Prolonged Disease-Free Survival in a Relapsed Adult Granulosa Cell Tumor of the Ovary Treated by Combined Leuprolide and Letrozole: Case Report
Abstract
1. Introduction
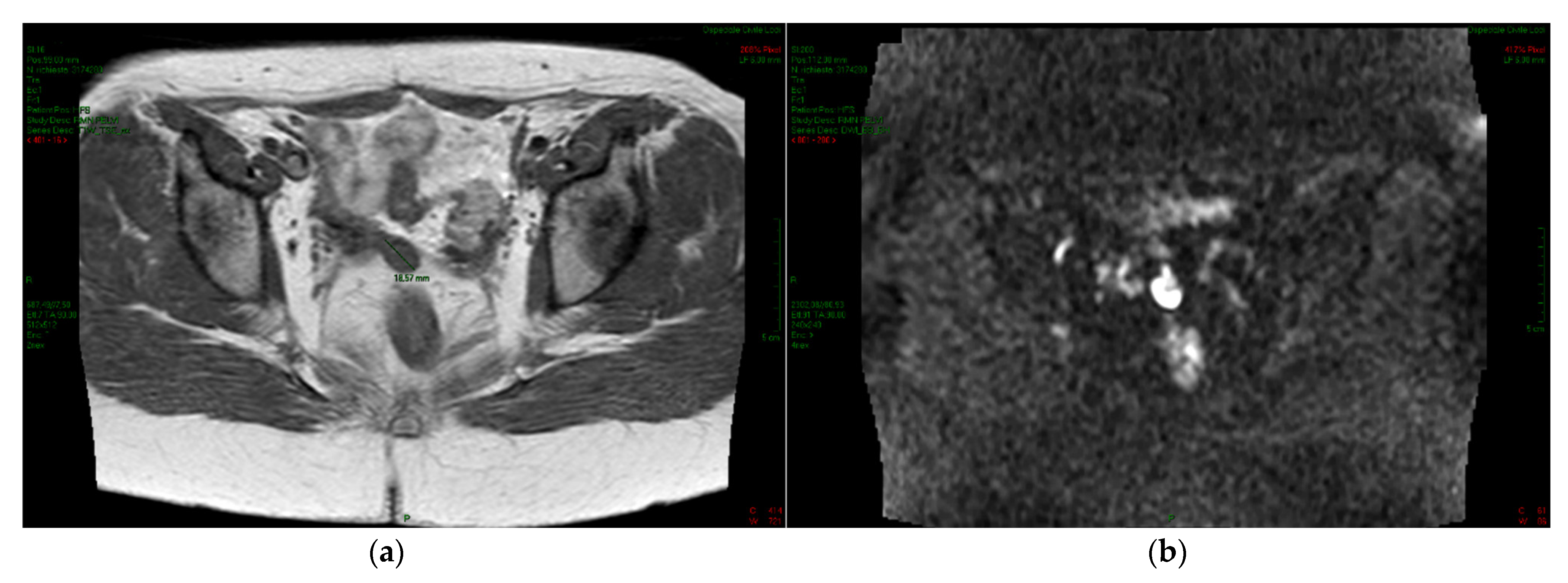
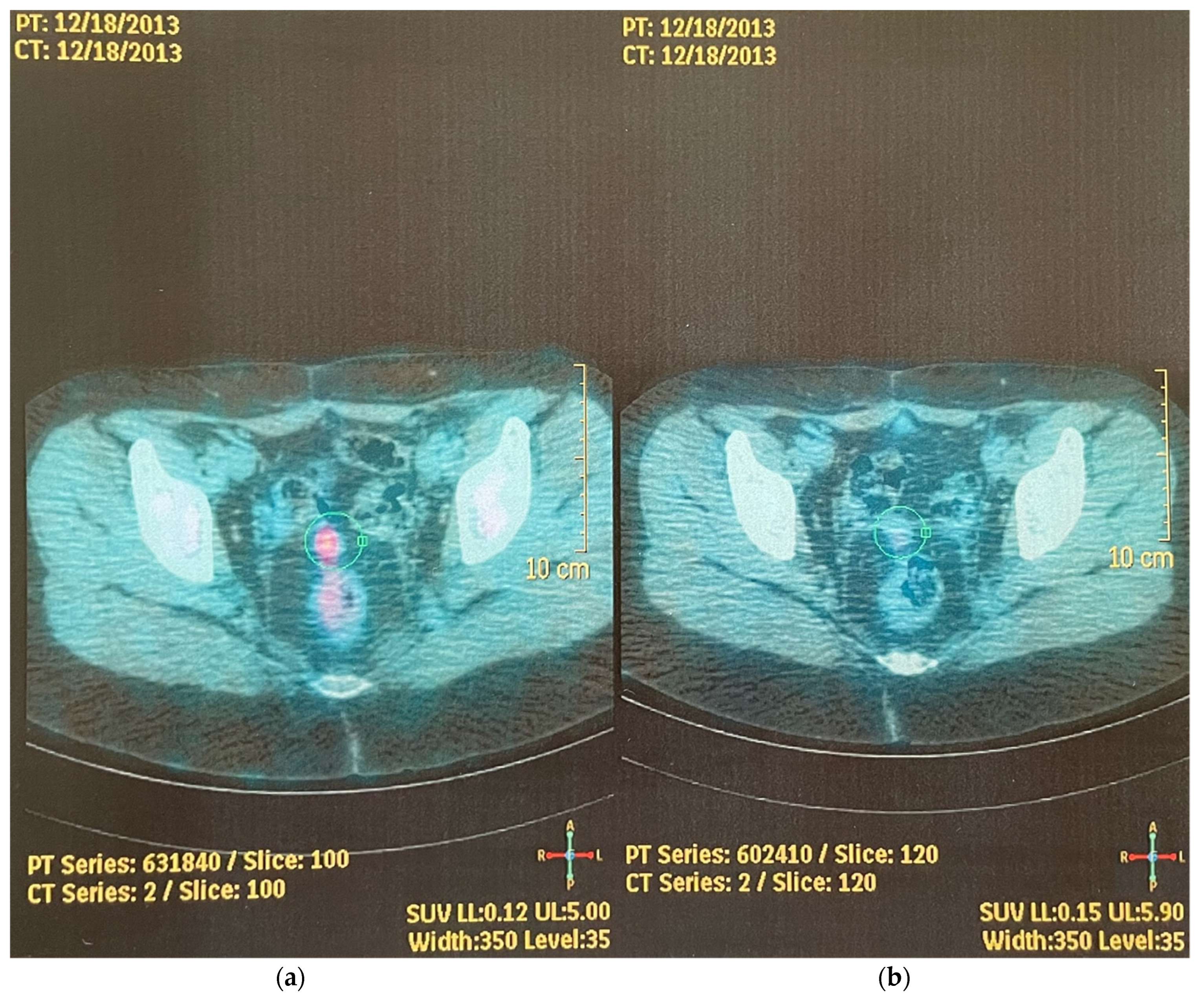
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shumer, S.T.; Cannistra, S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003, 21, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.; Fuller, P.J. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr. Rev. 2012, 33, 109–144. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Parma, G.; Zanagnolo, V.; Insinga, A. Management of stromal cell tumors. J. Clin. Oncol. 2007, 25, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Koike, E.; Murakami, K.; Takaya, H.; Kotani, Y.; Nakai, R.; Suzuki, A.; Aoki, M.; Matsumura, N.; Mandai, M. Clinical determinants affecting indications for surgery and chemotherapy in recurrent ovarian granulosa cell tumor. Healthcare 2019, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, H.S.; Van Lonkhuijzen, R.C.W.; Limpes, J.; Van der Valden, J.; Buist, M.R. Hormone therapy in ovarian granulosa cell tumors: A systematic review. Gynecol. Oncol. 2014, 134, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Benerjee, S.N.; Tang, M.; O’Connell, R.L.; Sjoquist, K.; Clamp, A.R.; Millan, D.; Nottley, S.; Lord, R. A phase 2 study of Anastrozole in patients with estrogen receptor and progesterone receptor positive recurrent/metastatic granulosa cell tumors/sex cord stromal tumors of the ovary: The PARAGON/ANZ GOG 0903 trial. Gynecol. Oncol. 2021, 163, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Portuesi, R.; Loppini, A.; Mancari, R.; Filippi, S.; Colombo, N. Role of inhibin B in detecting recurrence of granulosa cell tumors of the ovary in postmenopausal patients. Int. J. Gynecol. Cancer 2021, 31, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, S.; Liu, Y.; Yang, H. GnRH as a treatment for letrozole-resistant recurrent adult granulosa cell tumors: A case report. Medicine 2021, 100, e28343. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.T.; Willson, M.L.; Goel, S.; Beith, J.; Goodwin, A. Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst. Rev. 2020, 3, CDO013538. [Google Scholar] [CrossRef] [PubMed]
- Haltia, U.M.; Pihlajoki, M.; Andersson, N.; Makinen, L.; Tapper, J.; Cervera, A.; Horlings, H.M.; Turpeinen, V.; Anttonen, M.; Unkila-Kallio, L.; et al. Functional profiling of FSH and estradiol in ovarian granulosa cell tumors. J. Endocr. 2020, 41, bvaa034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Klausen, C.; Leung, P.L.K. Overexpression of wild-type but not C134W mutant FOXL2 enhances GnRH induced cell apoptosis by increasing GnRH receptor expression in human granulosa cell tumors. PLoS ONE 2013, 8, e55099. [Google Scholar] [CrossRef] [PubMed]
- Fleming, N.I.; Knower, K.C.; Lazarus, K.A.; Fuller, P.J.; Simpson, E.R.; Clyne, C.D. Aromatase is a direct target of FOXL2: C134W in granulosa cell tumors via a single highly conserved binding site in the ovarian specific promoter. PLoS ONE 2010, 5, e14389. [Google Scholar] [CrossRef] [PubMed]
- Brink, G.J.; Groeneweg, J.W.; Hooft, L.; Zweemer, R.P.; Witteeven, P.O. Response to systematic therapies in ovarian adult granulosa cell tumors: A literature review. Cancers 2022, 14, 2998. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, S.; Liu, Y.; Yang, H. Can adjuvant chemotherapy improve the prognosis of adult ovarian granulosa cell tumors? A narrative review. Medicine 2022, 101, e29062. [Google Scholar] [CrossRef]
- Van Meurs, H.S.; Van Der Velden, J.; Buist, M.R.; Van Driel, W.J.; Kenter, G.G.; Van Lonkhuijzen, L. Evaluation of response to hormone therapy in patients with measurable adult granulosa cell tumors of the ovary. Acta Obstet. Gynecol. Scand. 2015, 94, 1269–1275. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garuti, G.; Sagrada, P.F.; Delfrati, S.; Sogaro, L.; Soligo, M. Prolonged Disease-Free Survival in a Relapsed Adult Granulosa Cell Tumor of the Ovary Treated by Combined Leuprolide and Letrozole: Case Report. Reprod. Med. 2022, 3, 297-302. https://doi.org/10.3390/reprodmed3040023
Garuti G, Sagrada PF, Delfrati S, Sogaro L, Soligo M. Prolonged Disease-Free Survival in a Relapsed Adult Granulosa Cell Tumor of the Ovary Treated by Combined Leuprolide and Letrozole: Case Report. Reproductive Medicine. 2022; 3(4):297-302. https://doi.org/10.3390/reprodmed3040023
Chicago/Turabian StyleGaruti, Giancarlo, Paola Francesca Sagrada, Susanna Delfrati, Lorenzo Sogaro, and Marco Soligo. 2022. "Prolonged Disease-Free Survival in a Relapsed Adult Granulosa Cell Tumor of the Ovary Treated by Combined Leuprolide and Letrozole: Case Report" Reproductive Medicine 3, no. 4: 297-302. https://doi.org/10.3390/reprodmed3040023
APA StyleGaruti, G., Sagrada, P. F., Delfrati, S., Sogaro, L., & Soligo, M. (2022). Prolonged Disease-Free Survival in a Relapsed Adult Granulosa Cell Tumor of the Ovary Treated by Combined Leuprolide and Letrozole: Case Report. Reproductive Medicine, 3(4), 297-302. https://doi.org/10.3390/reprodmed3040023




