The New ISO/IEC Standard for Automated ECG Interpretation
Abstract
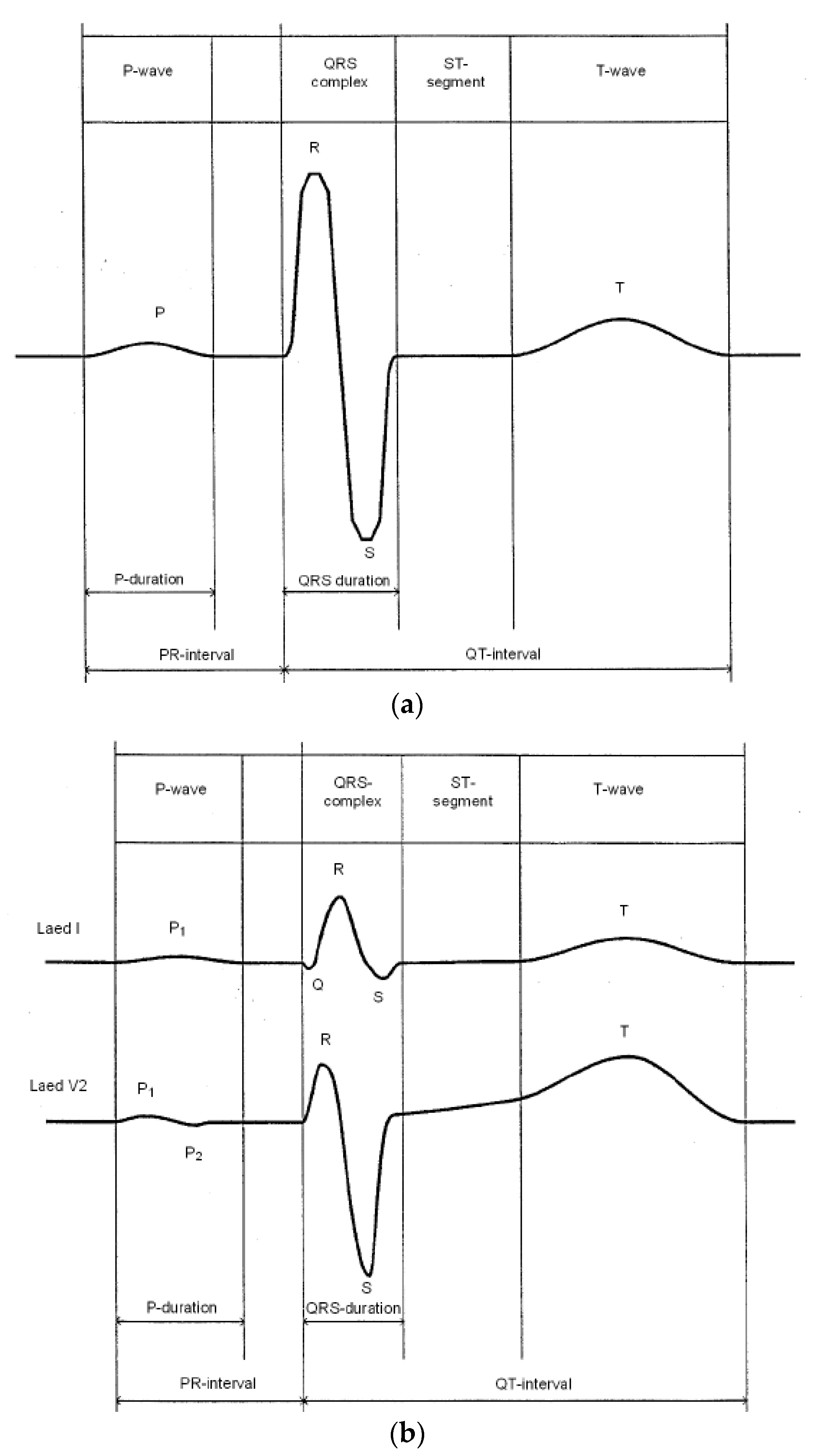
:1. Introduction
2. Background
3. Impact of 801601-2-86 on Automated ECG Interpretation
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO/IEC. 80601, Part 2-86: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographs, Including Diagnostic Equipment, Monitoring Equipment, Ambulatory Equipment, Electrodes, Cables, and Leadwires; Committee Draft; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- ANSI/AAMI/IEC. ANSI/AAMI/IEC 60601-2-25: 2011/(R)2016 Medical Electrical Equipment—Part 2-25: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographs; AAMI: Arlington, VA, USA, 2016. [Google Scholar]
- ANSI/AAMI/IEC. ANSI/AAMI/IEC 60601-2-27: 2011/(R)2016 Medical Electrical Equipment—Part 2-27: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographic Monitoring Equipment; AAMI: Arlington, VA, USA, 2016. [Google Scholar]
- ANSI/AAMI/IEC. ANSI/AAMI/IEC 60601-2-47: 2012/(R)2016 Medical Electrical Equipment—Part 2-47: Particular Requirements for the Basic Safety and Essential Performance of Ambulatory Electrocardiographic Systems; AAMI: Arlington, VA, USA, 2016. [Google Scholar]
- ANSI/AAMI. ANSI/AAMI EC12: 2000/(R)2015 Disposable ECG Electrodes; ANSI/AAMI: Arlington, VA, USA, 2015. [Google Scholar]
- ANSI/AAMI. ANSI/AAMI EC53: 2013 ECG Trunk Cables and Patient Leadwires; ANSI/AAMI: Arlington, VA, USA, 2013. [Google Scholar]
- ANSI/AAMI. ANSI/AAMI EC57-2012, Testing and Reporting Performance Results of Cardiac Rhythm and ST Segment Measurement Algorithms; ANSI/AAMI: Arlington, VA, USA, 2012. [Google Scholar]
- IEC (Ed.) International Standard IEC 60601-2-51:2003 Medical Electrical Equipment. Particular Requirements for Safety, Including Essential Performance, of Recording and Analysing Single Channel and Multichannel Electrocardiographs; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2003; p. 86. [Google Scholar]
- Young, B.; Schmid, J.J. Updates to IEC/AAMI ECG standards, a new hybrid standard. J. Electrocardiol. 2018, 51, S103–S105. [Google Scholar] [CrossRef] [PubMed]
- ANSI/AAMI. EC11:1991/(R)2001 Diagnostic Electrocardiographic Devices; ANSI/AAMI: Arlington, VA, USA, 1992; p. 39. [Google Scholar]
- ANSI/AAMI. EC13:2002 Cardiac Monitors, Heart Rate Meters, and Alarms; ANSI/AAMI: Arlington, VA, USA, 2002; p. 79. [Google Scholar]
- ANSI/AAMI. EC38:1998 Ambulatory Electrocardiographs; ANSI/AAMI: Arlington, VA, USA, 1999; p. 58. [Google Scholar]
- IEC (Ed.) International Standard 60601-2-25:2011 Medical Electrical Equipment. Particular Requirements for Safety, Including Essential Performance, of Recording and Analysing Single Channel and Multichannel Electrocardiographs; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2011; p. 190. [Google Scholar]
- Dower, G.E.; Yakush, A.; Nazzal, S.B.; Jutzy, R.V.; Ruiz, C.E. Deriving the 12-lead electrocardiogram from four (EASI) electrodes. J. Electrocardiol. 1988, 21, S182–S187. [Google Scholar] [CrossRef]
- Drew, B.J.; Pelter, M.M.; Brodnick, D.E.; Yadav, A.V.; Dempel, D.; Adams, M.G. Comparison of a new reduced lead set ECG with the standard ECG for diagnosing cardiac arrhythmias and myocardial ischemia. J. Electrocardiol. 2002, 35, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hongo, R.H.; Goldschlager, N. Status of Computerized Electrocardiography. Cardiol. Clin. 2006, 24, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Smulyan, H. The Computerized ECG: Friend and Foe. Am. J. Med. 2019, 132, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Willems, J.L. Common standards for quantitative electrocardiography. Second progress report. In Proceedings of the CSE Conference, Leuven, Belgium, 20 November 1982; pp. 1–246. [Google Scholar]
- Willems, J.L.; Arnaud, P.; van Bemmel, J.H.; Bourdillon, P.J.; Brohet, C.; Dalla Volta, S.; Andersen, J.D.; Degani, R.; Denis, B.; Demeester, M.; et al. Assessment of the performance of electrocardiographic computer programs with the use of a reference data base. Circulation 1985, 71, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Zywietz, C.; Mertins, V.; Willems, J.L. Quality assurance of interpretative electrocardiographs: Development of testing services in the European communities-first results. In Proceedings of the [1990] Proceedings Computers in Cardiology, Chicago, IL, USA, 23–26 September 1990; pp. 181–184. [Google Scholar]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part I: The Electrocardiogram and Its Technology. A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Circulation 2007, 115, 1325–1332. [Google Scholar]
- Schläpfer, J.; Wellens Hein, J. Computer-Interpreted Electrocardiograms. J. Am. Coll. Cardiol. 2017, 70, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Salerno, S.M.; Alguire, P.C.; Waxman, H.S. Competency in interpretation of 12-lead electrocardiograms: A summary and appraisal of published evidence. Ann. Intern. Med. 2003, 138, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Laks, M.M.; Selvester, R.H.S. Computerized Electrocardiography—An Adjunct to the Physician. N. Engl. J. Med. 1991, 325, 1803–1804. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.L.; Fridsma, D.B.; Gatti, G. Computer decision support as a source of interpretation error: The case of electrocardiograms. J. Am. Med. Inform. Assoc. 2003, 10, 478–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.P.; Rubin, S.A. Errors in the computerized electrocardiogram interpretation of cardiac rhythm. J. Electrocardiol. 2007, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- DeCamilla, J.; Xia, X.; Wang, M.; Wade, J.; Mykins, B.; Zareba, W.; Couderc, J.P. The multiple arrhythmia dataset evaluation database (MADAE). J. Electrocardiolog 2018, 51, S106–S112. [Google Scholar] [CrossRef] [PubMed]
- FDA (Ed.) Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SAMD) Action Plan; FDA: Silver Spring, MD, USA, 2021. [Google Scholar]
- Corabian, P. Accuracy and Reliability of Using Computerized Interpretation of Electrocardiograms for Routine Examinations; HTA 25: Series A; Alberta Heritage Foundation for Medical Research: Edmonton, AB, Canada, 2002. [Google Scholar]
- Farrell, R.M.; Rowlandson, G.I. The effects of noise on computerized electrocardiogram measurements. J. Electrocardiol. 2006, 39, S165–S173. [Google Scholar] [CrossRef] [PubMed]
- Zywietz, C.; Willems, J.L.; Arnaud, P.; van Bemmel, J.H.; Degani, R.; Macfarlane, P.W. Stability of computer ECG amplitude measurements in the presence of noise. Comput. Biomed. Res. 1990, 23, 10–31. [Google Scholar] [CrossRef]
| Standard Designation | Title |
|---|---|
| AAMI EC11 [10] | Diagnostic electrocardiographic devices |
| AAMI EC12 [5] | Disposable ECG Electrodes |
| AAMI EC13 [11] | Cardiac monitors, heart rate meters, and alarms |
| AAMI EC38 [12] | Ambulatory electrocardiographs |
| AAMI EC53 [6] | ECG cables and leadwires |
| AAMI EC57 [7] | Testing and reporting performance results of cardiac rhythm and ST segment measurement algorithms |
| IEC 60601-2-25 [2] | Medical electrical equipment—Part 2-25: Particular requirements for the basic safety and essential performance of electrocardiographs |
| IEC 60601-2-27 [3] | Medical electrical equipment—Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment |
| IEC 60601-2-47 [4] | Medical electrical equipment—Part 2-47: Particular requirements for the basic safety and essential performance of ambulatory electrocardiographic systems |
| IEC 60601-2-51 [8] | Medical electrical equipment—Part 2-51: Particular requirements for the basic safety and essential performance of recording and analyzing single channel and multichannel electrocardiographs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, B.; Schmid, J.-J. The New ISO/IEC Standard for Automated ECG Interpretation. Hearts 2021, 2, 410-418. https://doi.org/10.3390/hearts2030032
Young B, Schmid J-J. The New ISO/IEC Standard for Automated ECG Interpretation. Hearts. 2021; 2(3):410-418. https://doi.org/10.3390/hearts2030032
Chicago/Turabian StyleYoung, Brian, and Johann-Jakob Schmid. 2021. "The New ISO/IEC Standard for Automated ECG Interpretation" Hearts 2, no. 3: 410-418. https://doi.org/10.3390/hearts2030032
APA StyleYoung, B., & Schmid, J.-J. (2021). The New ISO/IEC Standard for Automated ECG Interpretation. Hearts, 2(3), 410-418. https://doi.org/10.3390/hearts2030032





