Monitoring, Delivery and Outcome in Early Onset Fetal Growth Restriction
Abstract
1. Introduction
2. Definition and Diagnosis
3. Monitoring Tools in Early-Onset Fetal Growth Restriction
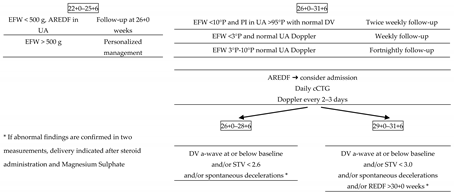
4. Management and Delivery in Early-Onset Fetal Growth Restriction: What We Have Learned from the TRUFFLE Study
5. Early-Onset Fetal Growth Restriction in the TRUFFLE Era: Delivery and Fetal Outcomes
6. Periviable Fetal Growth Restriction
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alberry, M.; Soothill, P. Management of Fetal Growth Restriction. Arch Dis Child Fetal Neonatal Ed 2007, 92, F62–F67. [Google Scholar] [CrossRef]
- Damodaram, M.; Story, L.; Kulinskaya, E.; Rutherford, M.; Kumar, S. Early Adverse Perinatal Complications in Preterm Growth-Restricted Fetuses. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzi, E.; Bozzo, M.; Rigano, S.; Bellotti, M.; Morabito, A.; Pardi, G.; Battaglia, F.C.; Galan, H.L. Temporal Sequence of Abnormal Doppler Changes in the Peripheral and Central Circulatory Systems of the Severely Growth-Restricted Fetus. Ultrasound Obstet. Gynecol. 2002, 19, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal Morbidity and Mortality in Early-Onset Fetal Growth Restriction: Cohort Outcomes of the Trial of Randomized Umbilical and Fetal Flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef]
- Gardosi, J.; Giddings, S.; Buller, S.; Southam, M.; Williams, M. Preventing Stillbirths through Improved Antenatal Recognition of Pregnancies at Risk Due to Fetal Growth Restriction. Public Health 2014, 128, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Gardosi, J.; Chang, A.; Kalyan, B.; Sahota, D.; Symonds, E.M. Customised Antenatal Growth Charts. Lancet 1992, 339, 283–287. [Google Scholar] [CrossRef]
- Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Optimizing the Definition of Intrauterine Growth Restriction: The Multicenter Prospective PORTO Study. Am. J. Obstet. Gynecol. 2013, 208, 290.e1–290.e6. [Google Scholar] [CrossRef] [PubMed]
- Lawin-O’Brien, A.R.; Dall’Asta, A.; Knight, C.; Sankaran, S.; Scala, C.; Khalil, A.; Bhide, A.; Heggarty, S.; Rakow, A.; Pasupathy, D.; et al. Short-Term Outcome of Periviable Small-for-Gestational-Age Babies: Is Our Counseling up to Date? Ultrasound Obstet. Gynecol. 2016, 48, 636–641. [Google Scholar] [CrossRef]
- Sibai, B.M.; Abdella, T.N.; Anderson, G.D. Pregnancy Outcome in 211 Patients with Mild Chronic Hypertension. Obs. Gynecol 1983, 61, 571–576. [Google Scholar]
- Villar, J.; Altman, D.G.; Purwar, M.; Noble, J.A.; Knight, H.E.; Ruyan, P.; Cheikh Ismail, L.; Barros, F.C.; Lambert, A.; Papageorghiou, A.T.; et al. The Objectives, Design and Implementation of the INTERGROWTH-21 St Project. BJOG Int. J. Obstet. Gynaecol. 2013, 120 (Suppl. S2), 9–26. [Google Scholar] [CrossRef]
- McIntire, D.D.; Bloom, S.L.; Casey, B.M.; Leveno, K.J. Birth Weight in Relation to Morbidity and Mortality among Newborn Infants. N. Engl. J. Med. 1999, 340, 1234–1238. [Google Scholar] [CrossRef]
- Jones, R.A.K.; Roberton, N.R.C. Problems of the Small-for-Dates Baby. Clin. Obstet. Gynaecol. 1984, 11, 499–524. [Google Scholar]
- Alkalay, A.L.; Graham, J.M.; Pomerance, J.J. Evaluation of Neonates Born with Intrauterine Growth Retardation: Review and Practice Guidelines. J. Perinatol. 1998, 18, 142–151. [Google Scholar] [PubMed]
- Barker, D.J.P.; Osmond, C.; Golding, J.; Kuh, D.; Wadsworth, M.E.J. Growth in Utero, Blood Pressure in Childhood and Adult Life, and Mortality from Cardiovascular Disease. Br. Med. J. 1989, 298, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Fetal Origins of Coronary Heart Disease. BMJ 1995, 311, 171. [Google Scholar] [CrossRef]
- Sharp, A.; Cornforth, C.; Jackson, R.; Harrold, J.; Turner, M.A.; Kenny, L.C.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): A multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health 2017, 2, 93–102. [Google Scholar] [CrossRef]
- Pels, A.; Derks, J.; Elvan-Taspinar, A.; van Drongelen, J.; de Boer, M.; Duvekot, H.; van Laar, J.; van Eyck, J.; Al-Nasiry, S.; Sueters, M.; et al. Maternal Sildenafil vs Placebo in Pregnant Women with Severe Early-Onset Fetal Growth Restriction: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e205323. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Cosmi, E.; Bilardo, C.M.; Wolf, H.; Berg, C.; Rigano, S.; Germer, U.; Moyano, D.; Turan, S.; Hartung, J.; et al. Predictors of Neonatal Outcome in Early-Onset Placental Dysfunction. Obstet. Gynecol. 2007, 109, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, Z.; Stampalija, T.; Dowswell, T. Fetal and Umbilical Doppler Ultrasound in High-Risk Pregnancies. Cochrane Database Syst. Rev. 2017, 6, CD007529. [Google Scholar] [CrossRef] [PubMed]
- Boers, K.E.; Vijgen, S.M.C.; Bijlenga, D.; van der Post, J.A.M.; Bekedam, D.J.; Kwee, A.; van der Salm, P.C.M.; van Pampus, M.G.; Spaanderman, M.E.A.; de Boer, K.; et al. Induction versus Expectant Monitoring for Intrauterine Growth Restriction at Term: Randomised Equivalence Trial (DIGITAT). BMJ 2010, 341, c7087. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 134. Obstet. Gynecol. 2013, 121, 1122–1133. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. The Investigation and Management of the Small-for-Gestational-Age Fetus; Guideline No. 31; RCOG: London, UK, 2002. [Google Scholar]
- Lausman, A.; Kingdom, J.; Gagnon, R.; Basso, M.; Bos, H.; Crane, J.; Davies, G.; Delisle, M.F.; Hudon, L.; Menticoglou, S.; et al. Intrauterine Growth Restriction: Screening, Diagnosis, And Management. J. Obstet. Gynaecol. Can. 2013, 35, 741–748. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus Definition of Fetal Growth Restriction: A Delphi Procedure. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Miller, J.; Baschat, A.A. Integrated Testing and Management in Fetal Growth Restriction. Semin Perinatol. 2008, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, A.; Brunelli, V.; Prefumo, F.; Frusca, T.; Lees, C.C. Early Onset Fetal Growth Restriction. Matern. Health Neonatol. Perinatol. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Italian Society of Ultrasound in Obstetrics and Gynecology (Società Italiana di Ecografia Ostetrico Ginecologica—SIEOG). SIEOG Guidelines; Editeam: Ferrara, Italy, 2015. [Google Scholar]
- Salomon, L.J.; Alfirevic, Z.; Bilardo, C.M.; Chalouhi, G.E.; Ghi, T.; Kagan, K.O.; Lau, T.K.; Papageorghiou, A.T.; Raine-Fenning, N.J.; Stirnemann, J.; et al. ISUOG Practice Guidelines: Performance of First-Trimester Fetal Ultrasound Scan. Ultrasound Obstet. Gynecol. 2013, 102–113. [Google Scholar] [CrossRef]
- Borrell, A.; Grande, M.; Meler, E.; Sabrià, J.; Mazarico, E.; Muñoz, A.; Rodriguez-Revenga, L.; Badenas, C.; Figueras, F. Genomic Microarray in Fetuses with Early Growth Restriction: A Multicenter Study. Fetal Diagn. Ther. 2017, 42, 174–180. [Google Scholar] [CrossRef]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and Management of Fetal Growth Restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A. Fetal Growth Restriction—From Observation to Intervention. J. Perinat. Med. 2010, 38, 239–246. [Google Scholar] [CrossRef]
- Harman, C.R.; Baschat, A.A. Comprehensive Assessment of Fetal Wellbeing: Which Doppler Tests Should Be Performed? Curr. Opin. Obstet. Gynecol. 2003, 15, 147–157. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacós, E. Update on the Diagnosis and Classification of Fetal Growth Restriction and Proposal of a Stage-Based Management Protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Romanini, C.; Rizzo, G. The Development of Abnormal Heart Rate Patterns after Absent End-Diastolic Velocity in Umbilical Artery: Analysis of Risk Factors. Am. J. Obstet. Gynecol. 1993, 168, 43–50. [Google Scholar] [CrossRef]
- Brar, H.S.; Platt, L.D. Reverse End-Diastolic Flow Velocity on Umbilical Artery Velocimetry in High-Risk Pregnancies: An Ominous Finding with Adverse Pregnancy Outcome. Am. J. Obstet. Gynecol. 1988, 159, 559–561. [Google Scholar] [CrossRef]
- Valcamonico, A.; Danti, L.; Frusca, T.; Soregaroli, M.; Zucca, S.; Abrami, F.; Tiberti, A. Absent End-Diastolic Velocity in Umbilical Artery: Risk of Neonatal Morbidity and Brain Damage. Am. J. Obstet. Gynecol. 1994, 170, 796–801. [Google Scholar] [CrossRef]
- Parra-Saavedra, M.; Crovetto, F.; Triunfo, S.; Savchev, S.; Peguero, A.; Nadal, A.; Gratacõs, E.; Figueras, F. Association of Doppler Parameters with Placental Signs of Underperfusion in Late-Onset Small-for-Gestational-Age Pregnancies. Ultrasound Obstet. Gynecol. 2014, 44, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Flood, K.; Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; Mcauliffe, F.M.; O’donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.; et al. The Role of Brain Sparing in the Prediction of Adverse Outcomes in Intrauterine Growth Restriction: Results of the Multicenter PORTO Study. Am. J. Obstet. Gynecol. 2014. [Google Scholar] [CrossRef]
- Meher, S.; Hernandez-Andrade, E.; Basheer, S.N.; Lees, C. Impact of Cerebral Redistribution on Neurodevelopmental Outcome in Small-for-Gestational-Age or Growth-Restricted Babies: A Systematic Review. Ultrasound Obstet. Gynecol. 2015, 46, 398–404. [Google Scholar] [CrossRef]
- Lees, C.C.; Marlow, N.; van Wassenaer-Leemhuis, A.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Calvert, S.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. 2 Year Neurodevelopmental and Intermediate Perinatal Outcomes in Infants with Very Preterm Fetal Growth Restriction (TRUFFLE): A Randomised Trial. Lancet 2015, 385, 2162–2172. [Google Scholar] [CrossRef]
- Ganzevoort, W.; Mensing Van Charante, N.; Thilaganathan, B.; Prefumo, F.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. How to Monitor Pregnancies Complicated by Fetal Growth Restriction and Delivery before 32 Weeks: Post-Hoc Analysis of TRUFFLE Study. Ultrasound Obstet. Gynecol. 2017, 49, 769–777. [Google Scholar] [CrossRef]
- Kiserud, T.; Kessler, J.; Ebbing, C.; Rasmussen, S. Ductus Venosus Shunting in Growth-Restricted Fetuses and the Effect of Umbilical Circulatory Compromise. Ultrasound Obstet. Gynecol. 2006, 28, 143–149. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Bellotti, M.; Galan, H.; Pennati, G.; Bozzo, M.; Rigano, S.; Battaglia, F.C. Doppler Investigation in Intrauterine Growth Restriction-From Qualitative Indices to Flow Measurements. Ann. N. Y. Acad. Sci. 2001, 943, 316–325. [Google Scholar] [CrossRef]
- Hecher, K.; Bilardo, C.M.; Stigter, R.H.; Ville, Y.; Hackelöer, B.J.; Kok, H.J.; Senat, M.V.; Visser, G.H.A. Monitoring of Fetuses with Intrauterine Growth Restriction: A Longitudinal Study. Ultrasound Obstet. Gynecol. 2001, 18, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; Ferrazzi, E.; Fratelli, N.; Frusca, T.; Ganzevoort, W.; Lees, C.C.; Napolitano, R.; Todros, T.; et al. Fetal Monitoring Indications for Delivery and 2-Year Outcome in 310 Infants with Fetal Growth Restriction Delivered before 32 Weeks’ Gestation in the TRUFFLE Study. Ultrasound Obstet. Gynecol. 2017, 50, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, E.; Ambrosini, G.; D’Antona, D.; Saccardi, C.; Mari, G. Doppler, Cardiotocography, and Biophysical Profile Changes in Growth-Restricted Fetuses. Obstet. Gynecol. 2005, 106, 1240–1245. [Google Scholar] [CrossRef]
- Baschat, A.A.; Gembruch, U.; Harman, C.R. The Sequence of Changes in Doppler and Biophysical Parameters as Severe Fetal Growth Restriction Worsens. Ultrasound Obstet. Gynecol. 2001, 18, 571–577. [Google Scholar] [CrossRef]
- Vieira Francisco, R.P.; Miyadahira, S.; Zugaib, M. Predicting PH at Birth in Absent or Reversed End-Diastolic Velocity in the Umbilical Arteries. Obstet. Gynecol. 2006, 107, 1042–1048. [Google Scholar] [CrossRef]
- Schwarze, A.; Gembruch, U.; Krapp, M.; Katalinic, A.; Germer, U.; Axt-Fliedner, R. Qualitative Venous Doppler Flow Waveform Analysis in Preterm Intrauterine Growth-Restricted Fetuses with ARED Flow in the Umbilical Artery—Correlation with Short-Term Outcome. Ultrasound Obstet. Gynecol. 2005, 25, 573–579. [Google Scholar] [CrossRef]
- van Wassenaer-Leemhuis, A.; Marlow, N.; Lees, C.; Wolf, H. The Association of Neonatal Morbidity with Long-Term Neurological Outcome in Infants Who Were Growth Restricted and Preterm at Birth: Secondary Analyses from TRUFFLE (Trial of Randomized Umbilical and Fetal Flow in Europe). BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- GRIT Study Group. A Randomised Trial of Timed Delivery for the Compromised Preterm Fetus: Short Term Outcomes and Bayesian Interpretation. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 27–32. [Google Scholar] [CrossRef]
- Savchev, S.; Figueras, F.; Sanz-Cortes, M.; Cruz-Lemini, M.; Triunfo, S.; Botet, F.; Gratacos, E. Evaluation of an Optimal Gestational Age Cut-off for the Definition of Early-and Late-Onset Fetal Growth Restriction. Fetal Diagn. Ther. 2014, 36, 99–105. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and Management of Small-for-gestational-age Fetus and Fetal Growth Restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- DeVore, G.R. The Importance of the Cerebroplacental Ratio in the Evaluation of Fetal Well-Being in SGA and AGA Fetuses. Am. J. Obstet. Gynecol. 2015, 1, 5–15. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. Stage-Based Approach to the Management of Fetal Growth Restriction. Prenat. Diagn. 2014, 34, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Stampalija, T.; Arabin, B.; Wolf, H.; Bilardo, C.M.; Lees, C.; Brezinka, C.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; Ferrazzi, E.; et al. Is Middle Cerebral Artery Doppler Related to Neonatal and 2-Year Infant Outcome in Early Fetal Growth Restriction? Am. J. Obstet. Gynecol. 2017, 216, 521.e1–521.e13. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.; Arabin, B.; Lees, C.C.; Oepkes, D.; Prefumo, F.; Thilaganathan, B.; Todros, T.; Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; et al. Longitudinal Study of Computerized Cardiotocography in Early Fetal Growth Restriction. Ultrasound Obstet. Gynecol. 2017, 50, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Savchev, S.; Figueras, F.; Cruz-Martinez, R.; Illa, M.; Botet, F.; Gratacos, E. Estimated Weight Centile as a Predictor of Perinatal Outcome in Small-for-Gestational-Age Pregnancies with Normal Fetal and Maternal Doppler Indices. Ultrasound Obstet. Gynecol. 2012, 39, 299–303. [Google Scholar] [CrossRef]
- Grivell, R.M.; Wong, L.; Bhatia, V. Regimens of Fetal Surveillance for Impaired Fetal Growth. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Severi, F.M.; Bocchi, C.; Visentin, A.; Falco, P.; Cobellis, L.; Florio, P.; Zagonari, S.; Pilu, G. Uterine and Fetal Cerebral Doppler Predict the Outcome of Third-Trimester Small-for-Gestational Age Fetuses with Normal Umbilical Artery Doppler. Ultrasound Obstet. Gynecol. 2002, 19, 225–228. [Google Scholar] [CrossRef]
- Ghosh, G.; Gudmundsson, S. Uterine and Umbilical Artery Doppler Are Comparable in Predicting Perinatal Outcome of Growth-Restricted Fetuses. Bjog: Int. J. Obstet. Gynaecol. 2009, 116, 424–430. [Google Scholar] [CrossRef]
- Raju, T.N.K.; Mercer, B.M.; Burchfield, D.J.; Joseph, G.F. Periviable Birth: Executive Summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Am. J. Obstet. Gynecol. 2014, 210, 1083–1096. [Google Scholar] [CrossRef]
- Vergani, P.; Roncaglia, N.; Andreotti, C.; Arreghini, A.; Teruzzi, M.; Pezzullo, J.C.; Ghidini, A. Prognostic Value of Uterine Artery Doppler Velocimetry in Growth-Restricted Fetuses Delivered near Term. Am. J. Obstet. Gynecol. 2002, 187, 932–936. [Google Scholar] [CrossRef]
- Frusca, T.; Todros, T.; Lees, C.; Bilardo, C.M.; Hecher, K.; Visser, G.H.A.; Papageorghiou, A.T.; Marlow, N.; Thilaganathan, B.; van Wassenaer-Leemhuis, A.; et al. Outcome in Early-Onset Fetal Growth Restriction Is Best Combining Computerized Fetal Heart Rate Analysis with Ductus Venosus Doppler: Insights from the Trial of Umbilical and Fetal Flow in Europe. Am. J. Obstet. Gynecol. 2018, 218, S783–S789. [Google Scholar] [CrossRef]
- Bilardo, C.M.; Hecher, K.; Visser, G.H.A.; Papageorghiou, A.T.; Marlow, N.; Thilaganathan, B.; van Wassenaer-Leemhuis, A.; Todros, T.; Marsal, K.; Frusca, T.; et al. Severe Fetal Growth Restriction at 26–32 Weeks: Key Messages from the TRUFFLE Study. Ultrasound Obstet. Gynecol. 2017, 50, 285–290. [Google Scholar] [CrossRef]
- Story, L.; Sankaran, S.; Mullins, E.; Tan, S.; Russell, G.; Kumar, S.; Kyle, P. Survival of Pregnancies with Small for Gestational Age Detected before 24 Weeks Gestation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 188, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Temming, L.A.; Dicke, J.M.; Stout, M.J.; Rampersad, R.M.; Macones, G.A.; Tuuli, M.G.; Cahill, A.G. Early Second-Trimester Fetal Growth Restriction and Adverse Perinatal Outcomes. Obstet. Gynecol. 2017, 130, 865–869. [Google Scholar] [CrossRef]
- Dall’Asta, A.; Girardelli, S.; Usman, S.; Lawin-O’Brien, A.; Paramasivam, G.; Frusca, T.; Lees, C.C. Etiology and Perinatal Outcome of Periviable Fetal Growth Restriction Associated with Structural or Genetic Anomaly. Ultrasound Obstet. Gynecol. 2020, 55, 368–374. [Google Scholar] [CrossRef]
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Asta, A.; Minopoli, M.; Ghi, T.; Frusca, T. Monitoring, Delivery and Outcome in Early Onset Fetal Growth Restriction. Reprod. Med. 2021, 2, 85-94. https://doi.org/10.3390/reprodmed2020009
Dall’Asta A, Minopoli M, Ghi T, Frusca T. Monitoring, Delivery and Outcome in Early Onset Fetal Growth Restriction. Reproductive Medicine. 2021; 2(2):85-94. https://doi.org/10.3390/reprodmed2020009
Chicago/Turabian StyleDall’Asta, Andrea, Monica Minopoli, Tullio Ghi, and Tiziana Frusca. 2021. "Monitoring, Delivery and Outcome in Early Onset Fetal Growth Restriction" Reproductive Medicine 2, no. 2: 85-94. https://doi.org/10.3390/reprodmed2020009
APA StyleDall’Asta, A., Minopoli, M., Ghi, T., & Frusca, T. (2021). Monitoring, Delivery and Outcome in Early Onset Fetal Growth Restriction. Reproductive Medicine, 2(2), 85-94. https://doi.org/10.3390/reprodmed2020009






