Outcomes and Safety of Direct Oral Anticoagulants (DOACs) versus Vitamin K Antagonists (VKAs) amongst Patients with Valvular Heart Disease (VHD): A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Endpoint Definitions
2.2. Search Criteria
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Study Selection Strategy
2.6. Data Extraction
2.7. Statistical Analysis
3. Results
3.1. Stroke–Vascular Events
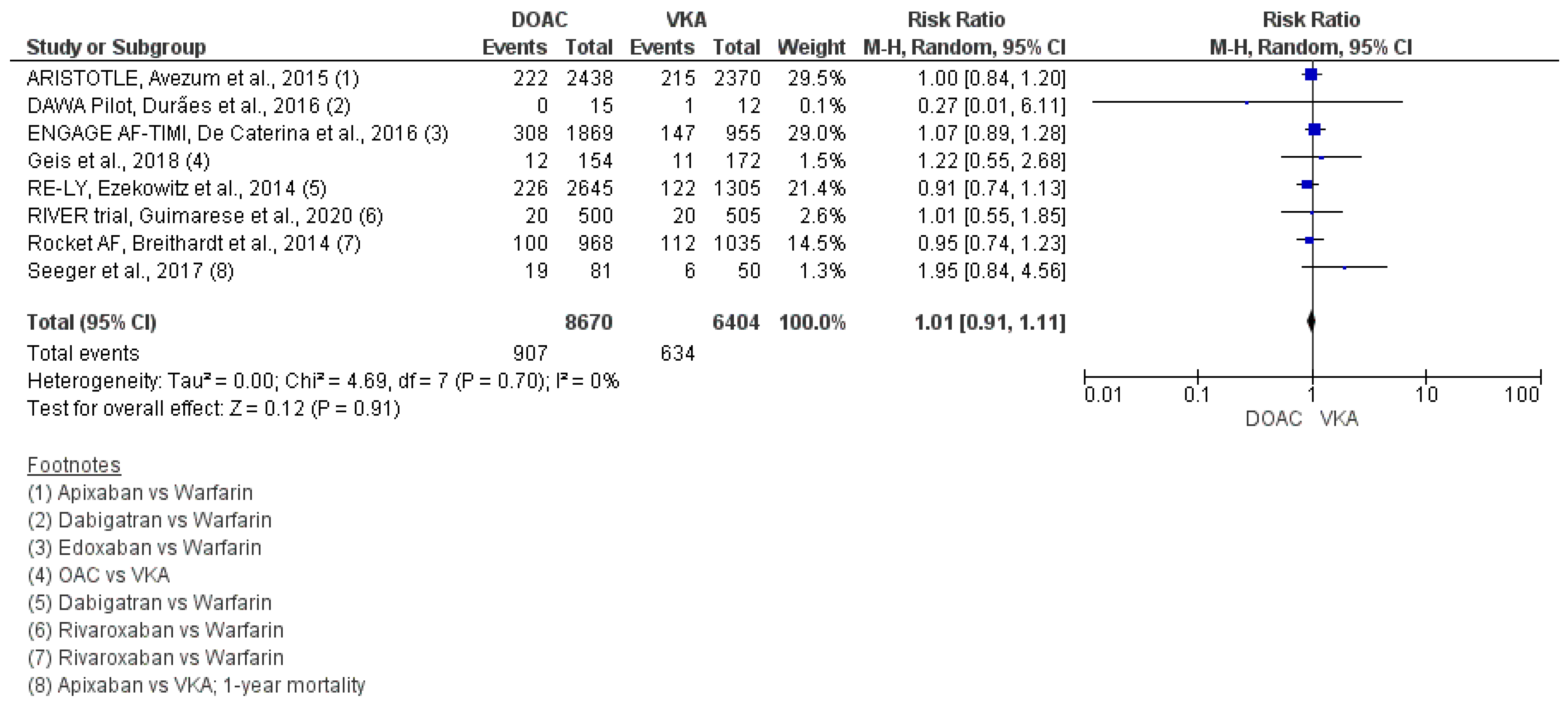
3.2. All-Cause Mortality
3.3. Major Bleeding
3.4. Intracranial Bleeding
3.5. Composite Poor Outcome Overall Event
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global epidemiology of atrial fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Camm, A.J. What is ‘valvular’ atrial fibrillation? A reappraisal. Eur. Heart J. 2014, 35, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of Atrial Fibrillation on the Risk of Death. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Yandrapalli, S.; Aronow, W.S.; Panza, J.A.; Cooper, H.A. Oral anticoagulants in atrial fibrillation with valvular heart disease and bioprosthetic heart valves. Heart 2019, 105, 1432–1436. [Google Scholar] [CrossRef]
- You, J.J.; Singer, D.E.; Howard, P.A.; Lane, D.A.; Eckman, M.H.; Fang, M.C.; Hylek, E.M.; Schulman, S.; Go, A.S.; Hughes, M.; et al. Antithrombotic Therapy for Atrial Fibrillation. Chest 2012, 141, e531S–e575S. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.H.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Ammentorp, B.; Darius, H.; De Caterina, R.; Le Heuzey, J.-Y.; Schilling, R.J.; Schmitt, J.; Zamorano, J.L. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: Primary results of the PREvention oF thromboemolic events—European Registry in Atrial Fibrillation (PREFER in AF). EP Eur. 2014, 16, 6–14. [Google Scholar] [CrossRef]
- Kirchhof, P.; Nabauer, M.; Gerth, A.; Limbourg, T.; Lewalter, T.; Goette, A.; Wegscheider, K.; Treszl, A.; Meinertz, T.; Oeff, M.; et al. Impact of the type of centre on management of AF patients: Surprising evidence for differences in antithrombotic therapy decisions. Thromb. Haemost. 2011, 105, 1010–1023. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Laroche, C.; Ioachim, P.M.; Rasmussen, L.H.; Vitali-Serdoz, L.; Petrescu, L.; Darabantiu, D.; Crijns, H.J.G.M.; Kirchhof, P.; Vardas, P.; et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: One Year Follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur. Heart J. 2014, 35, 3365–3376. [Google Scholar] [CrossRef]
- Durães, A.R.; de Souza Roriz, P.; de Almeida Nunes, B.; Albuquerque, F.P.E.; de Bulhões, F.V.; de Souza Fernandes, A.M.; Aras, R. Dabigatran Versus Warfarin After Bioprosthesis Valve Replacement for the Management of Atrial Fibrillation Postoperatively: DAWA Pilot Study. Drugs R D 2016, 16, 149–154. [Google Scholar] [CrossRef]
- Neree, C. Quality of oral anticoagulation in patients with atrial fibrillation: A cross-sectional study in general practice. Eur. J. Gen. Pract. 2006, 12, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gadisseur, A.P.A.; Kaptein, A.A.; Breukink-Engbers, W.G.M.; Van Der Meer, F.J.M.; Rosendaal, F.R. Patient self-management of oral anticoagulant care vs. management by specialized anticoagulation clinics: Positive effects on quality of life. J. Thromb. Haemost. 2004, 2, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, B.S.; Walton, S.M.; Sharp, L.K.; Galanter, W.L.; Lee, T.A.; Nutescu, E.A. High number of newly initiated direct oral anticoagulant users switch to alternate anticoagulant therapy. J. Thromb. Thrombolysis 2017, 44, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, A.; Mekaj, Y.; Duci, S.; Miftari, E. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, F.; Avierinos, J.-F.; Ling, L.H.; Scott, C.G.; Bailey, K.R.; Tajik, A.J.; Frye, R.L.; Enriquez-Sarano, M. Atrial fibrillation complicating the course of degenerative mitral regurgitation. J. Am. Coll. Cardiol. 2002, 40, 84–92. [Google Scholar] [CrossRef]
- Turpie, A.G.; Hirsh, J.; Gunstensen, J.; Nelson, H.; Gent, M. Randomised comparison of two intensities of oral anticoagulant therapy after tissue heart valve replacement. Lancet 1988, 331, 1242–1245. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Guimarães, P.O.; Pokorney, S.D.; Lopes, R.D.; Wojdyla, D.M.; Gersh, B.J.; Giczewska, A.; Carnicelli, A.; Lewis, B.S.; Hanna, M.; Wallentin, L.; et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: Insights from the ARISTOTLE trial. Clin. Cardiol. 2019, 42, 568–571. [Google Scholar] [CrossRef]
- De Caterina, R.; Renda, G.; Carnicelli, A.P.; Nordio, F.; Trevisan, M.; Mercuri, M.F.; Ruff, C.T.; Antman, E.M.; Braunwald, E.; Giugliano, R.P. Valvular Heart Disease Patients on Edoxaban or Warfarin in the ENGAGE AF-TIMI 48 Trial. J. Am. Coll. Cardiol. 2017, 69, 1372–1382. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Estes, N.A.M.; Fonarow, G.C.; Jurgens, C.Y.; Kittleson, M.M.; Marine, J.E.; McManus, D.D.; McNamara, R.L. 2020 Update to the 2016 ACC/AHA Clinical Performance and Quality Measures for Adults with Atrial Fibrillation or Atrial Flutter. J. Am. Coll. Cardiol. 2021, 77, 326–341. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Ezekowitz, M.D.; Nagarakanti, R.; Noack, H.; Brueckmann, M.; Litherland, C.; Jacobs, M.; Clemens, A.; Reilly, P.A.; Connolly, S.J.; Yusuf, S.; et al. Comparison of Dabigatran and Warfarin in Patients with Atrial Fibrillation and Valvular Heart Disease. Circulation 2016, 134, 589–598. [Google Scholar] [CrossRef]
- Breithardt, G.; Baumgartner, H.; Berkowitz, S.D.; Hellkamp, A.S.; Piccini, J.P.; Stevens, S.R.; Lokhnygina, Y.; Patel, M.R.; Halperin, J.L.; Singer, D.E.; et al. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur. Heart J. 2014, 35, 3377–3385. [Google Scholar] [CrossRef]
- Guimarães, H.P.; Lopes, R.D.; de Barros e Silva, P.G.M.; Liporace, I.L.; Sampaio, R.O.; Tarasoutchi, F.; Hoffmann-Filho, C.R.; de Lemos Soares Patriota, R.; Leiria, T.L.L.; Lamprea, D.; et al. Rivaroxaban in Patients with Atrial Fibrillation and a Bioprosthetic Mitral Valve. N. Engl. J. Med. 2020, 383, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Geis, N.A.; Kiriakou, C.; Chorianopoulos, E.; Uhlmann, L.; Katus, H.A.; Bekeredjian, R. NOAC monotherapy in patients with concomitant indications for oral anticoagulation undergoing transcatheter aortic valve implantation. Clin. Res. Cardiol. 2018, 107, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Seeger, J.; Gonska, B.; Rodewald, C.; Rottbauer, W.; Wöhrle, J. Apixaban in Patients With Atrial Fibrillation after Transfemoral Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 66–74. [Google Scholar] [CrossRef]
- Caldeira, D.; David, C.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease: Systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 111–118. [Google Scholar] [CrossRef]
- de Souza Lima Bitar, Y.; Neto, M.G.; Filho, J.A.L.; Pereira, L.V.; Travassos, K.S.O.; Akrami, K.M.; Roever, L.; Duraes, A.R. Comparison of the New Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation and Valvular Heart Disease: Systematic Review and Meta-Analysis. Drugs R D 2019, 19, 117–126. [Google Scholar] [CrossRef]
- Pan, K.; Singer, D.E.; Ovbiagele, B.; Wu, Y.; Ahmed, M.A.; Lee, M. Effects of Non–Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease: A Systematic Review and Meta—Analysis. J. Am. Heart Assoc. 2017, 6, e005835. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Jensen, M.; Melgaard, L.; Skjøth, F.; Nielsen, P.B.; Larsen, T.B. Stroke and bleeding risk scores in patients with atrial fibrillation and valvular heart disease: Evaluating ‘valvular heart disease’ in a nationwide cohort study. EP Eur. 2019, 21, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus Warfarin in Patients with Mechanical Heart Valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef] [PubMed]





| Study Name, Year | Country | Study Design | Population | Sample Size | Mean/Median Age (Years) | Female (%) | Intervention (DOAC vs. VKA) | Outcomes (Events) (Events in NOAC vs. Events in VKA) |
|---|---|---|---|---|---|---|---|---|
| RE-LY Ezekowitz et al., (2014) [23] | USA | Prospective, randomized trial | Atrial fibrillation and valvular heart disease | 3950 | 74.0 (68.0, 79.0) | 40.7% | Dabigatran vs. warfarin (2645 vs. 1305) | All-cause mortality (226/2645 vs. 122/1305) Stroke (77/2645 vs. 49/1305) Major bleeding (209/2645 vs. 132/1305) Intracranial bleeding (16/2645 vs. 24/1305) |
| Rocket AF Breithardt et al., (2014) [24] | USA | Multicenter, international, double-blind, double-dummy, randomized trial | Atrial fibrillation and valvular heart disease | 2003 | 75 (68.0, 79.0) | 39.4% | Rivaroxaban vs. warfarin (968 vs. 1035) | All-cause mortality (100/968 vs. 112/1035) Stroke (38/968 vs. 50/1035) Major bleeding (88/968 vs. 68/1035) Intracranial bleeding (13/968 vs. 12/1035) |
| ARISTOTLE Avezum et al., (2015) [18] | USA | Randomized, double-blind trial | Atrial fibrillation and valvular heart disease | 4808 | 71 (64.0, 77.0) | 40.3% | Apixaban vs. warfarin (2438 vs. 2370) | All-cause mortality (222/2438 vs. 215/2370) Stroke (60/2438 vs. 87/2370) Major bleeding (99/2438 vs. 119/2370) Intracranial bleeding (10/2438 vs. 34/2370) |
| ENGAGE AF-TIMI De Caterina, et al., (2017) [19] | USA | Randomized, double-blind, double-dummy trial | Atrial fibrillation and co-existing valvular heart disease | 2824 | 71.8 ± 9.4 | 42.2% | Edoxaban vs. warfarin (1869 vs. 955) | All-cause mortality (308/1869 vs. 147/955) Stroke (82/1869 vs. 50/955) Major bleeding (99/1869 vs. 89/955) Intracranial bleeding (11/1869 vs. 34/2370) |
| RIVER trial Guimarese et al., (2020) [25] | Brazil | Phase 4, multicenter, randomized, noninferiority, open-label design with blinded adjudication of outcomes | Atrial fibrillation and bioprosthetic mitral valve | 1005 | 59.3 ± 12.1 | 60.4% | Rivaroxaban vs. warfarin (500 vs. 505) | All-cause mortality (20/500 vs. 20/505) Stroke (3/500 vs. 12/505) Major bleeding (7/500 vs. 13/505) Intracranial bleeding (0/500 vs. 5/505) |
| Geis et al., (2018) [26] | Germany | Prospective cohort | Post TAVI | 326 | 83.1 ± 5.3 | 51% | DOAC vs. VKA (154 vs. 172) | All-cause mortality (12/154 vs. 11/172) Stroke (5/154 vs. 2/172) Major bleeding (3/154 vs. 3/172) Intracranial bleeding |
| Seeger et al., (2016) [27] | Germany | Prospective cohort | Post TAVR | 272 | 81.3 ± 5.9 | 40.5% | Apixaban vs. VKA (141 vs. 131) | All-cause mortality at 12 months (19/81 vs. 6/50) Stroke at 12 months (1/81 vs. 1/50) Major bleeding (5/141 vs. 7/131) Intracranial bleeding (1/141 vs. 0/131) |
| DAWA pilot, Durães et al., (2016) [10] | Brazil | Phase II, prospective, open label, randomized, pilot study | Bioprosthetic mitral and/or aortic valve replacement and post-op AF | 27 | Not given (intervention group—48.8 ± 10.4, control group—45.7 ± 6) | 63% | Dabigatran vs. warfarin (15 vs. 12) | All-cause mortality (0/15 vs. 1/12) Stroke (0/15 vs. 1/12) TIA (1/15 vs. 0/12) Major bleeding (1/15 vs. 2/12) Intracranial bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, G.; Iskandar, B.; Chelikam, N.; Jain, S.; Vyas, V.; Singla, T.; Dondapati, L.; Bombaywala, A.; Peela, A.S.; Khealani, M.; et al. Outcomes and Safety of Direct Oral Anticoagulants (DOACs) versus Vitamin K Antagonists (VKAs) amongst Patients with Valvular Heart Disease (VHD): A Systematic Review and Meta-Analysis. Hearts 2023, 4, 61-72. https://doi.org/10.3390/hearts4030008
Patel G, Iskandar B, Chelikam N, Jain S, Vyas V, Singla T, Dondapati L, Bombaywala A, Peela AS, Khealani M, et al. Outcomes and Safety of Direct Oral Anticoagulants (DOACs) versus Vitamin K Antagonists (VKAs) amongst Patients with Valvular Heart Disease (VHD): A Systematic Review and Meta-Analysis. Hearts. 2023; 4(3):61-72. https://doi.org/10.3390/hearts4030008
Chicago/Turabian StylePatel, Ghanshyam, Beshoy Iskandar, Nikhila Chelikam, Siddhant Jain, Vandit Vyas, Tanvi Singla, Lavanya Dondapati, Ali Bombaywala, Appala Suman Peela, Milan Khealani, and et al. 2023. "Outcomes and Safety of Direct Oral Anticoagulants (DOACs) versus Vitamin K Antagonists (VKAs) amongst Patients with Valvular Heart Disease (VHD): A Systematic Review and Meta-Analysis" Hearts 4, no. 3: 61-72. https://doi.org/10.3390/hearts4030008
APA StylePatel, G., Iskandar, B., Chelikam, N., Jain, S., Vyas, V., Singla, T., Dondapati, L., Bombaywala, A., Peela, A. S., Khealani, M., Mukesh, S., Korsapati, H. R., Korsapati, A. R., Regassa, H., Jain, N., Patel, U., & Venkata, V. S. (2023). Outcomes and Safety of Direct Oral Anticoagulants (DOACs) versus Vitamin K Antagonists (VKAs) amongst Patients with Valvular Heart Disease (VHD): A Systematic Review and Meta-Analysis. Hearts, 4(3), 61-72. https://doi.org/10.3390/hearts4030008







