, J.M.; Balemba, O.B.; Fortunato, E.A. Human Cytomegalovirus Compromises Development of Cerebral Organoids. J. Virol. 2019, 93, e00957-19. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Y.; Zhu, Y.; Tao, T.; Yin, F.; Guo, Y.; Liu, H.; Li, F.; Wang, P.; Chen, Y.; et al. Neurodevelopmental impairment induced by prenatal valproic acid exposure shown with the human cortical organoid-on-a-chip model. Microsyst. Nanoeng. 2020, 6, 49. [Google Scholar] [CrossRef]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.-K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef]
- Gabriel, E.; Albanna, W.; Pasquini, G.; Ramani, A.; Josipovic, N.; Mariappan, A.; Schinzel, F.; Karch, C.M.; Bao, G.; Gottardo, M.; et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell 2021, 28, 1740–1757.e8. [Google Scholar] [CrossRef]
- Schmidt, C.; Deyett, A.; Ilmer, T.; Caballero, A.T.; Haendeler, S.; Pimpale, L.; Netzer, M.A.; Ginistrelli, L.C.; Cirigliano, M.; Mancheno, E.J.; et al. Multi-chamber cardioids unravel human heart development and cardiac defects. bioRxiv 2022. [Google Scholar] [CrossRef]
- Skardal, A.; Aleman, J.; Forsythe, S.; Rajan, S.; Murphy, S.; Devarasetty, M.; Pourhabibi Zarandi, N.; Nzou, G.; Wicks, R.; Sadri-Ardekani, H.; et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication 2020, 12, 025017. [Google Scholar] [CrossRef]
- Han, Y.; Duan, X.; Yang, L.; Nilsson-Payant, B.E.; Wang, P.; Duan, F.; Tang, X.; Yaron, T.M.; Zhang, T.; Uhl, S.; et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 2021, 589, 270–275. [Google Scholar] [CrossRef]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell 2020, 27, 890–904.e8. [Google Scholar] [CrossRef]
- Lamers, M.M.; van der Vaart, J.; Knoops, K.; Riesebosch, S.; Breugem, T.I.; Mykytyn, A.Z.; Beumer, J.; Schipper, D.; Bezstarosti, K.; Koopman, C.D.; et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021, 40, e105912. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Feng, J.; Zhang, Y.; Sun, H.; Li, L.; Yang, X.; He, J.; Xiao, S.; Xiong, J.; Lin, Y.; et al. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection. Protein Cell 2021, 12, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.A.; Choi, S.S.; Rustagi, A.; Zhu, J.; van Unen, V.; de la O, S.M.; Flynn, R.A.; Margalef-Català, M.; Santos, A.J.M.; Ju, J.; et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 2020, 588, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.M.; Majd, H.; Richter, M.N.; Ghazizadeh, Z.; Zekavat, S.M.; Navickas, A.; Ramirez, J.T.; Asgharian, H.; Simoneau, C.R.; Bonser, L.R.; et al. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men. Cell Stem Cell 2020, 27, 876–889.e12. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Wang, S.; Smith, D.; Carlin, A.F.; Rana, T.M. Revealing Tissue-Specific SARS-CoV-2 Infection and Host Responses using Human Stem Cell-Derived Lung and Cerebral Organoids. Stem Cell Rep. 2021, 16, 437–445. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020, 27, 125–136.e7. [Google Scholar] [CrossRef]
- Balak, J.R.A.; Juksar, J.; Carlotti, F.; Lo Nigro, A.; de Koning, E.J.P. Organoids from the Human Fetal and Adult Pancreas. Curr. Diabetes Rep. 2019, 19, 160. [Google Scholar] [CrossRef]
- Andersen, P.I.; Ianevski, A.; Lysvand, H.; Vitkauskiene, A.; Oksenych, V.; Bjørås, M.; Telling, K.; Lutsar, I.; Dumpis, U.; Irie, Y.; et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020, 93, 268–276. [Google Scholar] [CrossRef]
- Lawrence, M.L.; Elhendawi, M.; Morlock, M.; Liu, W.; Liu, S.; Palakkan, A.; Seidl, L.F.; Hohenstein, P.; Sjögren, A.K.; Davies, J.A. Human iPSC-derived renal organoids engineered to report oxidative stress can predict drug-induced toxicity. iScience 2022, 25, 103884. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Benincasa, J.C.; Cruz, E.M.; Maricato, J.T.; Porcionatto, M.A. 3D culture models to study SARS-CoV-2 infectivity and antiviral candidates: From spheroids to bioprinting. Biomed. J. 2021, 44, 31–42. [Google Scholar] [CrossRef]

| Cell Model | Advantages | Disadvantages |
|---|---|---|
Placental cell lines  |
|
|
Isolated primary trophoblasts  |
|
|
Trophoblastic stem cells (TSCs)  |
|
|
3D placental organoids  |
|
|
Placental explants 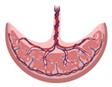 |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbek, S.L.; Smithgall, M.C.; Murphy, E.A.; Schwartz, R.E.; Chen, S.; Riley, L.E.; Stuhlmann, H.; Yang, Y.J.; Goswami, R. Human Maternal-Fetal Interface Cellular Models to Assess Antiviral Drug Toxicity during Pregnancy. Reprod. Med. 2022, 3, 303-319. https://doi.org/10.3390/reprodmed3040024
Herbek SL, Smithgall MC, Murphy EA, Schwartz RE, Chen S, Riley LE, Stuhlmann H, Yang YJ, Goswami R. Human Maternal-Fetal Interface Cellular Models to Assess Antiviral Drug Toxicity during Pregnancy. Reproductive Medicine. 2022; 3(4):303-319. https://doi.org/10.3390/reprodmed3040024
Chicago/Turabian StyleHerbek, Savannah L., Marie C. Smithgall, Elisabeth A. Murphy, Robert E. Schwartz, Shuibing Chen, Laura E. Riley, Heidi Stuhlmann, Yawei J. Yang, and Ria Goswami. 2022. "Human Maternal-Fetal Interface Cellular Models to Assess Antiviral Drug Toxicity during Pregnancy" Reproductive Medicine 3, no. 4: 303-319. https://doi.org/10.3390/reprodmed3040024
APA StyleHerbek, S. L., Smithgall, M. C., Murphy, E. A., Schwartz, R. E., Chen, S., Riley, L. E., Stuhlmann, H., Yang, Y. J., & Goswami, R. (2022). Human Maternal-Fetal Interface Cellular Models to Assess Antiviral Drug Toxicity during Pregnancy. Reproductive Medicine, 3(4), 303-319. https://doi.org/10.3390/reprodmed3040024







