Machine Learning Application in Different Imaging Modalities for Detection of Obstructive Coronary Artery Disease and Outcome Prediction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review Methods
- Human studies using coronary images
- Machine learning methods
- Discrimination statistics of prediction models reported
- Peer-reviewed studies published from January 2013–March 2023.
- Image processing studies
- Quantitative parameter calculations
- Absence of clinical outcomes
2.2. Meta-Analysis Methods
3. Results
3.1. Systematic Review Results
3.2. Meta-Analysis Results
4. Discussion
4.1. Diagnostic Studies
4.2. Prognostic Studies
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Leape, L.L.; Park, R.E.; Bashore, T.M.; Harrison, J.; Davidson, C.J.; Brook, R.H. Effect of Variability in the Interpretation of Coronary Angiograms on the Appropriateness of Use of Coronary Revascularization Procedures; Rand Corporation: Santa Monica, CA, USA, 2000. [Google Scholar]
- Zhang, H.; Mu, L.; Hu, S.; Nallamothu, B.K.; Lansky, A.J.; Xu, B.; Bouras, G.; Cohen, D.J.; Spertus, J.A.; Masoudi, F.A.; et al. Comparison of Physician Visual Assessment with Quantitative Coronary Angiography in Assessment of Stenosis Severity in China. JAMA Intern. Med. 2018, 178, 239–247. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Spertus, J.A.; Lansky, A.J.; Cohen, D.J.; Jones, P.G.; Kureshi, F.; Dehmer, G.J.; Drozda, J.P., Jr.; Walsh, M.N.; Brush, J.E., Jr.; et al. Comparison of clinical interpretation with visual assessment and quanti-tative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: The Assessing Angiography (A2) project. Circulation 2013, 127, 1793–1800. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Nakamura, M.; Räber, L.; Colleran, R.; Kadota, K.; Capodanno, D.; Wijns, W.; Akasaka, T.; Valgimigli, M.; Guagliumi, G.; et al. Current use of intracoronary imaging in interventional practice—Results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. EuroIntervention 2018, 14, e475–e484. [Google Scholar] [CrossRef]
- Bularga, A.; Saraste, A.; Fontes-Carvalho, R.; Holte, E.; Cameli, M.; Michalski, B.; Williams, M.C.; Podlesnikar, T.; D’Andrea, A.; Stankovic, I.; et al. EACVI survey on investigations and imaging modalities in chronic coro-nary syndromes. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter AC-CURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [PubMed]
- Livieratos, L. Technical Pitfalls and Limitations of SPECT/CT. Semin. Nucl. Med. 2015, 45, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Mézquita, A.J.V.; Biavati, F.; Falk, V.; Alkadhi, H.; Hajhosseiny, R.; Maurovich-Horvat, P.; Manka, R.; Kozerke, S.; Stuber, M.; Derlin, T.; et al. Clinical quantitative coronary artery stenosis and coronary atherosclerosis imaging: A Consensus Statement from the Quantitative Cardiovascular Imaging Study Group. Nat. Rev. Cardiol. 2023, 20, 696–714. [Google Scholar] [CrossRef]
- Henglin, M.; Stein, G.; Hushcha, P.V.; Snoek, J.; Wiltschko, A.B.; Cheng, S. Machine Learning Approaches in Cardiovascular Imaging. Circ. Cardiovasc. Imaging 2017, 10, e005614. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Manral, N.; McElhinney, P.; Killekar, A.; Matsumoto, H.; Kwiecinski, J.; Pieszko, K.; Razipour, A.; Grodecki, K.; Park, C.; et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: An international multicentre study. Lancet Digit. Health 2022, 4, e256–e265. [Google Scholar] [CrossRef] [PubMed]
- Freiman, M.; Manjeshwar, R.; Goshen, L. Unsupervised abnormality detection through mixed structure regularization (MSR) in deep sparse autoencoders. Med. Phys. 2019, 46, 2223–2231. [Google Scholar] [CrossRef]
- Tesche, C.; De Cecco, C.N.; Baumann, S.; Renker, M.; McLaurin, T.W.; Duguay, T.M.; Bayer, R.R.; Steinberg, D.H.; Grant, K.L.; Canstein, C.; et al. Coronary CT Angiography-derived Fractional Flow Reserve: Machine Learning Algorithm versus Computational Fluid Dynamics Modeling. Radiology 2018, 288, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Hao, G.; Cheng, X.; Ma, C.; Guo, N.; Hu, S.; Tao, Q.; Yao, F.; Hu, C. Diagnostic performance of deep learning-based vascular extraction and stenosis detection technique for coronary artery disease. Br. J. Radiol. 2020, 93, 20191028. [Google Scholar] [CrossRef]
- Li, Y.; Yu, M.; Dai, X.; Lu, Z.; Shen, C.; Wang, Y.; Lu, B.; Zhang, J. Detection of Hemodynamically Significant Coronary Stenosis: CT Myocardial Perfusion versus Machine Learning CT Fractional Flow Reserve. Radiology 2019, 293, 305–314. [Google Scholar] [CrossRef]
- Duguay, T.M.; Tesche, C.; Vliegenthart, R.; De Cecco, C.N.; Lin, H.; Albrecht, M.H.; Varga-Szemes, A.; De Santis, D.; Ebersberger, U.; Bayer, R.R.; et al. Coronary Computed Tomographic Angiography-Derived Fractional Flow Reserve Based on Machine Learning for Risk Stratification of Non-Culprit Coronary Narrowings in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2017, 120, 1260–1266. [Google Scholar] [CrossRef]
- Griffin, W.F.; Choi, A.D.; Riess, J.S.; Marques, H.; Chang, H.J.; Choi, J.H.; Doh, J.H.; Her, A.Y.; Koo, B.K.; Nam, C.W.; et al. AI Evaluation of Steno-sis on Coronary CTA, Comparison with Quantitative Coronary Angiography and Fractional Flow Reserve: A CREDENCE Trial Substudy. JACC Cardiovasc. Imaging 2023, 16, 193–205. [Google Scholar] [CrossRef]
- Xu, L.; He, Y.; Luo, N.; Guo, N.; Hong, M.; Jia, X.; Wang, Z.; Yang, Z. Diagnostic Accuracy and Generalizability of a Deep Learning-Based Fully Automated Algorithm for Coronary Artery Stenosis Detection on CCTA: A Multi-Centre Registry Study. Front. Cardiovasc. Med. 2021, 8, 707508. [Google Scholar] [CrossRef]
- Arsanjani, R.; Xu, Y.; Dey, D.; Vahistha, V.; Shalev, A.; Nakanishi, R.; Hayes, S.; Fish, M.; Berman, D.; Germano, G.; et al. Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. J. Nucl. Cardiol. 2013, 20, 553–562. [Google Scholar] [CrossRef]
- Betancur, J.; Commandeur, F.; Motlagh, M.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.; Sinusas, A.J.; et al. Deep Learning for Prediction of Obstructive Disease From Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc. Imaging 2018, 11, 1654–1663. [Google Scholar] [CrossRef]
- Kang, D.; Dey, D.; Slomka, P.J.; Arsanjani, R.; Nakazato, R.; Ko, H.; Berman, D.S.; Li, D.; Kuo, C.-C.J. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J. Med. Imaging 2015, 2, 014003. [Google Scholar] [CrossRef]
- Dey, D.; Gaur, S.; Ovrehus, K.A.; Slomka, P.J.; Betancur, J.; Goeller, M.; Hell, M.M.; Gransar, H.; Berman, D.S.; Achenbach, S.; et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: A multicentre study. Eur. Radiol. 2018, 28, 2655–2664. [Google Scholar] [CrossRef]
- Johnson, K.M.; Johnson, H.E.; Zhao, Y.; Dowe, D.A.; Staib, L.H. Scoring of Coronary Artery Disease Characteristics on Coronary CT Angiograms by Using Machine Learning. Radiology 2019, 292, 354–362. [Google Scholar] [CrossRef]
- van Rosendael, A.R.; Maliakal, G.; Kolli, K.K.; Beecy, A.; Al’aRef, S.J.; Dwivedi, A.; Singh, G.; Panday, M.; Kumar, A.; Ma, X.; et al. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J. Cardiovasc. Comput. Tomogr. 2018, 12, 204–209. [Google Scholar] [CrossRef]
- Motwani, M.; Dey, D.; Berman, D.S.; Germano, G.; Achenbach, S.; Al-Mallah, M.H.; Andreini, D.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: A 5-year multicentre prospective registry analysis. Eur. Heart J. 2016, 38, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Al’aRef, S.J.; Singh, G.; Choi, J.W.; Xu, Z.; Maliakal, G.; van Rosendael, A.R.; Lee, B.C.; Fatima, Z.; Andreini, D.; Bax, J.J.; et al. A Boosted Ensemble Algorithm for Determination of Plaque Stability in High-Risk Patients on Coronary CTA. JACC Cardiovasc. Imaging 2020, 13, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Al’aRef, S.J.; Maliakal, G.; Singh, G.; van Rosendael, A.R.; Ma, X.; Xu, Z.; Alawamlh, O.A.H.; Lee, B.; Pandey, M.; Achenbach, S.; et al. Machine learning of clinical variables and coronary artery calcium scoring for the prediction of obstructive coronary artery disease on coronary computed tomography angiography: Analysis from the CONFIRM registry. Eur. Heart J. 2019, 41, 359–367. [Google Scholar] [CrossRef] [PubMed]
- AlOthman, A.F.; Sait, A.R.W.; Alhussain, T.A. Detecting Coronary Artery Disease from Computed Tomography Images Using a Deep Learning Technique. Diagnostics 2022, 12, 2073. [Google Scholar] [CrossRef]
- Baskaran, L.; Ying, X.; Xu, Z.; Al’aRef, S.J.; Lee, B.C.; Lee, S.-E.; Danad, I.; Park, H.-B.; Bathina, R.; Baggiano, A.; et al. Machine learning insight into the role of imaging and clinical variables for the prediction of obstructive coronary artery disease and revascularization: An exploratory analysis of the CONSERVE study. PLoS ONE 2020, 15, e0233791. [Google Scholar] [CrossRef]
- Betancur, J.; Otaki, Y.; Motwani, M.; Fish, M.B.; Lemley, M.; Dey, D.; Gransar, H.; Tamarappoo, B.; Germano, G.; Sharir, T.; et al. Prognostic Value of Combined Clinical and Myocardial Perfusion Imaging Data Using Machine Learning. JACC Cardiovasc. Imaging 2018, 11, 1000–1009. [Google Scholar] [CrossRef]
- Ho, H.; Lee, J.G.; Kang, S.J.; Kim, W.J.; Choi, S.Y.; Ko, J.; Min, H.S.; Choi, G.H.; Kang, D.Y.; Lee, P.H.; et al. Angiography-Based Machine Learning for Predicting Fractional Flow Reserve in Interme-diate Coronary Artery Lesions. J. Am. Heart Assoc. 2019, 8, e011685. [Google Scholar]
- Commandeur, F.; Slomka, P.J.; Goeller, M.; Chen, X.; Cadet, S.; Razipour, A.; McElhinney, P.; Gransar, H.; Cantu, S.; Miller, R.J.H.; et al. Machine learning to predict the long-term risk of myocardi-al infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: A prospective study. Cardiovasc. Res. 2020, 116, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Kato, Y.; De Vasconcellos, H.D.; Ostovaneh, M.R.; Lima, J.A.C.; Ambale-Venkatesh, B. Deep learning-based end-to-end automated stenosis classification and localization on catheter coronary angiography. Front. Cardiovasc. Med. 2023, 10, 944135. [Google Scholar] [CrossRef]
- Du, T.; Xie, L.; Zhang, H.; Liu, X.; Wang, X.; Chen, D.; Xu, Y.; Sun, Z.; Zhou, W.; Song, L.; et al. Training and validation of a deep learning architecture for the automatic analysis of coronary angiography. EuroIntervention 2021, 17, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hae, H.; Kang, S.-J.; Kim, W.-J.; Choi, S.-Y.; Lee, J.-G.; Bae, Y.; Cho, H.; Yang, D.H.; Kang, J.-W.; Lim, T.-H.; et al. Machine learning assessment of myocardial ischemia using angiography: Development and retrospective validation. PLOS Med. 2018, 15, e1002693. [Google Scholar] [CrossRef]
- Han, D.; Lee, J.H.; Rizvi, A.; Gransar, H.; Baskaran, L.; Schulman-Marcus, J.; Hartaigh, B.Ó.; Lin, F.Y.; Min, J.K. Incremental role of resting myocardial computed tomography perfusion for predicting physiologically significant coronary artery disease: A machine learning approach. J. Nucl. Cardiol. 2017, 25, 223–233. [Google Scholar] [CrossRef]
- Han, D.; Kolli, K.K.; Al’Aref, S.J.; Baskaran, L.; van Rosendael, A.R.; Gransar, H.; Andreini, D.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; et al. Machine Learning Framework to Identify Individuals at Risk of Rapid Progression of Coronary Atherosclerosis: From the PARADIGM Registry. J. Am. Heart Assoc. 2020, 9, e013958. [Google Scholar] [CrossRef]
- Alonso, D.H.; Wernick, M.N.; Yang, Y.; Germano, G.; Berman, D.S.; Slomka, P. Prediction of cardiac death after adenosine myocardial perfusion SPECT based on machine learning. J. Nucl. Cardiol. 2018, 26, 1746–1754. [Google Scholar] [CrossRef]
- Hu, L.-H.; Betancur, J.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; Miller, E.J.; et al. Machine learning predicts per-vessel early coronary revascularization after fast myocardial perfusion SPECT: Results from multicentre REFINE SPECT registry. Eur. Heart J.-Cardiovasc. Imaging 2019, 21, 549–559. [Google Scholar] [CrossRef]
- Hu, L.H.; Miller, R.J.H.; Sharir, T.; Commandeur, F.; Rios, R.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; et al. Prognostically safe stress-only single-photon emission computed tomography myocardial perfusion imaging guided by machine learning: Report from REFINE SPECT. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 705–714. [Google Scholar] [CrossRef]
- Kwan, A.C.; McElhinney, P.A.; Tamarappoo, B.K.; Cadet, S.; Hurtado, C.; Miller, R.J.H.; Han, D.; Otaki, Y.; Eisenberg, E.; Ebinger, J.E.; et al. Prediction of revascularization by coronary CT angiography using a machine learning ischemia risk score. Eur. Radiol. 2020, 31, 1227–1235. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; He, J.; Jiang, W.; Wang, J.; Peng, Y.; Jia, Y.; Xiong, T.; Jia, K.; Yi, Z.; et al. Automatic coronary artery segmentation and diagnosis of stenosis by deep learning based on computed tomographic coronary angiography. Eur. Radiol. 2022, 32, 6037–6045. [Google Scholar] [CrossRef]
- Mahendiran, T.; Thanou, D.; Senouf, O.; Meier, D.; Dayer, N.; Aminfar, F.; Auberson, D.; Raita, O.; Frossard, P.; Pagnoni, M.; et al. Deep learning-based prediction of future myocardial infarction using invasive coronary angiography: A feasibility study. Open Heart 2023, 10, e002237. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Nakaura, T.; Funama, Y.; Okimoto, T.; Sato, T.; Higaki, T.; Noda, N.; Imada, N.; Baba, Y.; Awai, K. Machine-learning integration of CT histogram analysis to evaluate the composition of atherosclerotic plaques: Validation with IB-IVUS. J. Cardiovasc. Comput. Tomogr. 2018, 13, 163–169. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, D.Y.; Cha, W.C.; Chung, M.J.; Lee, K.-S.; Cho, B.H.; Choi, J.H. Automatic stenosis recognition from coronary angiography using convolutional neural networks. Comput. Methods Programs Biomed. 2021, 198, 105819. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Slomka, P.J.; Rios, R.; Betancur, J.; Blaha, M.J.; Nasir, K.; Miedema, M.D.; Rumberger, J.A.; Gransar, H.; Shaw, L.J.; et al. Machine Learning Adds to Clinical and CAC Assessments in Predicting 10-Year CHD and CVD Deaths. JACC Cardiovasc. Imaging 2021, 14, 615–625. [Google Scholar] [CrossRef]
- Ross, E.G.; Shah, N.H.; Dalman, R.L.; Nead, K.T.; Cooke, J.P.; Leeper, N.J. The use of machine learning for the identification of peripheral artery disease and future mortality risk. J. Vasc. Surg. 2016, 64, 1515–1522.e3. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.A.; Lopez-Rivera, V.; Barman, A.; Grotta, J.C.; Yoo, A.J.; Lee, S.; Inam, M.E.; Savitz, S.I.; Giancardo, L. Machine Learning–Enabled Automated Determination of Acute Ischemic Core From Computed Tomography Angiography. Stroke 2019, 50, 3093–3100. [Google Scholar] [CrossRef]
- Shu, Y.; Wu, X.; Ramirez, G. Deep Learning Based Coronary Angiography in Diagnosis of Myocardial Ischemia. Sci. Program. 2021, 2021, 8491976. [Google Scholar] [CrossRef]
- van Hamersvelt, R.W.; Zreik, M.; Voskuil, M.; Viergever, M.A.; Išgum, I.; Leiner, T. Deep learning analysis of left ventricular myocardium in CT angiographic intermediate-degree coronary stenosis improves the diagnostic accuracy for identification of functionally significant stenosis. Eur. Radiol. 2018, 29, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Zhou, Y.-J.; Zhao, Y.-X.; Shi, D.-M.; Liu, Y.-Y.; Liu, W.; Liu, X.-L.; Li, Y.-P. Diagnostic accuracy of a deep learning approach to calculate FFR from coronary CT angiography. J. Geriatr. Cardiol. 2019, 16, 42–48. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, C.; Chan, H.P.; Chughtai, A.; Agarwal, P.; Kuriakose, J.; Hadjiiski, L.; Patel, S.; Kazerooni, E. Computerized detec-tion of noncalcified plaques in coronary CT angiography: Evaluation of topological soft gradient prescreening method and luminal analysis. Med. Phys. 2014, 41, 081901. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Kola, D.; Heo, R.; Elmore, K.; Cho, I.; Min, J.K. Myocardial perfusion analysis in cardiac computed tomography angiographic images at rest. Med. Image Anal. 2015, 24, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Koo, B.-K.; Hoshino, M.; Lee, J.M.; Murai, T.; Park, J.; Zhang, J.; Hwang, D.; Shin, E.-S.; Doh, J.-H.; et al. CT Angiographic and Plaque Predictors of Functionally Significant Coronary Disease and Outcome Using Machine Learning. JACC Cardiovasc. Imaging 2021, 14, 629–641. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, B.; Chen, F.; Cao, X.; Yi, H.; Hou, Y.; He, X.; Liang, J. An automatic multi-class coronary atherosclerosis plaque detection and classification framework. Med. Biol. Eng. Comput. 2018, 57, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Zreik, M.; Lessmann, N.; van Hamersvelt, R.W.; Wolterink, J.M.; Voskuil, M.; Viergever, M.A.; Leiner, T.; Išgum, I. Deep learning analysis of the myocardium in coronary CT angiography for identification of patients with functionally significant coronary artery stenosis. Med. Image Anal. 2018, 44, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wu, G.; Suk, H.I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef]
- Hesamian, M.H.; Jia, W.; He, X.; Kennedy, P. Deep Learning Techniques for Medical Image Segmentation: Achievements and Challenges. J. Digit. Imaging 2019, 32, 582–596. [Google Scholar] [CrossRef]
- Litjens, G.; Ciompi, F.; Wolterink, J.M.; de Vos, B.D.; Leiner, T.; Teuwen, J.; Išgum, I. State-of-the-Art Deep Learning in Cardio-vascular Image Analysis. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 1549–1565. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Kelm, B.M.; Mittal, S.; Zheng, Y.; Tsymbal, A.; Bernhardt, D.; Vega-Higuera, F.; Zhou, S.K.; Meer, P.; Comaniciu, D. Detection, grading and classification of coronary stenoses in computed tomography angiography. Med. Image Comput. Comput. Assist. Interv. 2011, 14, 25–32. [Google Scholar] [CrossRef]
- Serruys, P.W.; Morice, M.-C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Van Den Brand, M.; Bass, E.J.; et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Chichareon, P.; Modolo, R.; Leaman, D.M.; Reiber, J.H.; Emanuelsson, H.; Di Mario, C.; Pijls, N.H.; Morel, M.-A.; Valgimigli, M.; et al. The SYNTAX score on its way out or … towards artificial intelligence: Part I. EuroIntervention 2020, 16, 44–59. [Google Scholar] [CrossRef]
- Pugliese, F.; Hunink, M.G.M.; Gruszczynska, K.; Alberghina, F.; Malagó, R.; van Pelt, N.; Mollet, N.R.; Cademartiri, F.; Weustink, A.C.; Meijboom, W.B.; et al. Learning Curve for Coronary CT Angiography: What Constitutes Sufficient Training? Radiology 2009, 251, 359–368. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Hoe, J. Quantification of Coronary Arterial Stenoses by Multidetector CT Angiography in Comparison With Conventional Angiography. JACC Cardiovasc. Imaging 2011, 4, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Al’aRef, S.J.; Anchouche, K.; Singh, G.; Slomka, P.J.; Kolli, K.K.; Kumar, A.; Pandey, M.; Maliakal, G.; van Rosendael, A.R.; Beecy, A.N.; et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur. Heart J. 2018, 40, 1975–1986. [Google Scholar] [CrossRef]
- Wolterink, J.M.; van Hamersvelt, R.W.; Viergever, M.A.; Leiner, T.; Išgum, I. Coronary artery centerline extraction in cardiac CT angiography using a CNN-based orientation classifier. Med. Image Anal. 2019, 51, 46–60. [Google Scholar] [CrossRef]
- Huang, W.; Huang, L.; Lin, Z.; Huang, S.; Chi, Y.; Zhou, J.; Zhang, J.; Tan, R.-S.; Zhong, L. Coronary Artery Segmentation by Deep Learning Neural Networks on Computed Tomographic Coronary Angiographic Images. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 608–611. [Google Scholar]
- Mariani, A.; Spaccarotella, C.A.M.; Rea, F.S.; Franzone, A.; Piccolo, R.; Castiello, D.S.; Indolfi, C.; Esposito, G. Artificial Intelligence and Its Role in the Diagnosis and Prediction of Adverse Events in Acute Coronary Syndrome: A Narrative Review of the Literature. Life 2025, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, P.P. Machine learning in cardiovascular medicine: Are we there yet? Heart 2018, 104, 1156–1164. [Google Scholar] [CrossRef] [PubMed]

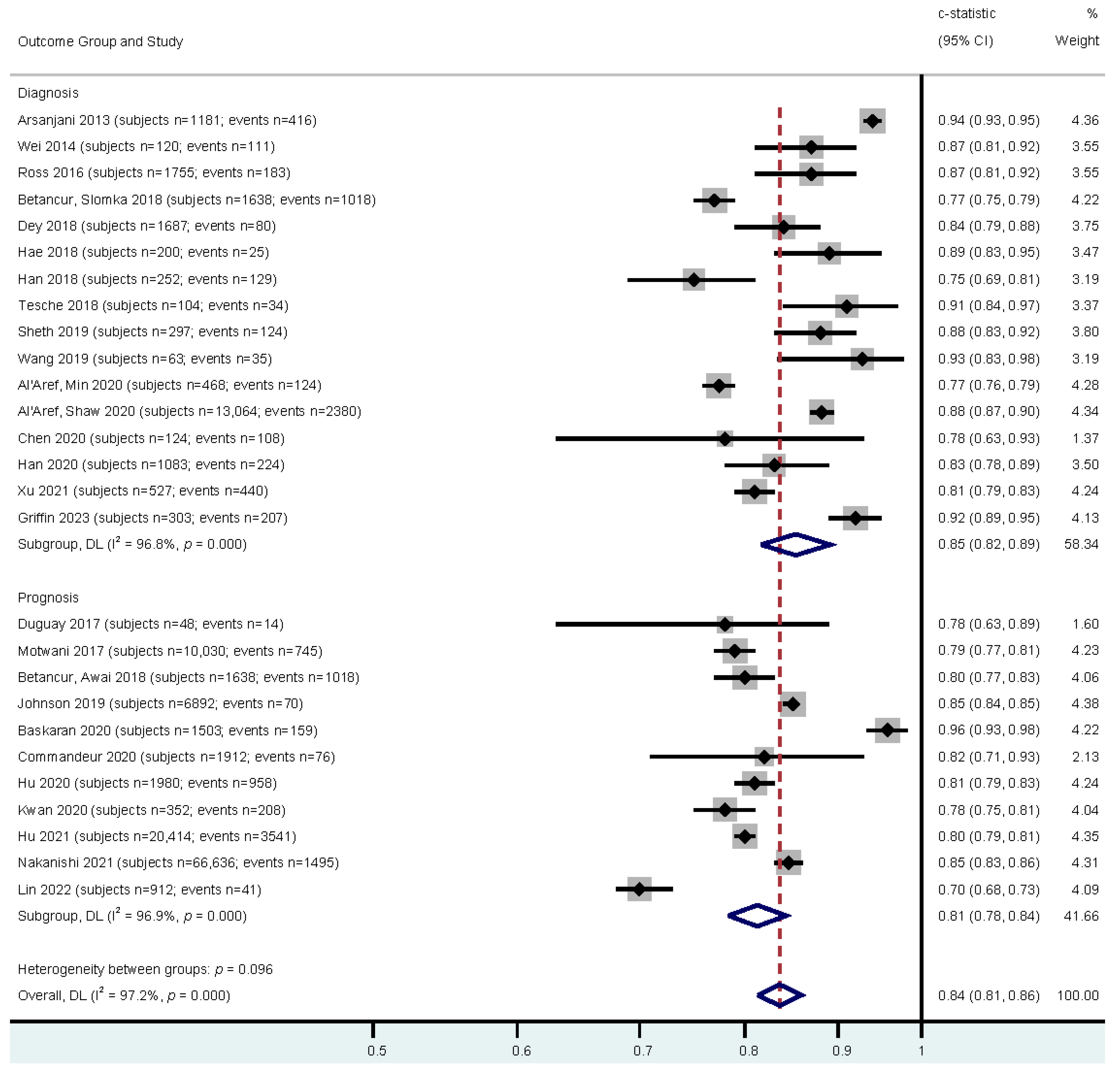
| Study | Effect Size | Outcome | Sample Size | Events | Population | Model | Image Modality |
|---|---|---|---|---|---|---|---|
| Lin 2022 [15] | 0.7 (0.68–0.73) | Prediction of MI | 912 | 41 | Patients with suspected CAD | DNN | CT |
| Freiman 2019 [16] | 0.94 | Detection of severe stenosis | 90 | Patients with suspected CAD | DNN | CT | |
| Tesche 2018 [17] | 0.91 (0.84–0.97) | Detection of lesion specific ischemia | 104 | 34 | Patients with CAD | DNN | CT |
| Chen 2020 [18] | 0.78 (0.63–0.93) | Diagnosis of obstructive CAD | 124 | 108 | Patients with suspected CAD | DNN | CT |
| Li 2019 [19] | 0.85 | Detection of functionally significant stenosis | 157 | 99 | Patients with stable angina | DNN | CT |
| Duguay 2017 [20] | 0.78 (0.63–0.89) | Prediction of MACE | 48 | 14 | Patients with suspected ACS | DNN | CT |
| Griffin 2023 [21] | 0.92 (0.89–0.95) | Detection of severe stenosis | 303 | 207 | Patients with suspected CAD | DNN | CT |
| Xu 2021 [22] | 0.81 (0.79–0.83) | Detection of severe stenosis | 527 | 440 | Patients with suspected CAD | DNN | CT |
| Arsanjani 2013 [23] | 0.94 (0.93–0.95) | Diagnosis of obstructive CAD | 1181 | 416 | Patients with suspected CAD | LogitBoost | ICA |
| Betancur, Slomka 2018 [24] | 0.77 (0.75–0.79) | Diagnosis of obstructive CAD | 1638 | 1018 | Patients without CAD | CNN | SPECT |
| Kang 2015 [25] | 0.94 (±0.03) | Detection of obstructive and nonobstructive lesions | 42 | 21 | Patients with and without CAD | SVM | SPECT |
| Dey 2018 [26] | 0.84 (0.79–0.88) | Detection of lesion specific ischemia | 1687 | 80 | Patients with suspected CAD | LogitBoost | CT |
| Johnson 2019 [27] | 0.85 (0.84–0.85) | Prediction of CVD Death | 6892 | 70 | Patients with suspected CAD | KNN | CT |
| van Rosendael 2018 [28] | 0.771 | Prediction of MI and death | 8844 | 609 | Patients without CAD | XGboost | CT |
| Motwani 2017 [29] | 0.79 (0.77–0.81) | Prediction of 5-year all-cause mortality | 10,030 | 745 | Patients with suspected CAD | LogitBoost | CT |
| Al’Aref, Min 2020 [30] | 0.77 (0.76–0.79) | Detection of culprit lesion precursors | 468 | 124 | Patients with ACS | XGboost | CT |
| Al’Aref, Shaw 2020 [31] | 0.88 (0.87–0.90) | Diagnosis of obstructive CAD | 13,064 | 2380 | Patients with suspected or previously established CAD | XGboost | CT |
| AlOthman 2022 [32] | 0.93 | Detection of stenosis | 200 | 100 | Patients with suspected CAD | CNN | CT |
| Baskaran 2020 [33] | 0.96 (0.93–0.98) | Prediction of necessity for revascularization | 1503 | 159 | Patients with suspected CAD | XGboost | CT |
| Betancur, Awai 2018 [34] | 0.8 (0.77–0.83) | Prediction of MACE | 1638 | 1018 | Patients without CAD | CNN | SPECT |
| Cho 2019 [35] | 0.87 (±0.16) | Detection of lesion specific ischemia | 1501 | 700 | Patients with CAD | XGboost | ICA |
| Commandeur 2020 [36] | 0.82 (0.71–0.93) | Prediction of MI and cardiac death | 1912 | 76 | Patients with suspected CAD | CNN | CT |
| Cong 2023 [37] | 0.86 | Characterization of stenosis | 194 | 194 | Patients with suspected CAD | DNN | CT |
| Du 2021 [38] | 0.86 | Characterization of vessel lesion | 20,612 | 12,184 | Patients with and without CAD | cGAN | ICA |
| Hae 2018 [39] | 0.89 (0.83–0.95) | Detection of lesion specific ischemia | 200 | 25 | Patients with suspected CAD | Light GBM | CT |
| Han 2018 [40] | 0.75 (0.69–0.81) | Detection of lesion specific ischemia | 252 | 129 | Patients with suspected CAD | GBM | CT |
| Han 2020 [41] | 0.83 (0.78–0.89) | Detection of rapid coronary plaque progression | 1083 | 224 | Patients with suspected CAD | LogitBoost | CT |
| Haro Alonso 2019 [42] | 0.83 | Prediction of cardiac death | 8321 | 551 | Patients with suspected or previously established CAD | SVM | CT |
| Hu 2020 [43] | 0.81 (0.79–0.83) | Prediction of necessity for revascularization | 1980 | 958 | Patients with suspected CAD | LogitBoost | SPECT |
| Hu 2021 [44] | 0.8 (0.79–0.81) | Prediction of MACE | 20,414 | 3541 | Patients with suspected CAD | XGboost | SPECT |
| Kwan 2020 [45] | 0.78 (0.75–0.81) | Prediction of Revascularization | 352 | 208 | Patients with suspected CAD | LogitBoost | CT |
| Li 2022 [46] | 0.737 | Detection of stenosis | 443 | 154 | Patients with suspected CAD | DNN | CT |
| Mahendiran 2022 [47] | 0.81 | Prediction of future culprit lesion | 746 | 203 | Patients with MI | DNN | ICA |
| Masuda 2018 [48] | 0.92 | Characterization of coronary plaque | 78 | 78 | Patients with CAD | XGboost | CT |
| Moon 2021 [49] | 0.971 | Detection of functionally significant stenosis | 452 | 221 | Patients with suspected CAD | CNN | ICA |
| Nakanishi 2021 [50] | 0.85 (0.83–0.86) | Prediction of CVD Death | 66,636 | 1495 | Patients with suspected CAD | LogitBoost | CT |
| Ross 2016 [51] | 0.87 (0.83–0.98) | Diagnosis of PAD | 1755 | 183 | Patients with suspected CAD | RF | ICA |
| Sheth 2019 [52] | 0.88 (0.83–0.92) | Detection of large vessel occlusion | 297 | 124 | Patients with and without TIA or AIS | CNN | CT |
| Shu 2021 [53] | 82.7% (Accuracy) | Diagnosis of Myocardial Ischemia | 75 | 50 | Patients with suspected CAD | CNN | ICA |
| van Hamersvelt 2019 [54] | 0.76 (±0.02) | Detection of functionally significant stenosis | 136 | 81 | Patients with suspected CAD | SVM | CT |
| Wang 2019 [55] | 0.93 (±0.01) | Detection of lesion specific ischemia | 63 | 35 | Patients with CAD | RNN | CT |
| Wei 2014 [56] | 0.87 (0.81–0.92) | Detection of noncalcified plaques | 120 | 111 | Patients with CAD | Linear Discrimnant Classifier | CT |
| Xiong 2015 [57] | 0.73 | Detection of obstructive coronary artery stenoses | 140 | 56 | Patients with and without CAD | AdaBoost | CT |
| Yang 2021 [58] | 0.706 | Prediction of cardiac death, MI, or revascularization | 643 | 177 | Patients with suspected CAD | RF | CT |
| Zhao 2019 [59] | 92.6 (Precision) | Detection and classification of atherosclerotic plaque | 14,628 | 1786 | Patients with suspected CAD | SVM | CT |
| Zreik 2018 [60] | 0.74 (±0.02) | Detection of functionally significant stenosis | 166 | 126 | Patients with CAD | SVM | CT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGranaghan, P.; Schoeppenthau, D.; Popp, A.; Saxena, A.; Kothakapu, S.; Rubens, M.; Jiménez, G.; Gordillo, P.; Veledar, E.; Abd El Al, A.; et al. Machine Learning Application in Different Imaging Modalities for Detection of Obstructive Coronary Artery Disease and Outcome Prediction: A Systematic Review and Meta-Analysis. Hearts 2025, 6, 21. https://doi.org/10.3390/hearts6030021
McGranaghan P, Schoeppenthau D, Popp A, Saxena A, Kothakapu S, Rubens M, Jiménez G, Gordillo P, Veledar E, Abd El Al A, et al. Machine Learning Application in Different Imaging Modalities for Detection of Obstructive Coronary Artery Disease and Outcome Prediction: A Systematic Review and Meta-Analysis. Hearts. 2025; 6(3):21. https://doi.org/10.3390/hearts6030021
Chicago/Turabian StyleMcGranaghan, Peter, Doreen Schoeppenthau, Antonia Popp, Anshul Saxena, Sharat Kothakapu, Muni Rubens, Gabriel Jiménez, Pablo Gordillo, Emir Veledar, Alaa Abd El Al, and et al. 2025. "Machine Learning Application in Different Imaging Modalities for Detection of Obstructive Coronary Artery Disease and Outcome Prediction: A Systematic Review and Meta-Analysis" Hearts 6, no. 3: 21. https://doi.org/10.3390/hearts6030021
APA StyleMcGranaghan, P., Schoeppenthau, D., Popp, A., Saxena, A., Kothakapu, S., Rubens, M., Jiménez, G., Gordillo, P., Veledar, E., Abd El Al, A., Hennemuth, A., Falk, V., & Meyer, A. (2025). Machine Learning Application in Different Imaging Modalities for Detection of Obstructive Coronary Artery Disease and Outcome Prediction: A Systematic Review and Meta-Analysis. Hearts, 6(3), 21. https://doi.org/10.3390/hearts6030021








