Systematic Review and Meta-Analysis of Cardiac MRI T1 and ECV Measurements in Pre-Heart Failure Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Reporting Guidelines
2.2. Search Strategy
2.3. Inclusion/Exclusion Criteria
- Studies investigating T1 and/or ECV mapping on CMR in pre-HF populations (asymptomatic individuals with risk factors or subclinical changes, without overt HF).
- Patients aged 18 years and above.
- Prospective, retrospective, cohort, cross-sectional studies, systematic reviews, or meta-analyses.
- Studies solely on established HF (e.g., HFrEF, HFpEF) without a pre-HF subgroup.
- Studies on chronic kidney disease (CKD) populations (e.g., uremic cardiomyopathy).
- Non-human studies.
- Case series/reports, consensus statements, or conference abstracts.
- Studies not reporting T1/ECV data or not using CMR.
2.4. Study Selection, Data Extraction, and Quality Assessment
2.5. Systematic Review Registration
2.6. Statistical Analysis
3. Results
3.1. Search Results
3.2. Methodological Characteristics and Quality of Studies
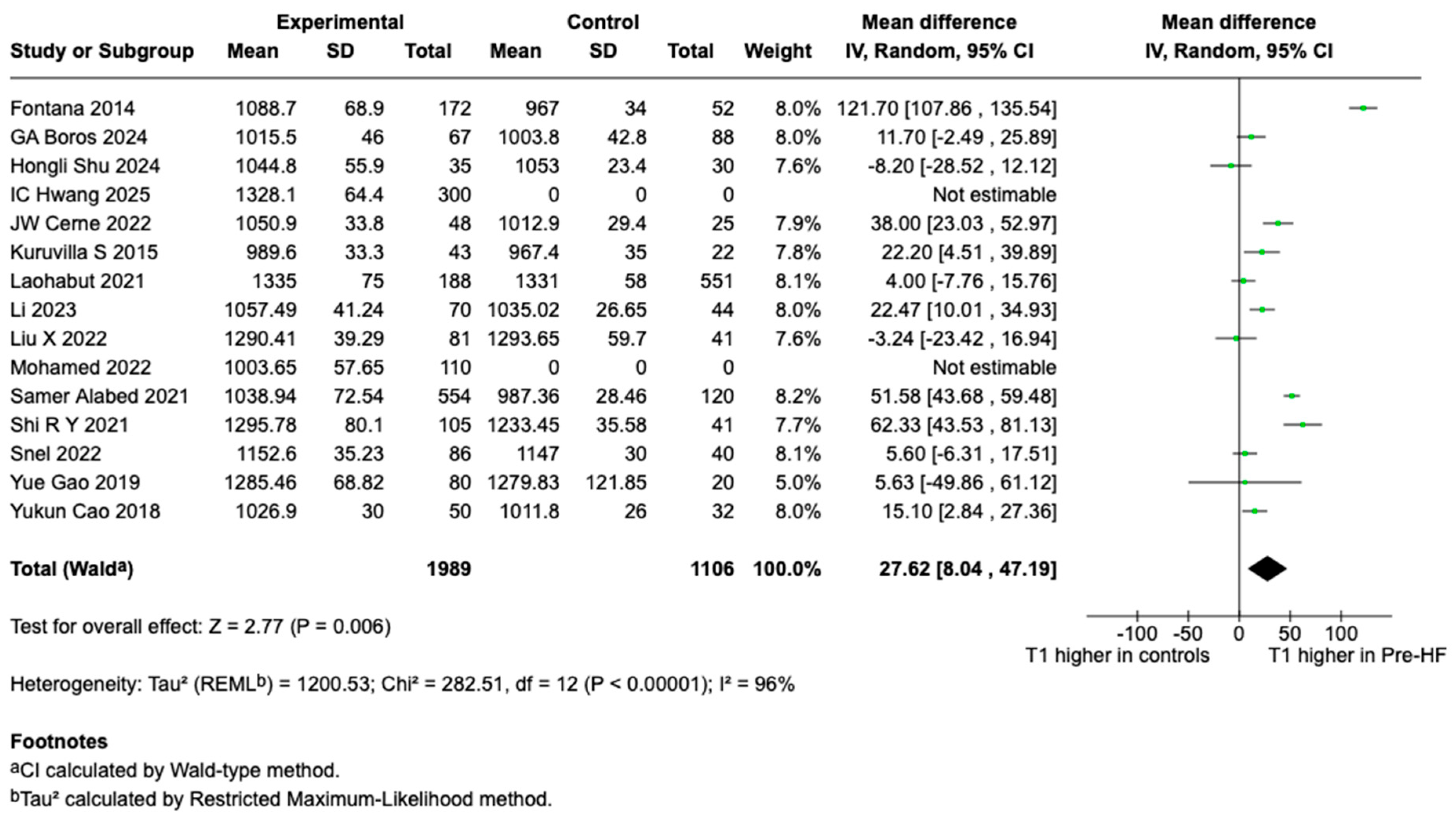
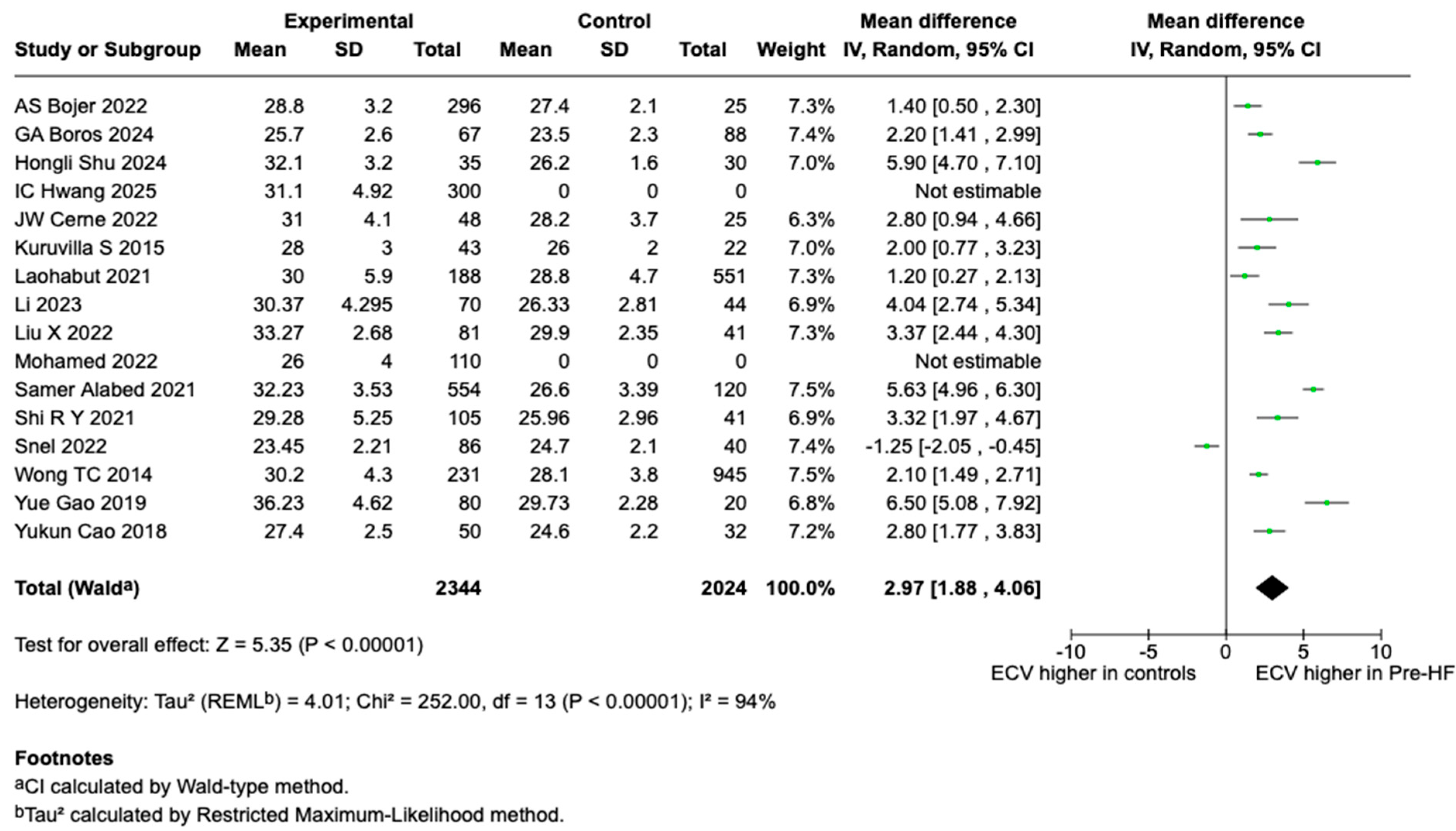
3.3. T1 and ECV Findings and Meta-Analysis Results
4. Discussion
4.1. T1 Meta-Analysis Findings
4.2. ECV Meta-Analysis Findings
4.3. T1 and ECV as Biomarkers in Pre-HF
4.4. Prognostic Implications
4.5. Clinical Applications
4.6. Methodological Variability and Quality
4.7. Clinical Implications and Future Directions
4.8. Risk Stratification
- Low-Risk: GLS > −18% (normal range) or T1 < 1000 ms (1.5 T) or <1200 ms (3 T) AND ECV < 26%. Indicates minimal myocardial changes. Recommend standard preventive care (lifestyle modifications: diet, exercise, smoking cessation) with reassessment every 3–5 years via repeat screening (AI-ECG/NP preferred over CMR for cost).
- Moderate-Risk: GLS −16% to −18% or T1 1000–1100 ms (1.5 T) or 1200–1300 ms (3 T) OR ECV 26–30%. Suggests early interstitial expansion/fibrosis. Initiate enhanced monitoring (e.g., quarterly clinical visits, home BP/glucose tracking) and intensified lifestyle interventions (e.g., structured exercise programs, weight loss targets). Consider adjunctive therapies like ACE inhibitors/ARBs if hypertension is present. Reassess with AI-ECG/NP, STE, or CMR every 1–2 years.
- High-Risk: GLS < −16% or T1 > 1100 ms (1.5 T) or >1300 ms (3 T) OR ECV > 30%. Indicates significant subclinical changes with high HF progression risk. Trigger evidence-based preventive pharmacotherapy (e.g., SGLT2 inhibitors for T2DM/hypertension) and frequent surveillance (e.g., biannual AI-ECG/NP or STE, annual CMR). Refer to cardiology for a comprehensive evaluation.
4.9. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef]
- cMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Committees, Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2016, 19, 75. [Google Scholar] [CrossRef]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; E Arai, A.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.-L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Piechnik, S.K.; Ferreira, V.M.; Lewandowski, A.J.; AB Ntusi, N.; Banerjee, R.; Holloway, C.; Hofman, M.B.; Sado, D.M.; Maestrini, V.; White, S.K.; et al. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J. Cardiovasc. Magn. Reson. 2013, 15, 13. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Ledwidge, M.; Dodd, J.D.; Ryan, F.; Sweeney, C.; McDonald, K.; Fox, R.; Shorten, E.; Zhou, S.; Watson, C.; Gallagher, J.; et al. Effect of Sacubitril/Valsartan vs Valsartan on Left Atrial Volume in Patients with Pre–Heart Failure with Preserved Ejection Fraction: The PARABLE Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 December 2019).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Wong, T.C.; Piehler, K.M.; Kang, I.A.; Kadakkal, A.; Kellman, P.; Schwartzman, D.S.; Mulukutla, S.R.; Simon, M.A.; Shroff, S.G.; Kuller, L.H.; et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur. Heart J. 2013, 35, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Snel, G.J.H.; Slart, R.H.J.A.; Velthuis, B.K.; Boomen, M.v.D.; Nguyen, C.T.; Sosnovik, D.E.; van Deursen, V.M.; Dierckx, R.A.J.O.; Borra, R.J.H.; Prakken, N.H.J.; et al. Interpretation of pre-morbid cardiac 3T MRI findings in overweight and hypertensive young adults. PLoS ONE 2022, 17, e0278308. [Google Scholar] [CrossRef]
- Fontana, M.; Banypersad, S.M.; Treibel, T.A.; Maestrini, V.; Sado, D.M.; White, S.K.; Pica, S.; Castelletti, S.; Piechnik, S.K.; Robson, M.D.; et al. Native T1 Mapping in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 157–165. [Google Scholar] [CrossRef]
- Mohamed, A.T.; Georgiopoulos, G.; Faconti, L.; Asher, C.; Vennin, S.; McNally, R.; Vasileios, S.; Alfakih, K.; Lamata, P.; Keehn, L.; et al. Ethnicity-specific myocardial remodelling in hypertensive heart disease by multi-parametric cardiovascular magnetic resonance. Eur. Heart J. 2022, 43, ehac544.251. [Google Scholar] [CrossRef]
- Hwang, I.-C.; Chun, E.J.; Kim, P.K.; Kim, M.; Park, J.; Choi, H.-M.; Yoon, Y.E.; Cho, G.-Y.; Choi, B.W.; Limongelli, G. Automated extracellular volume fraction measurement for diagnosis and prognostication in patients with light-chain cardiac amyloidosis. PLoS ONE 2025, 20, e0317741. [Google Scholar] [CrossRef]
- Cerne, J.W.; Pathrose, A.; Sarnari, R.; Veer, M.; Chow, K.; Subedi, K.; Allen, B.D.; Avery, R.J.; Markl, M.; Carr, J.C. Left Ventricular Fibrosis Assessment by Native T1, ECV, and LGE in Pulmonary Hypertension Patients. Diagnostics 2022, 13, 71. [Google Scholar] [CrossRef]
- Alabed, S.; Saunders, L.; Garg, P.; Shahin, Y.; Alandejani, F.; Rolf, A.; Puntmann, V.O.; Nagel, E.; Wild, J.M.; Kiely, D.G.; et al. Myocardial T1-mapping and extracellular volume in pulmonary arterial hypertension: A systematic review and meta-analysis. Magn. Reson. Imaging 2021, 79, 66–75. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, Z.-G.; Ren, Y.; Liu, X.; Jiang, L.; Xie, L.-J.; Hu, B.-Y.; Shen, M.-T.; Xu, H.-Y.; Li, Z.-L.; et al. Evaluation of myocardial fibrosis in diabetes with cardiac magnetic resonance T1-mapping: Correlation with the high-level hemoglobin A1c. Diabetes Res. Clin. Pract. 2019, 150, 72–80. [Google Scholar] [CrossRef]
- Kuruvilla, S.; Janardhanan, R.; Antkowiak, P.; Keeley, E.C.; Adenaw, N.; Brooks, J.; Epstein, F.H.; Kramer, C.M.; Salerno, M. Increased Extracellular Volume and Altered Mechanics Are Associated with LVH in Hypertensive Heart Disease, Not Hypertension Alone. JACC Cardiovasc. Imaging 2015, 8, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Laohabut, I.; Songsangjinda, T.; Kaolawanich, Y.; Yindeengam, A.; Krittayaphong, R. Myocardial Extracellular Volume Fraction and T1 Mapping by Cardiac Magnetic Resonance Compared Between Patients with and Without Type 2 Diabetes, and the Effect of ECV and T2D on Cardiovascular Outcomes. Front. Cardiovasc. Med. 2021, 8, 771363. [Google Scholar] [CrossRef]
- Shu, H.; Xu, H.; Pan, Z.; Liu, Y.; Deng, W.; Zhao, R.; Sun, Y.; Wang, Z.; Yang, J.; Gao, H.; et al. Early detection of myocardial involvement by non-contrast T1ρ mapping of cardiac magnetic resonance in type 2 diabetes mellitus. Front. Endocrinol. 2024, 15, 1335899. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Guo, Y.-K.; Xia, C.-C.; Shi, R.; Jiang, L.; Shen, M.-T.; Xie, L.-J.; Peng, W.-L.; Qian, W.-L.; et al. Cardiac magnetic resonance T1 mapping for evaluating myocardial fibrosis in patients with type 2 diabetes mellitus: Correlation with left ventricular longitudinal diastolic dysfunction. Eur. Radiol. 2022, 32, 7647–7656. [Google Scholar] [CrossRef]
- Li, Z.; Han, D.; Qi, T.; Deng, J.; Li, L.; Gao, C.; Gao, W.; Chen, H.; Zhang, L.; Chen, W. Hemoglobin A1c in type 2 diabetes mellitus patients with preserved ejection fraction is an independent predictor of left ventricular myocardial deformation and tissue abnormalities. BMC Cardiovasc. Disord. 2023, 23, 49. [Google Scholar] [CrossRef]
- Cao, Y.; Zeng, W.; Cui, Y.; Kong, X.; Wang, M.; Yu, J.; Zhang, S.; Song, J.; Yan, X.; Greiser, A.; et al. Increased myocardial extracellular volume assessed by cardiovascular magnetic resonance T1 mapping and its determinants in type 2 diabetes mellitus patients with normal myocardial systolic strain. Cardiovasc. Diabetol. 2018, 17, 7. [Google Scholar] [CrossRef]
- Boros, G.A.B.; Hueb, W.; Rezende, P.C.; Rochitte, C.E.; Nomura, C.H.; Lima, E.G.; Ribeiro, M.d.O.L.; Dallazen, A.R.; Garcia, R.M.R.; Ramires, J.A.F.; et al. Unveiling myocardial microstructure shifts: Exploring the impact of diabetes in stable CAD patients through CMR T1 mapping. Diabetol. Metab. Syndr. 2024, 16, 156. [Google Scholar] [CrossRef]
- Bojer, A.S.; Sørensen, M.H.; Gæde, P.; Madsen, P.L. Myocardial Extracellular Volume Expansion in Type 2 Diabetes Is Associated With Ischemic Heart Disease, Autonomic Neuropathy, and Active Smoking. Diabetes Care 2022, 45, 3032–3039. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.-Y.; Wu, R.; An, D.-A.; Chen, B.-H.; Wu, C.-W.; Du, L.; Jiang, M.; Xu, J.-R.; Wu, L.-M. Texture analysis applied in T1 maps and extracellular volume obtained using cardiac MRI in the diagnosis of hypertrophic cardiomyopathy and hypertensive heart disease compared with normal controls. Clin. Radiol. 2021, 76, 236.e9–236.e19. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients with Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Ledwidge, M.; Gallagher, J.; Conlon, C.; Tallon, E.; O’Connell, E.; Dawkins, I.; Watson, C.; O’Hanlon, R.; Bermingham, M.; Patle, A.; et al. Natriuretic peptide-based screening and collaborative care for heart failure: The STOP-HF randomized trial. Jama 2013, 310, 66–74. [Google Scholar] [CrossRef]
- Tseng, A.S.; Thao, V.; Borah, B.J.; Attia, I.Z.; Inojosa, J.M.; Kapa, S.; Carter, R.E.; Friedman, P.A.; Lopez-Jimenez, F.; Yao, X.; et al. Cost Effectiveness of an Electrocardiographic Deep Learning Algorithm to Detect Asymptomatic Left Ventricular Dysfunction. Mayo Clin. Proc. 2021, 96, 1835–1844. [Google Scholar] [CrossRef]
- van Giessen, A.; Boonman-de Winter, L.J.M.; Rutten, F.H.; Cramer, M.J.; Landman, M.J.; Liem, A.H.; Hoes, A.W.; Koffijberg, H. Cost-effectiveness of screening strategies to detect heart failure in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Osenenko, K.M.; Kuti, E.; Deighton, A.M.; Pimple, P.; Szabo, S.M. Burden of hospitalization for heart failure in the United States: A systematic literature review. J. Manag. Care Speéc. Pharm. 2022, 28, 157–167. [Google Scholar] [CrossRef]
- Moschetti, K.; Kwong, R.Y.; Petersen, S.E.; Lombardi, M.; Garot, J.; Atar, D.; Rademakers, F.E.; Sierra-Galan, L.M.; Mavrogeni, S.; Li, K.; et al. Cost-Minimization Analysis for Cardiac Revascularization in 12 Health Care Systems Based on the EuroCMR/SPINS Registries. JACC Cardiovasc. Imaging 2022, 15, 607–625. [Google Scholar] [CrossRef]
- Murphy, T.; Jones, D.A.; Friebel, R.; Uchegbu, I.; Mohiddin, S.A.; Petersen, S.E. A cost analysis of cardiac magnetic resonance imaging in the diagnostic pathway of patients presenting with unexplained acute myocardial injury and culprit-free coronary angiography. Front. Cardiovasc. Med. 2021, 8, 749668. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Torretta, P.; Nicolosi, G.L.; Lombardo, M. Left ventricular mechanics assessment in amyloidosis patients: A systematic review and meta-analysis. Minerva Cardiol. Angiol. 2025. [Google Scholar] [CrossRef]
- Vitel, A.; Sporea, I.; Mare, R.; Banciu, C.; Bordejevic, D.A.; Parvanescu, T.; Citu, I.M.; Tomescu, M.C. Association Between Subclinical Left Ventricular Myocardial Systolic Dysfunction Detected by Strain and Strain-Rate Imaging and Liver Steatosis and Fibrosis Detected by Elastography and Controlled Attenuation Parameter in Patients with Metabolic Syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 2021, 188, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Matthews, P.M.; Francis, J.M.; Robson, M.D.; Zemrak, F.; Boubertakh, R.; Young, A.A.; Hudson, S.; Weale, P.; Garratt, S.; et al. UK Biobank’s cardiovascular magnetic resonance protocol. J. Cardiovasc. Magn. Reson. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Design | Population | Sample Size | CMR Protocol | Field Strength | Sequence | Contrast Agent |
|---|---|---|---|---|---|---|---|
| Wong et al. (2014) [14] | Prospective cohort | T2DM, controls | 1176 | ECV only | 1.5 T | MOLLI | Gadoteridol |
| Snel et al. (2022) [15] | Prospective cross-sectional | Overweight, HTN, controls | 126 | T1, ECV | 3 T | MOLLI | Gadoteric acid |
| Fontana et al. (2014) [16] | Cross-sectional | ATTR amyloidosis, controls | 270 | T1 only | 1.5 T | ShMOLLI | Gadoterate meglumine |
| Mohamed et al. (2024) [17] | Prospective observational | HTN (ethnic groups), controls | 110 | T1 only | 1.5 T | MOLLI | Unspecified gadolinium-based contrast |
| Hwang et al. (2025) [18] | Retrospective observational | AL amyloidosis, controls | 300 | T1 only | 3 T | MOLLI | Gadobutrol |
| Cerne et al. (2023) [19] | Prospective observational | PH (PrePH, IpcPH), controls | 73 | T1, ECV, LGE | 1.5 T | MOLLI | Gadobutrol |
| Alabed et al. (2021) [20] | Systematic review/meta-analysis | PAH, controls | 606 | T1, ECV | 1.5, 3 T | MOLLI, ShMOLLI | Varying gadolinium-based contrasts |
| Gao et al. (2019) [21] | Prospective observational | T2DM, controls | 100 | T1, ECV | 3 T | MOLLI | Gadobenate dimeglumine |
| Kuruvilla et al. (2015) [22] | Cross-sectional observational | HTN (LVH, non-LVH), controls | 65 | T1, ECV | 1.5 T | MOLLI | Gadopentetate dimeglumine |
| Laohabut et al. (2021) [23] | Retrospective cohort | T2DM, controls (CAD suspected) | 739 | T1, ECV | 3 T | MOLLI | Unspecified gadolinium-based contrast |
| Shu et al. (2024) [24] | Prospective observational | T2DM, controls | 65 | T1, ECV | 1.5 T | MOLLI | Gadolinium-based contrast |
| Liu et al. (2022) [25] | Prospective cross-sectional | T2DM, controls | 122 | T1, ECV | 3 T | MOLLI | Gadobenate dimeglumine |
| Li et al. (2023) [26] | Cross-sectional | T2DM with preserved EF, controls | 114 | T1, ECV | 1.5 T | MOLLI | Gadobutrol |
| Cao et al. (2018) [27] | Prospective observational | T2DM, controls | 82 | T1, ECV | 1.5 T | MOLLI | Gadolinium-diethylenetriamine pentaacetic acid |
| GA Boros et al. (2024) [28] | Prospective observational | T2DM, controls (CAD) | 155 | T1, ECV | 1.5 T | ShMOLLI | Gadoterate meglumine |
| AS Bojer et al. (2022) [29] | Cross-sectional observational | T2DM, controls | 264 | ECV only | 3 T | MOLLI | Gadobutrol |
| Shi et al. (2021) [30] | Retrospective | HCM, HHD, controls | 146 | T1, ECV | 3 T | MOLLI | Gadopentetate dimeglumine |
| Study | Population | Sample Size | T1 (ms ± SD) Pre-HF | T1 (ms ± SD) Controls | T1 p-Value | ECV (% ± SD) Pre-HF | ECV (% ± SD) Controls | ECV p-Value | Key Findings/Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Wong et al. (2014) [14] | T2DM, controls | 1176 | - | - | - | 30.2 ± 4.3 | 28.1 ± 3.8 | p < 0.001 | ECV > 30% predicted HF admission (HR: 1.52, p < 0.01) |
| Snel et al. (2022) [15] | Overweight, HTN, controls | 126 | 1152.6 ± 35.23 | 1147 ± 30 | p > 0.05 | 23.45 ± 2.21 | 24.7 ± 2.1 | p < 0.01 | ECV lower in overweight/HTN |
| Fontana et al. (2014) [16] | ATTR amyloidosis, controls | 270 | 1088.7 ± 68.9 | 967 ± 34 | p < 0.001 | - | - | - | T1 elevation tracks amyloid burden |
| Mohamed et al. (2024) [17] | HTN (ethnic groups), controls | 110 | 1003.65 ± 57.65 | - | - | - | - | - | No ethnic differences in ECV |
| Hwang et al. (2025) [18] | AL amyloidosis, controls | 300 | 1328.1 ± 64.4 | - | - | - | - | - | T1 and ECV diagnostic for amyloidosis |
| Cerne et al. (2023) [19] | PH (PrePH, IpcPH), controls | 73 | 1050.9 ± 33.8 | 1012.9 ± 29.4 | p < 0.05 | 31.0 ± 4.1 | 28.2 ± 3.7 | p < 0.05 | PrePH had higher septal T1 |
| Alabed et al. (2021) [20] | PAH, controls | 606 | 1038.94 ± 72.54 | 987.36 ± 28.46 | p < 0.05 | 32.23 ± 3.53 | 26.6 ± 3.39 | p < 0.003 | T1 and ECV elevated in PAH |
| Gao et al. (2019) [21] | T2DM, controls | 100 | 1285.46 ± 68.82 | 1279.83 ± 121.85 | p > 0.017 | 36.23 ± 4.62 | 29.73 ± 2.28 | p < 0.001 | ECV increased with HbA1c levels |
| Kuruvilla et al. (2015) [22] | HTN (LVH, non-LVH), controls | 65 | 989.6 ± 33.3 | 967.4 ± 35.0 | p < 0.05 | 28.0 ± 3.0 | 26.0 ± 2.0 | p < 0.05 | ECV linked to strain impairment |
| Laohabut et al. (2021) [23] | T2DM, controls (CAD suspected) | 739 | 1335 ± 75 | 1311 ± 58 | p = 0.516 | 30.0 ± 5.9 | 28.8 ± 4.7 | p = 0.004 | ECV predicted CV outcomes (p = 0.004) |
| Shu et al. (2024) [24] | T2DM, controls | 65 | 1044.8 ± 55.9 | 1053.0 ± 23.4 | p = 0.264 | 32.1 ± 3.2 | 26.2 ± 1.6 | p < 0.001 | Non-contrast T1ρ mapping feasible |
| Liu et al. (2022) [25] | T2DM, controls | 122 | 1290.41 ± 39.29 | 1293.65 ± 59.70 | p < 0.05 | 33.27 ± 2.68 | 29.90 ± 2.35 | p < 0.05 | ECV correlated with diastolic dysfunction |
| Li et al. (2023) [26] | T2DM with preserved EF, controls | 114 | 1057.49 ± 41.24 | 1035.02 ± 26.65 | p < 0.05 | 30.37 ± 4.295 | 26.33 ± 2.81 | p < 0.05 | ECV linked to HbA1c levels |
| Cao et al. (2018) [27] | T2DM, controls | 82 | 1026.9 ± 30.0 | 1011.8 ± 26.0 | p = 0.022 | 27.4 ± 2.5 | 24.6 ± 2.2 | p < 0.001 | ECV linked to systolic strain impairment |
| GA Boros et al. (2024) [28] | T2DM, controls (CAD) | 155 | 1015.5 ± 46.0 | 1003.8 ± 42.8 | p = 0.10 | 25.7 ± 2.6 | 23.5 ± 2.3 | p < 0.01 | ECV increased in T2DM with CAD |
| AS Bojer et al. (2022) [29] | T2DM, controls | 264 | - | - | - | 28.8 ± 3.2 | 27.4 ± 2.1 | p < 0.004 | ECV associated with ischemic heart disease |
| Shi et al. (2021) [30] | HCM, HHD, controls | 146 | 1295.78 ± 80.10 | 1233.45 ± 35.58 | p < 0.001 | 29.28 ± 5.25 | 25.96 ± 2.96 | p < 0.0001 | T1 and ECV diagnostic for HCM/HHD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, R.S.; Walsh, R.; Walsh, J.; Temperley, H.C.; McCormick, J.; Giblin, G. Systematic Review and Meta-Analysis of Cardiac MRI T1 and ECV Measurements in Pre-Heart Failure Populations. Hearts 2025, 6, 22. https://doi.org/10.3390/hearts6030022
Doyle RS, Walsh R, Walsh J, Temperley HC, McCormick J, Giblin G. Systematic Review and Meta-Analysis of Cardiac MRI T1 and ECV Measurements in Pre-Heart Failure Populations. Hearts. 2025; 6(3):22. https://doi.org/10.3390/hearts6030022
Chicago/Turabian StyleDoyle, Robert S., Ross Walsh, Jamie Walsh, Hugo C. Temperley, John McCormick, and Gerard Giblin. 2025. "Systematic Review and Meta-Analysis of Cardiac MRI T1 and ECV Measurements in Pre-Heart Failure Populations" Hearts 6, no. 3: 22. https://doi.org/10.3390/hearts6030022
APA StyleDoyle, R. S., Walsh, R., Walsh, J., Temperley, H. C., McCormick, J., & Giblin, G. (2025). Systematic Review and Meta-Analysis of Cardiac MRI T1 and ECV Measurements in Pre-Heart Failure Populations. Hearts, 6(3), 22. https://doi.org/10.3390/hearts6030022







