A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload
Abstract
1. Introduction
2. Epidemiology
3. Pathophysiology
4. Clinical Manifestations
4.1. Myocardial Disorders
4.2. Conduction Disturbances/Arrhythmia
4.3. Myocardial Infarction/Coronary Artery Disease
4.4. Valvular Heart Diseases
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; de Graaff, B.; McLaren, C.E.; Loréal, O. Haemochromatosis. Nat. Rev. Dis. Primers 2018, 4, 18016. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Brown, K.E.; Ahn, J.; Sundaram, V. ACG Clinical Guideline: Hereditary Hemochromatosis. Am. J. Gastroenterol. 2019, 114, 1202–1218. [Google Scholar] [CrossRef]
- Gulati, V.; Harikrishnan, P.; Palaniswamy, C.; Aronow, W.S.; Jain, D.; Frishman, W.H. Cardiac Involvement in Hemochromatosis. Cardiol. Rev. 2014, 22, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A.; Dormishian, F.; Domingo, R.; Ellis, M.C.; Fullan, A.; et al. A Novel MHC Class I-like Gene Is Mutated in Patients with Hereditary Haemochromatosis. Nat. Genet. 1996, 13, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Samuels, M.E.; Ludwig, E.H.; MacDonald, M.L.E.; Franchini, P.L.; Dubé, M.-P.; Andres, L.; MacFarlane, J.; Sakellaropoulos, N.; Politou, M.; et al. Mutations in HFE2 Cause Iron Overload in Chromosome 1q-Linked Juvenile Hemochromatosis. Nat. Genet. 2004, 36, 77–82. [Google Scholar] [CrossRef]
- Camaschella, C.; Roetto, A.; Calì, A.; De Gobbi, M.; Garozzo, G.; Carella, M.; Majorano, N.; Totaro, A.; Gasparini, P. The Gene TFR2 Is Mutated in a New Type of Haemochromatosis Mapping to 7q22. Nat. Genet. 2000, 25, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Montosi, G.; Donovan, A.; Totaro, A.; Garuti, C.; Pignatti, E.; Cassanelli, S.; Trenor, C.C.; Gasparini, P.; Andrews, N.C.; Pietrangelo, A. Autosomal-Dominant Hemochrom-Atosis Is Associated with a Mutation in the Ferroportin (SLC11A3) Gene. J. Clin. Investig. 2001, 108, 619–623. [Google Scholar] [CrossRef]
- Ahmed, S.; Peterson, S.J.; Parikh, M.A.; Frishman, W.H. Cardiovascular Manifestations of Hemochromatosis: A Review of Pathophysiology, Mechanisms, and Treatment Options. Cardiol. Rev. 2023, 33, 359–364. [Google Scholar] [CrossRef]
- Elmberg, M.; Hultcrantz, R.; Simard, J.F.; Stål, P.; Pehrsson, K.; Askling, J. Risk of Ischaemic Heart Disease and Cardiomyopathy in Patients with Haemochromatosis and in Their First-Degree Relatives: A Nationwide, Population-Based Study. J. Intern. Med. 2012, 272, 45–54. [Google Scholar] [CrossRef]
- Bardou-Jacquet, E.; Morcet, J.; Manet, G.; Lainé, F.; Perrin, M.; Jouanolle, A.-M.; Guyader, D.; Moirand, R.; Viel, J.-F.; Deugnier, Y. Decreased Cardiovascular and Extrahepatic Cancer-Related Mortality in Treated Patients with Mild HFE Hemochromatosis. J. Hepatol. 2015, 62, 682–689. [Google Scholar] [CrossRef]
- Kölmel, S.; Nowak, A.; Krayenbuehl, P.-A. Iron Overload Associated Symptoms and Laboratory Changes in the Swiss Haemochromatosis Cohort-When a Clinician Should Become Attentive. Swiss Med. Wkly. 2020, 150, w20294. [Google Scholar] [CrossRef] [PubMed]
- Pilling, L.C.; Tamosauskaite, J.; Jones, G.; Wood, A.R.; Jones, L.; Kuo, C.-L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Common Conditions Associated with Hereditary Haemochromatosis Genetic Variants: Cohort Study in UK Biobank. BMJ 2019, 364, k5222. [Google Scholar] [CrossRef] [PubMed]
- Mottelson, M.; Helby, J.; Nordestgaard, B.G.; Ellervik, C.; Mandrup-Poulsen, T.; Petersen, J.; Bojesen, S.E.; Glenthøj, A. Mortality and Risk of Diabetes, Liver Disease, and Heart Disease in Individuals with Haemochromatosis HFE C282Y Homozygosity and Normal Concentrations of Iron, Transferrin Saturation, or Ferritin: Prospective Cohort Study. BMJ 2024, 387, e079147. [Google Scholar] [CrossRef]
- Adams, P.C.; Barton, J.C. How I Treat Hemochromatosis. Blood 2010, 116, 317–325. [Google Scholar] [CrossRef]
- Powell, L.W.; Seckington, R.C.; Deugnier, Y. Haemochromatosis. Lancet 2016, 388, 706–716. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hereditary Hemochromatosis: Pathogenesis, Diagnosis, and Treatment. Gastroenterology 2010, 139, 393–408, 408.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Udani, K.; Chris-Olaiya, A.; Ohadugha, C.; Malik, A.; Sansbury, J.; Paari, D. Cardiovascular Manifestations in Hospitalized Patients with Hemochromatosis in the United States. Int. J. Cardiol. 2021, 342, 117–124. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-Star (T2*) Magnetic Resonance for the Early Diagnosis of Myocardial Iron Overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Forni, G.L.; Grazzini, G.; Boudreaux, J.; Agostini, V.; Omert, L. Global Burden and Unmet Needs in the Treatment of Transfusion-Dependent β-Thalassemia. Front. Hematol. 2023, 2, 1187681. [Google Scholar] [CrossRef]
- Kottam, A.; Hanneman, K.; Schenone, A.; Daubert, M.A.; Sidhu, G.D.; Gropler, R.J.; Garcia, M.J. American Heart Association Council on Cardiovascular Radiology and Intervention State-of-the-Art Imaging of Infiltrative Cardiomyopathies: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2023, 16, e000081. [Google Scholar] [CrossRef]
- Ganz, T. Systemic Iron Homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Gujja, P.; Rosing, D.R.; Tripodi, D.J.; Shizukuda, Y. Iron Overload Cardiomyopathy: Better Understanding of an Increasing Disorder. J. Am. Coll. Cardiol. 2010, 56, 1001–1012. [Google Scholar] [CrossRef]
- Wood, J.C. Impact of Iron Assessment by MRI. Hematology Am. Soc. Hematol. Educ. Program 2011, 2011, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Recalcati, S.; Rybinska, I.; Buratti, P.; Cairo, G. Iron-Induced Damage in Cardiomyopathy: Oxidative-Dependent and Independent Mechanisms. Oxid. Med. Cell. Longev. 2015, 2015, 230182. [Google Scholar] [CrossRef]
- Murphy, C.J.; Oudit, G.Y. Iron-Overload Cardiomyopathy: Pathophysiology, Diagnosis, and Treatment. J. Card. Fail. 2010, 16, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qian, M.; Li Campian, J.; Marshall, J.; Zhou, Z.; Roberts, A.M.; Kang, Y.J.; Prabhu, S.D.; Sun, X.-F.; Eaton, J.W. Mitochondrial Dysfunction May Explain the Cardiomyopathy of Chronic Iron Overload. Free Radic. Biol. Med. 2010, 49, 401–407. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Sukumaran, A.; Chang, J.; Han, M.; Mintri, S.; Khaw, B.-A.; Kim, J. Iron Overload Exacerbates Age-Associated Cardiac Hypertrophy in a Mouse Model of Hemochromatosis. Sci. Rep. 2017, 7, 5756. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Johnson, W.; Jayalakshmamma, B.; Green, A.A. Fatal “Iron Heart” in an Adolescent: Biochemical and Ultrastructural Aspects of the Heart. Pediatrics 1975, 55, 336–341. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D. Iron Overload Cardiomyopathy in Clinical Practice. Circulation 2011, 124, 2253–2263. [Google Scholar] [CrossRef]
- Cascales, A.; Sánchez-Vega, B.; Navarro, N.; Pastor-Quirante, F.; Corral, J.; Vicente, V.; de la Peña, F.A. Clinical and Genetic Determinants of Anthracycline-Induced Cardiac Iron Accumulation. Int. J. Cardiol. 2012, 154, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- James, T.N. Pathology of the Cardiac Conduction System in Hemochromatosis. N. Engl. J. Med. 1964, 271, 92–94. [Google Scholar] [CrossRef]
- Schmitt, B.; Golub, R.M.; Green, R. Screening Primary Care Patients for Hereditary Hemochromatosis with Transferrin Saturation and Serum Ferritin Level: Systematic Review for the American College of Physicians. Ann. Intern. Med. 2005, 143, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Aronson, M.; Fitterman, N.; Snow, V.; Weiss, K.B.; Owens, D.K. Clinical Efficacy Assessment Subcommittee of the American College of Physicians Screening for Hereditary Hemochromatosis: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2005, 143, 517–521. [Google Scholar] [CrossRef]
- Świątczak, M.; Rozwadowska, K.; Sikorska, K.; Młodziński, K.; Świątczak, A.; Raczak, G.; Daniłowicz-Szymanowicz, L. The Potential Impact of Hereditary Hemochromatosis on the Heart Considering the Disease Stage and Patient Age—The Role of Echocardiography. Front. Cardiovasc. Med. 2023, 10, 1202961. [Google Scholar] [CrossRef]
- Candell-Riera, J.; Lu, L.; Serés, L.; González, J.B.; Batlle, J.; Permanyer-Miralda, G.; García-del-Castillo, H.; Soler-Soler, J. Cardiac Hemochromatosis: Beneficial Effects of Iron Removal Therapy. An Echocardiographic Study. Am. J. Cardiol. 1983, 52, 824–829. [Google Scholar] [CrossRef]
- Palka, P.; Macdonald, G.; Lange, A.; Burstow, D.J. The Role of Doppler Left Ventricular Filling Indexes and Doppler Tissue Echocardiography in the Assessment of Cardiac Involvement in Hereditary Hemochromatosis. J. Am. Soc. Echocardiogr. 2002, 15, 884–890. [Google Scholar] [CrossRef]
- Aypar, E.; Alehan, D.; Hazirolan, T.; Gümrük, F. The Efficacy of Tissue Doppler Imaging in Predicting Myocardial Iron Load in Patients with Beta-Thalassemia Major: Correlation with T2* Cardiovascular Magnetic Resonance. Int. J. Cardiovasc. Imaging 2010, 26, 413–421. [Google Scholar] [CrossRef]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular Function and Treatment in β-Thalassemia Major: A Consensus Statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Positano, V.; Santarelli, M.F.; Sorrentino, F.; Cracolici, E.; De Marchi, D.; Maggio, A.; Midiri, M.; Landini, L.; Lombardi, M. Multislice Multiecho T2* Cardiovascular Magnetic Resonance for Detection of the Heterogeneous Distribution of Myocardial Iron Overload. J. Magn. Reson. Imaging 2006, 23, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.A.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.A.; Anderson, L.J.; et al. Cardiac T2* Magnetic Resonance for Prediction of Cardiac Complications in Thalassemia Major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef]
- Cheong, B.; Huber, S.; Muthupillai, R.; Flamm, S.D. Evaluation of Myocardial Iron Overload by T2* Cardiovascular Magnetic Resonance Imaging. Tex. Heart Inst. J. 2005, 32, 448–449. [Google Scholar]
- Olson, L.J.; Edwards, W.D.; Holmes, D.R.; Miller, F.A.; Nordstrom, L.A.; Baldus, W.P. Endomyocardial Biopsy in Hemochromatosis: Clinicopathologic Correlates in Six Cases. J. Am. Coll. Cardiol. 1989, 13, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar] [CrossRef]
- Bacon, B.R.; Adams, P.C.; Kowdley, K.V.; Powell, L.W.; Tavill, A.S. Diagnosis and Management of Hemochromatosis: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 328–343. [Google Scholar] [CrossRef]
- Zoller, H.; Schaefer, B.; Vanclooster, A.; Griffiths, B.; Bardou-Jacquet, E.; Corradini, E.; Porto, G.; Ryan, J.; Cornberg, M. EASL Clinical Practice Guidelines on Haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef]
- Barton, J.C.; McDonnell, S.M.; Adams, P.C.; Brissot, P.; Powell, L.W.; Edwards, C.Q.; Cook, J.D.; Kowdley, K.V. Management of Hemochromatosis. Hemochromatosis Management Working Group. Ann. Intern. Med. 1998, 129, 932–939. [Google Scholar] [CrossRef]
- Olynyk, J.K.; Ramm, G.A. Hemochromatosis. N. Engl. J. Med. 2022, 387, 2159–2170. [Google Scholar] [CrossRef]
- Porter, J.B. Practical Management of Iron Overload. Br. J. Haematol. 2001, 115, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, H.; El, R.B.; Link, G.; Breuer, W.; Konijn, A.M.; Hershko, C.; Nick, H.; Cabantchik, Z.I. Action of Chelators in Iron-Loaded Cardiac Cells: Accessibility to Intracellular Labile Iron and Functional Consequences. Blood 2006, 108, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Pibiri, M.; Nair, S.V.; Walker, J.M.; Pennell, D.J. Combined Chelation Therapy in Thalassemia Major for the Treatment of Severe Myocardial Siderosis with Left Ventricular Dysfunction. J. Cardiovasc. Magn. Reson. 2008, 10, 12. [Google Scholar] [CrossRef]
- Cohen, A.R.; Galanello, R.; Piga, A.; De Sanctis, V.; Tricta, F. Safety and Effectiveness of Long-Term Therapy with the Oral Iron Chelator Deferiprone. Blood 2003, 102, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Dabestani, A.; Child, J.S.; Henze, E.; Perloff, J.K.; Schon, H.; Figueroa, W.G.; Schelbert, H.R.; Thessomboon, S. Primary Hemochromatosis: Anatomic and Physiologic Characteristics of the Cardiac Ventricles and Their Response to Phlebotomy. Am. J. Cardiol. 1984, 54, 153–159. [Google Scholar] [CrossRef]
- Caines, A.E.; Kpodonu, J.; Massad, M.G.; Chaer, R.; Evans, A.; Lee, J.C.; Geha, A.S. Cardiac Transplantation in Patients with Iron Overload Cardiomyopathy. J. Heart Lung Transplant. 2005, 24, 486–488. [Google Scholar] [CrossRef]
- Raichlin, E.; Daly, R.C.; Rosen, C.B.; McGregor, C.G.; Charlton, M.R.; Frantz, R.P.; Clavell, A.L.; Rodeheffer, R.J.; Pereira, N.L.; Kremers, W.K.; et al. Combined Heart and Liver Transplantation: A Single-Center Experience. Transplantation 2009, 88, 219–225. [Google Scholar] [CrossRef]
- Shizukuda, Y.; Tripodi, D.J.; Zalos, G.; Bolan, C.D.; Yau, Y.-Y.; Leitman, S.F.; Waclawiw, M.A.; Rosing, D.R. Incidence of Cardiac Arrhythmias in Asymptomatic Hereditary Hemochromatosis Subjects with C282Y Homozygosity. Am. J. Cardiol. 2012, 109, 856–860. [Google Scholar] [CrossRef]
- Sripetchwandee, J.; KenKnight, S.B.; Sanit, J.; Chattipakorn, S.; Chattipakorn, N. Blockade of Mitochondrial Calcium Uniporter Prevents Cardiac Mitochondrial Dysfunction Caused by Iron Overload. Acta Physiol. 2014, 210, 330–341. [Google Scholar] [CrossRef]
- Nanavaty, D.; Singh, S.; Sanghvi, A.; Thakre, A.; Kumar, V.; Devarakonda, P.K.; Manoharan, S.; Patibandla, S.; Bhavsar, D.; Dhulipala, V.; et al. PO-05-164 IMPACT OF ARRHYTHMIAS IN PATIENTS WITH HEMOCHROMATOSIS: A NATIONAL INPATIENT SAMPLE (NIS) ANALYSIS. Heart Rhythm. 2023, 20, S660. [Google Scholar] [CrossRef]
- Zacharski, L.R.; McKernan, L.J.; Metzger, M.E.; Malone, M.G.; Samnotra, V.; Bhargava, A.; Steiner, P.R.; Rauwerdink, C.; Ornstein, D.L.; Cornell, C.J. Remission of Paroxysmal Atrial Fibrillation with Iron Reduction in Hemophilia A. Blood 2009, 114, 5100. [Google Scholar] [CrossRef]
- Li, J.-Y.; Liu, S.-Q.; Yao, R.-Q.; Tian, Y.-P.; Yao, Y.-M. A Novel Insight Into the Fate of Cardiomyocytes in Ischemia-Reperfusion Injury: From Iron Metabolism to Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 799499. [Google Scholar] [CrossRef] [PubMed]
- Gaenzer, H.; Marschang, P.; Sturm, W.; Neumayr, G.; Vogel, W.; Patsch, J.; Weiss, G. Association between Increased Iron Stores and Impaired Endothelial Function in Patients with Hereditary Hemochromatosis. J. Am. Coll. Cardiol. 2002, 40, 2189–2194. [Google Scholar] [CrossRef]
- Marques, V.B.; Nascimento, T.B.; Ribeiro, R.F.; Broseghini-Filho, G.B.; Rossi, E.M.; Graceli, J.B.; dos Santos, L. Chronic Iron Overload in Rats Increases Vascular Reactivity by Increasing Oxidative Stress and Reducing Nitric Oxide Bioavailability. Life Sci. 2015, 143, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis Is Aggravated by Iron Overload and Ameliorated by Dietary and Pharmacological Iron Restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Siri-Angkul, N.; Xie, L.-H.; Chattipakorn, S.C.; Chattipakorn, N. Cellular Electrophysiology of Iron-Overloaded Cardiomyocytes. Front. Physiol. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Failla, M.; Giannattasio, C.; Piperno, A.; Vergani, A.; Grappiolo, A.; Gentile, G.; Meles, E.; Mancia, G. Radial Artery Wall Alterations in Genetic Hemochromatosis before and after Iron Depletion Therapy. Hepatology 2000, 32, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Margaritis, M.; Sanna, F.; Antoniades, C. Statins and Oxidative Stress in the Cardiovascular System. Curr. Pharm. Des. 2017, 23, 7040–7047. [Google Scholar] [CrossRef]
- Shishehbor, M.H.; Brennan, M.-L.; Aviles, R.J.; Fu, X.; Penn, M.S.; Sprecher, D.L.; Hazen, S.L. Statins Promote Potent Systemic Antioxidant Effects through Specific Inflammatory Pathways. Circulation 2003, 108, 426–431. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, N.; Song, Y.; Si, H.; Qin, Q.; Guo, Z. Effect of Iron Overload on Endothelial Cell Calcification and Its Mechanism. Ann. Transl. Med. 2021, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Mahmood, A.M.; Garcia, R.; Christel, C.; Khan, S.; Waqar, F.; Alexander, T. Abstract 13476: Prevalence of Hemochromatosis in Non-Rheumatic Valvular Heart Disease and Its Effect on Prognosis: A National Cohort Analysis. Circulation 2022, 146, A13476. [Google Scholar] [CrossRef]
- Olson, L.J.; Edwards, W.D.; McCall, J.T.; Ilstrup, D.M.; Gersh, B.J. Cardiac Iron Deposition in Idiopathic Hemochromatosis: Histologic and Analytic Assessment of 14 Hearts from Autopsy. J. Am. Coll. Cardiol. 1987, 10, 1239–1243. [Google Scholar] [CrossRef]
- Cortés, P.; Elsayed, A.A.; Stancampiano, F.F.; Barusco, F.M.; Shapiro, B.P.; Bi, Y.; Heckman, M.G.; Peng, Z.; Kempaiah, P.; Palmer, W.C. Clinical and Genetic Predictors of Cardiac Dysfunction Assessed by Echocardiography in Patients with Hereditary Hemochromatosis. Int. J. Cardiovasc. Imaging 2024, 40, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Montvilaitė-Laurinavičienė, A.; Dirsienė, R.; Neverauskaitė-Piliponienė, G.; Banišauskaitė, A.; Šukys, M.; Šakalytė, G.; Ereminienė, E. Heart Failure of Very Rare Aetiology—Haemochromatosis Type 3: A Case Report. Eur. Heart J. Case Rep. 2024, 8, ytae637. [Google Scholar] [CrossRef]
- Laguna-Fernandez, A.; Carracedo, M.; Jeanson, G.; Nagy, E.; Eriksson, P.; Caligiuri, G.; Franco-Cereceda, A.; Bäck, M. Iron Alters Valvular Interstitial Cell Function and Is Associated with Calcification in Aortic Stenosis. Eur. Heart J. 2016, 37, 3532–3535. [Google Scholar] [CrossRef]
- Xu, R.; Huang, Y.; Zhu, D.; Guo, J. Iron Promotes Slc7a11-Deficient Valvular Interstitial Cell Osteogenic Differentiation: A Possible Mechanism by Which Ferroptosis Participates in Intraleaflet Hemorrhage-Induced Calcification. Free Radic. Biol. Med. 2022, 184, 158–169. [Google Scholar] [CrossRef]
- Barton, J.C.; Parker, C.J. HFE-Related Hemochromatosis. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993. [Google Scholar]
- Piperno, A.; Bertola, F.; Bentivegna, A. Juvenile Hemochromatosis. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993. [Google Scholar]
- Luis Moríñigo, J.; Martín Luengo, C.; Ledesma, C.; Arribas, A.; Nieto, A.A.; Rodríguez, J. Ventricular tachycardia and cardiac hemochromatosis. Rev. Esp. Cardiol. 2001, 54, 1328–1331. [Google Scholar] [CrossRef]
- Vidula, M.K.; Bravo, P.E. Multimodality Imaging for the Diagnosis of Infiltrative Cardiomyopathies. Heart 2022, 108, 98–104. [Google Scholar] [CrossRef]
- Ptaszek, L.M.; Price, E.T.; Hu, M.Y.; Yang, P.C. Early Diagnosis of Hemochromatosis-Related Cardiomyopathy with Magnetic Resonance Imaging. J. Cardiovasc. Magn. Reson. 2005, 7, 689–692. [Google Scholar] [CrossRef]
- Wood, J.C. Diagnosis and Management of Transfusion Iron Overload: The Role of Imaging. Am. J. Hematol. 2007, 82, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.C.; Pennell, D.J. Imaging of the Heart: Historical Perspective and Recent Advances. Postgrad. Med. J. 2016, 92, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.H.; Auger, D.; Smith, G.C.; He, T.; Vassiliou, V.; Baksi, A.J.; Wage, R.; Drivas, P.; Feng, Y.; Firmin, D.N.; et al. T1 at 1.5T and 3T Compared with Conventional T2* at 1.5T for Cardiac Siderosis. J. Cardiovasc. Magn. Reson. 2015, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Hemochromatosis Classification: Update and Recommendations by the BIOIRON Society|Blood|American Society of Hematology. Available online: https://ashpublications.org/blood/article/139/20/3018/477138/Hemochromatosis-classification-update-and (accessed on 14 May 2025).


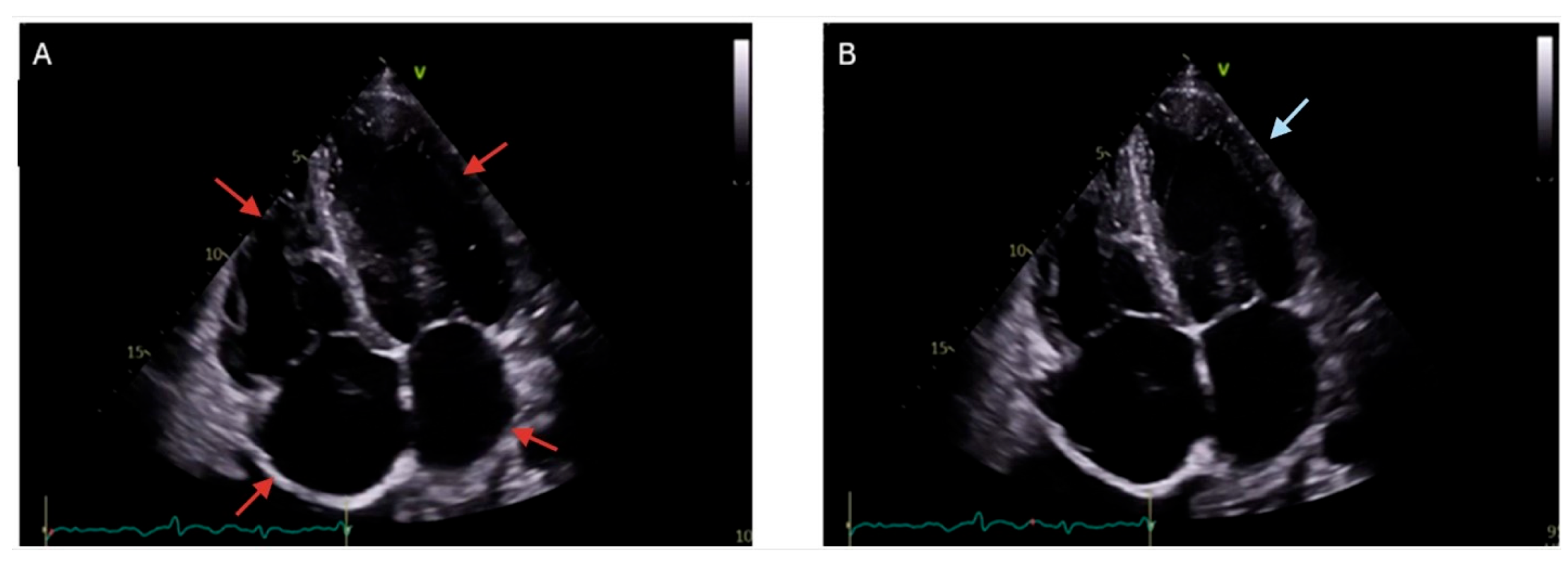
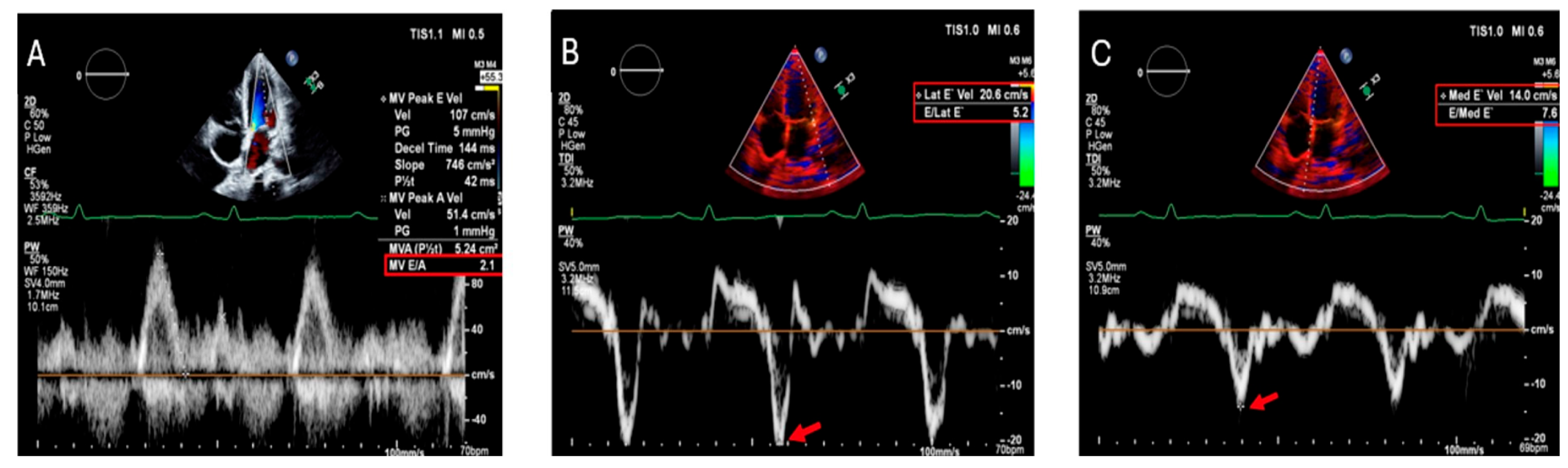

| Manifestation | Description | Symptom Presentation |
|---|---|---|
| Cardiomyopathy [2,17,78,79] | Can be restrictive or dilated; characterized by iron deposition in the myocardium | Dyspnea on exertion, fatigue, signs of heart failure |
| Heart Failure [2,17,78,79] | Often secondary to cardiomyopathy; presents with symptoms like dyspnea and fatigue | Dyspnea, orthopnea, paroxysmal nocturnal dyspnea, peripheral edema |
| Arrhythmias/Electrical Abnormalities [2,17,78,79,80] | Includes atrial fibrillation, supraventricular tachycardia, sick sinus syndrome, ventricular arrhythmias, low QRS complex voltage, and nonspecific ST-T-wave changes | Palpitations, dizziness, syncope, irregular heartbeat |
| Conduction Disorders [17,78,79] | Atrioventricular blocks and other conduction abnormalities due to iron deposition | Bradycardia, syncope, fatigue |
| Pulmonary Hypertension [17] | Increased pulmonary arterial pressure, more common in secondary hemochromatosis | Dyspnea, fatigue, chest pain, syncope |
| Sudden Cardiac Death [2,17] | Can occur due to severe arrhythmias or advanced cardiomyopathy | Sudden collapse, no preceding symptoms in some cases |
| Coronary Artery Disease/Myocardial Infarction [17] | Iron deposition in the coronary arteries leading to oxidative stress, endothelial dysfunction, and lipid plaque formation within the coronary arteries | Chest pain, dyspnea, myocardial infarction, angina |
| Valvular Heart Disease [17] | Iron deposition in the valves leading to thickening, fibrosis, and impaired valve mobility | Chest pain, murmurs, syncope, dyspnea, peripheral edema, palpitations |
| Imaging | Findings | Strengths | Limitations |
|---|---|---|---|
| Echocardiography [81] | Increased wall thickness, diastolic dysfunction | Widely available, non-invasive, first-line imaging, real-time assessment of cardiac function | Limited sensitivity for early iron deposition, operator-dependent, poor tissue characterization |
| Cardiac Magnetic Resonance (CMR): T2* Mapping and Quantification [19,50,79,82,83] | Decreased T2* signal, myocardial iron deposition, LV dysfunction | High sensitivity and specificity, non-invasive, quantifies iron load, excellent tissue characterization, proven when guiding therapy to improve outcomes | Expensive, limited availability, requires breath holding, contraindicated in patients with certain implants |
| T1 Mapping [83,84,85] | Low T1 values indicating iron overload | Non-invasive, high diagnostic accuracy, differentiates severity, reproducible | Requires specialized software, less widely available, variability between scanners |
| T2 Mapping [83,84,85] | Low T2 values indicating iron overload; T2 < 20 ms* indicating presence of myocardial iron overload, <10 ms* associated with high risk of heart failure; <6 ms* indicating severe myocardial iron overload | Non-invasive, high diagnostic accuracy, differentiates severity, reproducible | Requires specialized software, less widely available, variability between scanners |
| Nuclear Imaging [81] | Not typically used for iron overload | Useful for other infiltrative cardiomyopathies, can assess myocardial perfusion and viability | Limited role in iron overload cardiomyopathy, radiation exposure, less specific for iron deposition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, A.; El Dahdah, J.; Haroun, E.; Arockiam, A.D.; Safdar, A.; Sorathia, S.; Dong, T.; Griffin, B.; Wang, T.K.M. A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts 2025, 6, 17. https://doi.org/10.3390/hearts6030017
Agrawal A, El Dahdah J, Haroun E, Arockiam AD, Safdar A, Sorathia S, Dong T, Griffin B, Wang TKM. A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts. 2025; 6(3):17. https://doi.org/10.3390/hearts6030017
Chicago/Turabian StyleAgrawal, Ankit, Joseph El Dahdah, Elio Haroun, Aro Daniela Arockiam, Ahmad Safdar, Sharmeen Sorathia, Tiffany Dong, Brian Griffin, and Tom Kai Ming Wang. 2025. "A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload" Hearts 6, no. 3: 17. https://doi.org/10.3390/hearts6030017
APA StyleAgrawal, A., El Dahdah, J., Haroun, E., Arockiam, A. D., Safdar, A., Sorathia, S., Dong, T., Griffin, B., & Wang, T. K. M. (2025). A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts, 6(3), 17. https://doi.org/10.3390/hearts6030017






