Abstract
Ever since the first publication of the standard communication protocol for computer-assisted electrocardiography (SCP-ECG), prENV 1064, in 1993, by the European Committee for Standardization (CEN), SCP-ECG has become a leading example in health informatics, enabling open, secure, and well-documented digital data exchange at a low cost, for quick and efficient cardiovascular disease detection and management. Based on the experiences gained, since the 1970s, in computerized electrocardiology, and on the results achieved by the pioneering, international cooperative research on common standards for quantitative electrocardiography (CSE), SCP-ECG was designed, from the beginning, to empower personalized medicine, thanks to serial ECG analysis. The fundamental concept behind SCP-ECG is to convey the necessary information for ECG re-analysis, serial comparison, and interpretation, and to structure the ECG data and metadata in sections that are mostly optional in order to fit all use cases. SCP-ECG is open to the storage of the ECG signal and ECG measurement data, whatever the ECG recording modality or computation method, and can store the over-reading trails and ECG annotations, as well as any computerized or medical interpretation reports. Only the encoding syntax and the semantics of the ECG descriptors and of the diagnosis codes are standardized. We present all of the landmarks in the development and publication of SCP-ECG, from the early 1990s to the 2009 International Organization for Standardization (ISO) SCP-ECG standards, including the latest version published by CEN in 2020, which now encompasses rest and stress ECGs, Holter recordings, and protocol-based trials.
1. Introduction
The electrocardiogram was the very first biosignal ever processed by computers, pioneered by Cesar Caceres at the National Institute of Health (NIH) and Hubert Pipberger from the Veterans Administration (VA) [1]. In the early 1960s, the problem with digital ECG processing was the lack of commercial amplifiers and frequency modulation (FM) recorders for multichannel recordings of the 12-lead ECGs. The standard 12-lead ECG signals were transmitted—lead-by-lead or 3-leads at a time—in analog form by means of FM modems to central computing facilities, and the interpretation results were returned in a textual format by means of teletypes.
Significant changes took place in computer-based ECG analysis and interpretation in the late 1970s and in the 1980s, thanks to the introduction of minicomputers and PCs and the development of microcomputer-based ECG interpreting carts [1,2,3]. Machine intelligence could be brought at the bedside of the patient.
Early evaluation studies of computer-based ECG measurements and interpretations demonstrated the need for the development of reference databases to assess the performance of ECG measurements and interpretation programs [4,5,6,7,8,9], and the use of ECG-independent evidence as the gold standard to assess the diagnosis accuracy of ECG interpretation programs [6,7,8,10]. There was a lack of agreement concerning the definitions of waves, common measurements, standardized criteria for classification, and common terminology for reporting. This created a situation whereby large differences in measurements by different computer programs hampered not only the exchange of diagnostic criteria, but also of ECG measurements and their uses for decision support and clinical studies [4,5,6,7,8,9]. To solve these issues, a group of several European investigators, led by the late Jos L Willems (J.L.W.), proposed, in 1978, a concerted action to different advisory councils of the European Union (at that time, called European Community, abbreviated EC), aimed at establishing “Common Standards for Quantitative Electrocardiography” (abbreviated CSE) [11]. The proposal was officially approved in 1980 with three other European projects, in the second research and development program for medical and public health research of the EC, and extended during the two following European research and development programs (up until 1990). The objectives were to standardize the ECG measurement procedures in quantitative terms, and to develop methodologies and reference databases to assess the accuracy of ECG measurement programs and the diagnostic performances of computer interpretation programs against the combined wave delineation and interpretative results of a panel of highly skilled cardiologists (and against the clinical truth).
The (approximate) 100 person-years pioneering work is a leading example of how to assess medical information processing techniques [10,11,12,13,14,15,16,17,18,19,20,21,22]. CSE databases and measurement libraries are now widely used to assess the quality of ECG measurements and interpretation programs as required by some clauses of the International Electrotechnical Commission (IEC) 60621-2-25 and IEC 60621-2-51 standards [23,24], and are the starting points of new research programs to improve ECG processing and interpretation methodologies.
One interesting result (among the lessons learned during CSE) is that, as is also the case for panel reviews, computer interpretation could be further improved by combining the interpretation results of individual programs [10,17,20,21], a direct result of the application of the Central Limit Theorem. However, it was also shown that, when analyzing a single standard 10 s rest ECG, at the very best, a diagnostic accuracy of about 80 to 82% can be obtained [10,17,20,21], and that further improving the diagnostic performance of the ECG can only be obtained by analyzing, as recommended by several international cardiology societies [25,26], the trend of serial ECG changes, with reference to baseline recordings [25,26,27,28,29], and/or by designing advanced, data fusion-based artificial intelligence (AI) decision support systems, merging ECG signal data (or measurements) and demographic and clinical data featured in the patient’s electronic health record (EHR) [30,31]. However, the lack of open communication and interlinking of various types of ECG equipment and software, and the difficulty of overcoming the proprietary solutions proposed by the manufacturers for the exchange of ECG data, had to be solved.
To open this field, J.L.W., Jan van Bemmel, P.W.M., P.R. and the late Rosanna Degani and Christoph Zywietz (C.Z.) launched, in 1989, an international collaboration project called SCP-ECG, aimed at developing a “Standard Communications Protocol for computerized electrocardiography”, consisting of standards for the interchange, encoding, and storage of digital ECG data [32,33,34,35,36]. The development was achieved under the aegis of the preliminary Advanced Medical Informatics (AIM) program of the EC, in close collaboration with representatives from academia and industry.
In the following, we first present the main achievements of the AIM A1015 SCP-ECG project. We then recall the different steps of the development of the first two main versions, V1.x and V2.x, of European Norm (EN) 1064 and the International Organization for Standardization (ISO) 11073-91064 “standard communication protocol–computer-assisted electrocardiography” standards [37,38]. Finally, we present the main content of the latest version SCP-ECG V3.0 of the standard, approved by the European Committee for Standardization, or, in French, Comité Européen de Normalisation (CEN) as EN 1064:2020 22 June 2020 [39].
2. Main Achievements of the AIM A1015 SCP-ECG Project
The basic aim of the SCP-ECG project was to arrive at a standard communications protocol for computerized electrocardiography, to make possible the interconnection and exchange of ECG data between different computerized ECG acquisition and analysis devices of various manufacturers, preserve the quality of the original signals, to provide the data and metadata that are relevant for decision support and/or for reanalysis and interpretation by another device or system, and to facilitate the integration of ECG signals and data with other information systems for departmental, hospital, or ambulatory care.
To reach this goal, the project was divided into three closely related work packages, respectively dealing with the exchange of digital ECG data (Work Package 1), encoding and compression (Work Package 2), and storage (Work Package 3) [32,33]. The three work packages were simultaneously worked out and their issues extensively discussed during three working conferences held in Leuven (Belgium) in 1989 and 1990. The conferences were attended by 45 experts in the field, from 11 countries (USA, Japan, and Europe), including representatives of 13 leading manufacturers, responsible, at that time, for the production of over 80% of the world market share of computerized electrocardiographs.
Extensive proceedings were published for the first conference. During the last SCP-ECG conference, consensus was obtained among the different participants with respect to the final SCP-ECG project report [40], which provided a set of recommendations and a first draft of the agreed SCP-ECG standard communication protocol.
In the following, we briefly present the different achievements performed by the three work packages WP1–WP3. In Chapter 3, we present the different steps performed to obtain the draft SCP-ECG communication protocol approved by CEN and, further, by the US American National Standards Institute (ANSI) and ISO.
2.1. WP1: Standards for Digital ECG Data Interchange
The main goal of this work package was to develop a universal protocol, acting as a functional standard, and making recommendations as to how (and when) a given standard should be used for two-way digital ECG data transmission and communication between heterogeneous ECG computer systems. Three specific objectives were addressed:
- Definition of the data content and format of the ECG records to be exchanged, including the ECG signal data, demographic and acquisition data, as well as measurement and interpretation results;
- Definition of specific query and control messages, to initiate and control the flow of digital ECG data between different devices or users;
- Selection of the transport communication protocol and application services (A profiles) for the transfer of digital ECG data, such as File Transfer, Access and Management (FTAM), the Message Handling Services of X.400, Electronic Data Interchange For Administration, Commerce and Transport (EDIFACT), or others.
Various existing protocols from different research groups and manufacturers of computerized electrocardiographs were first examined. The VA transmission protocol [41] was then used as a starting point to define the content and data formats for the interchange. After several discussions and amendments, agreement was achieved on structuring the digital ECG data and metadata into the following 11 data sections, corresponding to the different steps of data acquisition and analysis, preceded by a header called Section 0, and containing pointers to Sections 1 to 11 in the record:
- Section 0: pointers to data areas in the record;
- Section 1: header information—patient data/ECG acquisition data;
- Section 2: Huffman tables used in encoding of ECG data, if used;
- Section 3: ECG lead definition section;
- Section 4: QRS locations, if medians are encoded;
- Section 5: encoded median data, if medians are stored;
- Section 6: encoded rhythm data if no medians are stored, or “error signal” after median subtraction, if medians are stored;
- Section 7: global measurements, wave onsets and offsets as well as global intervals;
- Section 8: textual diagnosis from the “interpretative” device;
- Section 9: manufacturer specific diagnostic and overreading data from the “interpretative” device;
- Section 10: lead measurement results, including the duration and amplitudes of major ECG waves (P+,P-,Q,R,S,R’,S’,J,T+,T-);
- Section 11: universal interpretative statement codes.
The messaging part of the agreed draft SCP-ECG standard describes the type of information that could be requested and transmitted, and the format of the message headers. Standard query request functions for networked or remotely connected ECG machines, terminals, workstations, ECG management systems, and hospital information systems were also defined, but in these early times, it was only required that every system, at minimum, be able to produce on demand the final interpretative report and the final measurement matrix. Future systems should provide means for interactive communication sessions, with requests that will automatically be transformed into Structured Query Language (SQL) queries.
2.2. WP2: Standards for Digital ECG Data Encoding
Although to a lesser degree than medical imaging, electrocardiography results in large amounts of digital data compared to other medical data, such as patient history, diagnostic codes, and biomedical laboratory data. Thus, data reduction was (and still is) highly desirable, especially in these early times, when transmission was performed over the normal telephone network. This required the use of efficient encoding and, eventually, encryption techniques.
The SCP-ECG project has opened up this field by soliciting publication and comparison of various algorithms. These experiments have demonstrated that, at the end, compression ratios of more than 20 could be obtained without significant loss of information for the so-called residual rhythm record, and with no loss at all for the representative ECG cycle (Figure 1). Based on this analysis, recommendations on minimum performance requirements were proposed. Manufacturers were free to apply their own methods. However, each manufacturer had to clearly define the degree to which signals could be reproduced after decompression, and to classify their systems into one of the following three categories:
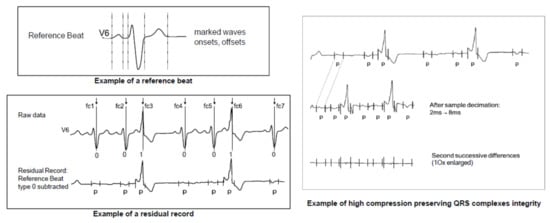
Figure 1.
Example of the high compression obtained by subtracting a reference beat from all complexes (including extrasystoles), filtering and sample decimation of the non-protected areas, and second difference calculations [42]. These types of lossy compression schemes, e.g., filtering, sample decimation, and beat subtraction, are no longer allowed in SCP-ECG V3.0 (See Chapter 4 and [39]). Figure adapted from EN 1064:2020 [39].
Category A: systems that could provide a fully reproducible ECG record, so that each sample can be reconstructed, as it has been obtained from the analog to digital converter with its original specifications.
Category B: systems that could provide a fully reproducible representative cycle and a minimally distorted so-called residual record. Consensus was obtained on the minimum requirements for data loss and on a general approach to ECG compression [42].
Category C: systems that perform data compression, but do not specify the amplitude/time distortion of their algorithms.
2.3. WP3: Conceptual Reference Model for Digital ECG Data Storage
The main objective of this work package was to develop a conceptual reference model for digital ECG storage and retrieval, according to different scenarios of use, e.g., for serial comparison, display on a remote workstation, integration in the patient’s EHR, different managerial and research purposes, such as statistics for management, evaluation of program performance and, eventually, re-analysis by a modified or totally different ECG program.
The conceptual reference model developed during the SCP-ECG project [43,44] was then further refined throughout the Open European Data Interchange and Processing for computerized Electrocardiography (OEDIPE) project, to fully comply with SCP-ECG V1.0, to improve its performance, in terms of implementation and use, and to support the setting up of generic, dynamic, and patient-specific management and control strategies of serial analysis processes [27,45,46,47,48].
The overall core model was designed around the concept of a relational database, which allows various data objects to be linked together via defined relations, based on the J. Martins entity–relationship formalism [48]. It was split into six sub-models, each centered on a major processing task that an ECG database should be able to support. Such a modular architecture was proven to ease the set-up of the different databases implemented during the OEDIPE project, whatever the clinical applications and the scenarios of use. The complete conceptual core data model was then obtained by merging the six sub-models into one model that was composed of about 50 tables and 200 attributes. It allowed the storage of multiple analysis and interpretation results of a given ECG, as well as their traceability. A snapshot of the ECG data acquisition model is displayed in Figure 2.
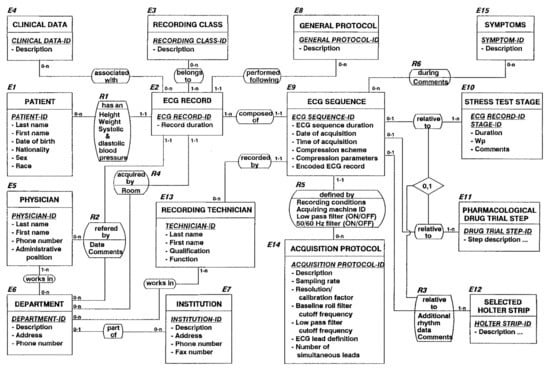
Figure 2.
Snapshot of the conceptual ECG data acquisition reference model developed during the AIM A1015 SCP-ECG project and fully implemented during the OEDIPE project [40,43,48,49,50,51].
Application area include rest, stress testing and Holter ECG, and the management of ECGs during pharmacological drug trials [44].
3. Development of SCP-ECG Versions V1.x and V2.x and Finalization of the First Versions of the Official EN 1064, ANSI EC71, and ISO 11073-91064 Standards
The final document of the recommendations and agreed transmission protocol developed from 1 June 1989 to 31 December 1990 during the AIM A1015 project was then further worked out with the support of DG XIII/F (Directorate-General XIII for Telecommunications, Information Industries, and Innovation, renamed DG CNECT: Directorate-General for Communications Networks, Content and Technology in 2012) of the European Commission, until February 1993, when it was approved by CEN as an official pre-standard, known as SCP-ECG V1.0 and identified by CEN as prENV 1064:1993.
Preparation of the final draft of this first version of the official SCP-ECG standard was undertaken by project team PT007 (coordinated by J.LW., C.Z. and P.R.) of the newly created CEN TC 251, the so-called Technical Committee (TC) for Medical Informatics established by the technical board (BT) of the European Standardization Committee CEN in March 1990. CEN TC 251 was later renamed Technical Committee on Health Informatics, and is nowadays the counterpart of ISO TC 215, which was created in 1998.
Concomitantly to the finalization of the SCP-ECG V1.0 draft—different research teams, led by J.F., P.R., J.L.W., and C.Z., and some associated industrial partners fully implemented the proposed SCP-ECG standard and carried out different experimentations within the frame of the AIM A2026 OEDIPE project (1992–1995) [27,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59], and J.L.W. and C.Z. set-up Conformance Testing Services (CTS) within the frame of the CTS-ECG project [60].
In the following, we briefly present the main SCP-ECG related achievements of the OEDIPE and CTS-ECG projects. We then briefly describe the content and the differences between SCP-ECG versions V1.x and V2.x, and we summarize the objectives and accomplishments of the OpenECG project [61].
3.1. Objectives and Main Achievements of the AIM A2026 OEDIPE Project
The ultimate goal of the OEDIPE Project (project leader P.R.) was to develop demonstration systems for the follow-up of cardiac-diseased patients, integrating serial analysis, decision support, open databases, and communication protocols [27,46,47]. OEDIPE successfully implemented and fully tested the SCP-ECG standard communications protocol and the related compression algorithms on stand-alone, industry standard microprocessor-based ECG carts from two different manufacturers [52]. Another successful step towards the electronic data interchange of digital ECGs was the implementation and the operational testing of a low level, Xmodem-based ECG file transfer protocol and an Enhanced Xmodem file transfer protocol [53], along with implementation and testing of the previously mentioned conceptual ECG data storage reference model, and the development of a general scheme, called “file to database”, which allowed the extraction of the data contained in an SCP-ECG file and the automation of their storage in different fields of a relational database (and vice versa) [49,50,51,62], as illustrated by Figure 3.
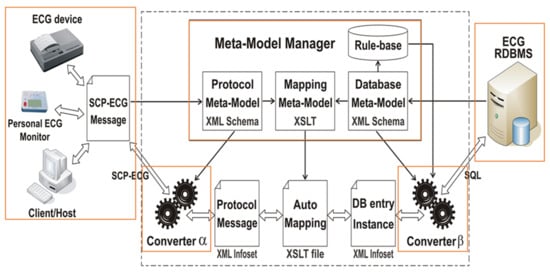
Figure 3.
Generic bi-directional SCP-ECG message to database interface schema. This is an updated version of the original “File to Database” schema developed by the OEDIPE project [49], where eXtensible Markup Language (XML) and eXtensible Stylesheet Language Transformations (XSLT) tools were based on Abstract Syntax Notation 1 (ASN.1) [50,51]. The interface updates the database with electrocardiographic information coming from the messages and gives the message handler data retrieved from the database. The solution contains generic software modules independent of the database and SCP-ECG protocol layout. It accesses a descriptive data dictionary containing the database structure, the data format layout, and the mapping between both. The design involves issues related to structure description and standard query language generation and allows automating the development of SCP-ECG Vx.i to Vy.j converters. For more details, see [50,62,63].
Operational testing of the implemented SCP-ECG protocol also included (i) the follow-up of patients who suffered from acute myocardial infarction during transportation in the emergency car or after admission to a coronary care unit; (ii) the follow-up of patients during experimental drug studies; and (iii) the follow-up of heart transplant patients.
OEDIPE was nominated as one of the two best Advanced Informatics in Medicine projects and selected for demonstration during the showcase “Building the Information Society for the Citizens of the World”, organized at the initiative of (then) U.S. Vice President Al Gore, within the frame of the G7 Ministerial Conference on Global Information Society held in Brussels, 25–26 February 1995. The objective of the showcase, entitled “Cardiology Network Berlin, Lyon”, was to demonstrate a comprehensive healthcare scenario covering several phases of treatment of patients suffering from a chronic coronary disease. An OEDIPE cardiology workstation was used to retrieve and display the patient’s health records (clinical data, ECGs, images, etc.), to transmit an ECG from a real ambulance, using an SCP-ECG compliant electrocardiograph via a global system for mobile (GSM) communication-based digital service (note that general packet radio services (GPRSs) were not yet operational), to use the patient’s smart card to identify and access health records, retrieve the patient’s previous ECG records from the SCP-ECG conceptual reference model-compliant Lyon Cardiology Hospital ECG database management system (DBMS) in near-real time, by means of an 128 Kbps Integrated Services Digital Network (ISDN) link; and retrieve some of the patient’s cardiac ultrasound and angiography images from the Deutsches HerzZentrum Picture Archiving and Communication System (PACS) in Berlin, by means of a broadband 35 Mbps asynchronous transfer mode (ATM) network.
A similar demonstrator, using an OEDIPE workstation, an SCP-ECG compliant interpreting electrocardiograph, and an International Maritime Satellite organization (INMARSAT-C) satellite link was set-up to conduct remote follow-up of a subject with cardiac disease (a former French Minister) during his transatlantic race in November 1995.
3.2. Objectives and Main Achievements of the CTS-ECG Project
Concomitantly to the SCP-ECG project, J.L.W. and C.Z. worked out a proposal for the Conformance Testing Services (CTS) program of DG XIII, of the European Commission [60]. The objective was to promote harmonized European testing services by setting-up at least two laboratories in two different countries that would be capable of implementing, executing and, if necessary, further refining IEC specifications, with regard to the performance and safety of computer-based electrocardiographs, and the minimum requirements worked out by other international groups or standards (e.g., the American Heart Association (AHA), CSE, Association for the Advancement of Medical Instrumentation (AAMI), SCP-ECG, EN, ISO, Consultative Committee on International Telephone and Telegraph, now International Telecommunication Union-Technical (CCITT)), along various technical steps, from signal acquisition to the measurement, and the transmission and communication of ECG data.
The 4-year CTS-ECG project (July 1989–June 1993) successfully implemented two pilot test centers, at the University of Leuven (Belgium) and at the Medical School in Hanover (Germany), and developed a PC-based Conformance Testing Services test bench, as well as a set of tools, including a database of test and calibration signals to test both analogue-based conventional electrocardiographs and electrocardiographs with integrated signal processing capabilities, e.g., filtering, ECG measurement and interpretation, signal compression and communication, according to the SCP-ECG standard [64,65,66].
The CTS-ECG database includes 16 calibration and three analytical ECGs, five type of noise signals, and a few ECGs, to be used for testing the conformance with the minimum requirements for ECG compression and decompression of the SCP-ECG standard. A subset of this database and the corresponding reference values, also known as CTS ECG Test Atlas, containing 14 calibration and three analytical signals, have been included in the IEC 60601-2-25:2011 standard [23], and are currently used to test the essential performance and accuracy of some of the characteristics and measurement functions of electrocardiographs.
3.3. Outline of prENV 1064:1993 SCP-ECG V1.0
prENV 1064:1993 consists of a 145-page document organized in eight chapters (called Clauses) and five annexes. It relates to the conventional recording of the electrocardiogram, i.e., the so-called standard 12-lead electrocardiogram and the vectorcardiogram (VCG). The clinical need to transmit specialized recordings such as body surface maps, Holter and long-term ECG recordings was very low in these early times, and was therefore not considered in this SCP-ECG V1.0 version of the official SCP-ECG standard.
SCP-ECG V1.0 defines the common conventions required for digital transmission of patient-specific data (demographic, recording metadata, etc.), ECG signal data, ECG measurement, and ECG interpretation results between digital electrocardiographs (ECG carts), computer ECG management systems (ECG DBMS), and any other type of computer system (hosts).
The standard specifies the content and structure of the information, which is to be interchanged, provides minimum requirements for encoding and compression of digital ECG data, and defines a set of specific query and control messages that may be used to initiate and control the flow of data for cart-to-host and host-to-host communication between different users.
The various data that may be transmitted by means of the standard ECG communications protocol are defined in Clause 5 of SCP-ECG V1.0. The data are organized in 11 sections as defined during the SCP-ECG A1015 project and enumerated in 2.1. For the sake of usability of the standard, only sections 0 and 1 are mandatory, all other sections are optional.
Although a large number of parameters of Section 1 may be transmitted, most devices will only send a subset of that number. The formatting of data in Section 1 was, thus, made flexible by introducing tagged fields (illustrating the use of tagged fields can be found hereafter in Figure 4), with the provision that, at minimum, the following set of patient demographics and ECG acquisition data must be present: Patient ID number; patient name; sex and birthdate; identification of acquiring cardiograph; date and time of ECG recording; identification of the analyzing device. Accurate patient identification of ECG data and reports is, of course, of utmost importance for data queries and replies.
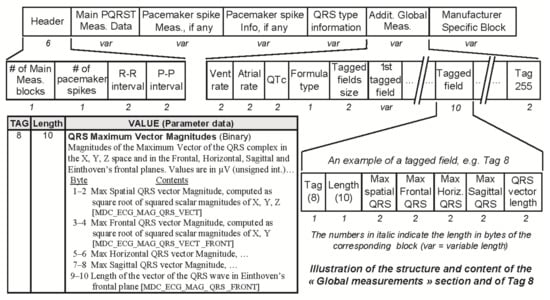
Figure 4.
Snapshot of the data part of Section 7 (global measurements), highlighting the structure of the additional global measurements data block and of one of the optional tagged fields, e.g., Tag 8, “QRS Maximum Vector Magnitudes”. SCP-ECG V3.0 defines 17 tagged global ECG measurement data fields, numbered from 0 to 16. The structure and content of tag 8 are detailed in the bottom left (tag, length, value) table. The number of tagged fields actually stored may vary from one SCP-ECG record to another.
Minimum requirements for data encoding and compression are defined in Clause 6. A minimum set of control and query messages for cart-to-cart and cart-to-host interchange are defined in Clause 7. A low-level transport protocol for the exchange of data between an ECG cart and a host based on an enhanced X-Modem protocol is defined in Clause 8.
Annex A provides specifications for encoding alphanumeric ECG data in a multilingual environment, so that the standard can be applied worldwide (the nowadays widely used UTF-8, universal coded character set transformation format–8-bit, did not exist yet). Annex B presents a detailed description of the universal ECG interpretation statement codes (to be used for coding Section 11) that were developed during the SCP-ECG project and extended under the PT007 mandate. Annex C (informative) provides a detailed description of the recommended signal compression methodology and outlines the minimum conformance requirements for ECG data compression. Annex D specifies how to encode the level of compatibility of the devices and systems with the SCP-ECG standard, according to four levels of compliance. The last three annexes (informative) provide a glossary of specific terms used in SCP-ECG (Annex E), a list of additional references (Annex F), and a subject index (Annex G).
3.4. From SCP-ECG V1.0 to V1.3
In 1997, AAMI set-up a working group with the mission to reformat and update the contents of ENV 1064:1993 and to adopt it as an ANSI/AAMI standard. Several updated proposals were drafted by the AAMI working group in close cooperation with CEN TC 251 WG4, and then extensively discussed during seven AAMI SCP-ECG WG meetings, also attended by experts having developed ENV 1064:1993.
The final draft, dated 11 September 1999, and identified as SCP-ECG V1.3, was then approved on 11 May 2001 by ANSI as an official ANSI standard catalogued ANSI/AAMI EC71:2001. The ANSI/AAMI standard was revised in 2007 (ANSI/AAMI EC71:2001/(R)2007) and reaffirmed by ANSI 10 September 2013 (ANSI/AAMI EC71:2001/(R)2013).
The main differences between SCP-ECG V1.0 and V1.3 (other than the renumbering of a few clauses and annexes and the introduction of some additional explanatory sentences for the purpose of clarification) are listed hereafter:
- Deprecation of the acquiring device identification encoding scheme, which is now identified by a text string;
- Extension of the language support encoding scheme;
- Amendment of the ECG device capabilities encoding scheme;
- Introduction of two new tags to support electrode configuration and date time zone encoding, and a tagged field to store the patient’s medical history in free text;
- Extension of the number of supported ECG leads from 65 to 85;
- Extension of the content of Section 7 global ECG measurements and provision of means to store the QRS type for each detected QRS, the type and source of each detected pacemaker spike (if any), and some additional global measurements in tagged fields (see example of tagged global ECG measurements data field in Figure 4);
- Extension of the confirmation status encoding possibilities in sections 8 and 11;
- Extension of the number of supported per-lead ST measurements;
- Introduction of a set of state diagrams in Clause 7 “Definition of a minimum set of control and query messages for the interchange of ECG data”, describing the process by which ID messages are exchanged by cart and host devices;
- Amendment and substantial extension of former Annex D “Definition of compliance with the SCP-ECG standard” (normative), which becomes Annex B (normative).
3.5. From ENV 1064:1993 to EN 1064:2005+A1:2007 and ISO 11073-91064:2009
On 17 December 2004, CEN approved a slightly reformatted version of ANSI/AAMI EC71:2001 as EN 1064:2005, also known as SCP-ECG V2.1. The main differences between SCP-ECG V1.3 and SCP-ECG V2.1 are the move of Clause 7 “Definition of a minimum set of control and query messages for the interchange of ECG data” and Clause 8 “Standard low-level ECG-Cart to host protocol” (both normative) to annexes D and E (both informative).
In 2006, CEN TC 251 Working Group 4 (WG4) further worked out SCP-ECG to take account of new requirements. This updated version identified as SCP-ECG V2.3 was then approved by CEN on 15 January 2007, as standard EN 1064:2005+A1:2007 and by ISO in May 2009 as standard ISO 11073-91064:2009. The latter was reconfirmed by ISO in 2017.
The main differences between SCP-ECG V2.1 and SCP-ECG V2.3 are:
- Extension of the number of supported ECG leads from 85 to 184;
- Amendment of the ECG leads descriptions in order to cross reference the SCP-ECG electrode names and codes with the MDC_ECG_LEAD REFIDs (nomenclature code REFerence IDentifier) from the newly adopted ISO/IEEE 11073-10101:2004 standard;
- Reduction from four to two, concerning the number of data format categories (specified in Annex B “Definition of compliance with the SCP-ECG standard”). The latter are used to encode the type of SCP-ECG related features and information content provided by a specific device.
3.6. Objectives and Main Achievements of the OpenECG Project
Most smaller companies have successfully implemented the different versions of SCP-ECG, but not the largest companies, which preferred to protect their market shares by selling quite expensive integrated departmental ECG analysis and management systems uniquely based on their old proprietary interchange protocols. Increased need for interoperability between medical devices and systems and new requirements coming from new applications, e.g., in the context of image processing using Digital Imaging and Communications in Medicine (DICOM), clinical studies, drug approval by the USA Food and Drugs Administration (FDA), telemedicine and home care, and the EHR, triggered the set-up of the two-year (July 2002–June 2004) European Information Society Technologies (IST)–Thematic Networks Program IST-2001-37711 OpenECG project (project leader Catherine Chronacki) [61,67,68]. The objective was to promote the consistent use of existing interchange format and communication standards for computer-assisted resting electrocardiography, to consolidate expertise, assist integration, and support correct implementations of the ECG interoperability standards, to pave the way towards developing similar standards for exercise and Holter ECGs [61,67,68,69,70,71].
To reach these goals, OpenECG set up an internet portal (www.OpenECG.net, which is unfortunately no longer active since the original OpenECG domain has expired in 2014) [70] to support information exchanges between a steadily growing network of registered OpenECG members (682 members from 56 countries in September 2006) [71] and to grant the OpenECG community access to data and tools for SCP-ECG conformance testing, a database containing annotated samples of real SCP-ECG files, Open Source SCP-ECG viewers [72], and two way converters between SCP-ECG and other standards such as HL7 (Health Level Seven) aECG [73] and DICOM 3.0 (supplement 30) [74], etc.
OpenECG also organized two workshops, in October 2002 in Crete (Greece) and in April 2004 in Berlin (Germany), bringing together about 60 users, manufacturers, healthcare providers, and standardization experts from CEN, AAMI, IEEE, ISO, and IEC, with the objective to consolidate experience and provide practical feedback on user experiences and manufacturer thoughts, ECG interchange formats and viewers, medical devices interoperability with the EHR, harmonization with the different ECG formats from other application areas, related R&D initiatives and trends in Europe, and future challenges [68,69].
The experience and feedback gained during the OpenECG project was taken into account for the development of SCP-ECG V2.3 and V3.0.
4. Development of SCP-ECG V3.0 and EN 1064:2020
According to the policy of CEN, each standard needs to be revised every 5 years. To this end, an EN 1064 SCP-ECG revision kickoff meeting was organized by A.V., convenor of CEN/TC 251/WG IV, in the premises of the CEN and the European Committee for Electrotechnical Standardization (CENELEC) meeting Center in Brussels (Belgium), 25 November 2013. An SCP-ECG revision project team lead by P.R. and A.S. has been nominated, with the mission of keeping useful and up-to-date parts of the standard more or less intact, removing or revising the outdated parts and, if relevant, extending the standard by including new rest ECG measurements and additional recording modalities with related metadata, measurements, and annotations.
The SCP-ECG revision project team first performed a “strengths, weaknesses, opportunities, and threats” (SWOT) analysis and an in depth review of the literature on the SCP-ECG standard [75,76,77,78,79] and complementary standards such as the newly approved ISO/IEEE 11073-10102 “Nomenclature—Annotated ECG” standard [80], the under revision version of the ISO/IEEE 11073-10101 “Nomenclature” standard [81], ANSI/HL7 V3 ECG [82,83,84], DICOM [85], and the medical waveform description format encoding rules (MFER) series of standards [86,87,88,89], as well as the recommendations from leading scientific societies, e.g., AHA, American College of Cardiology (ACC), European Society of Cardiology (ESC), etc. [90,91,92,93,94]. SCP-ECG updates and new section proposals were then drafted by and circulated within the project team members, and some stakeholders in the field of quantitative electrocardiology, and have been amply discussed over 30 WebEx meetings and during a special session on “standardization” held during the Staff 2015 meeting in Vence (France), with different representatives from the industry.
SCP-ECG V3.0 was approved by CEN, on 22 June 2020, as EN 1064:2020 standard [39]. Institutional contacts have been taken by CEN, asking ISO TC 215 to amend some of the terms used in ISO/IEEE/FDIS 11073-10101:2020 [81] and to confirm SCP-ECG V3.0 as an ISO standard that would supersede ISO 11073-91064:2009 [38].
In the following, we summarize the changes that were performed on the already existing sections in SCP-ECG versions 1.x and 2.x, briefly present the new sections that were included in version 3.0, and conclude by recalling the medical challenges of SCP-ECG and why it is a unique standard that is different from other signal-related standards.
4.1. Outline of SCP-ECG V3.0
EN 1064:2020 consists of a 240-page document that comprises a comprehensive, five-page introduction, describing the scene and the application areas, five clauses, eight annexes, and the bibliography. It specifies the means to be used to encode and exchange standard and medium- to long-term electrocardiogram waveforms, and the related metadata acquired in physiological laboratories, hospital wards, clinics, and during primary care medical check-ups, ambulatory, and homecare. It covers electrocardiograms, such as 12-lead, 15-lead, 18-lead, Cabrera lead, Nehb lead, Frank lead, XYZ lead, Holter ECGs, and exercise ECGs that were recorded, measured, and analyzed by equipment, such as electrocardiographs, patient monitors, and wearable devices. It also covers intracardiac electrograms recorded by implantable devices, as well as the analysis results produced by ECG analysis and interpretation systems and software that are compatible with SCP-ECG.
ECG waveforms and data that are not in the scope of this technical specification include real-time ECG waveform encoding and transmission (used for physiological monitors), which are covered by other standards, and intra-cardiac or extra-cardiac ECG mappings.
The various ECG data and related metadata and formats addressed by EN 1064:2020 are, as for the previous versions, specified in Clause 5. They are now structured into 18 sections that are summarized in Table 1. Sections 0, 1, 3, and at least one of the signal Sections 6, 12, or 14 are required. Section 13 is required if Section 14 is present. All other sections are optional.

Table 1.
List of SCP-ECG V3.0 data sections. Sections 0, 1, 3, and at least one of the signal sections 6, 12, or 14 are required. Section 13 is required if Section 14 is present. Section 4 was deprecated and will no longer be used. All other sections are optional.
Annex A (normative) provides a set of supplementary information for the encoding of drugs, of the functionalities of implanted cardiac devices, the filtering methods used for ECG processing, and the physical units and/or the type of measurements and annotations.
Annex B “Universal ECG interpretation statements codes” provides, as in the previous versions of SCP-ECG, a “pragmatic” approach to the problem of mapping computer interpretation statements onto a common and understandable lexicon. It was substantially amended to comply with the latest cardiology societies recommendations, and was extended by cross-referencing semantically equivalent statement codes and acronyms from AHA recommendations and related nomenclature standards.
Annex C provides a set of SCP-ECG compliance definitions and specifications, and outlines a testing procedure that may be followed by manufacturers who intend to state SCP-ECG compliance for their devices and/or systems or software.
Annex D recalls the recommended ECG compression techniques that were used in the previous versions of SCP-ECG. This annex was previously normative and is now only informative. It was kept for educational reasons, to support understanding of Huffman encoding and decoding, and conversion of high compressed ECGs from legacy SCP-ECG version 1.x and 2.x files into SCP-ECG version V3.0 files.
Annex E provides some cross-references to the coding schemes used by other ECG-related standards namely AHA recommendations [92], CDISC (Clinical Data Interchange Standards Consortium) C-Code [95], ISO/IEEE 11073-10102 aECG [80], and DICOM code values [85].
Annexes F, G, and H, respectively, provide some implementation recommendations, a short glossary of the terms used in EN 1064:2020 that are beyond the usual backgrounds (in electrocardiography and in computer science of the potential readers), and a revision history, since the initial release of the EN 1064 standard in 1993.
Sections 0 to 11 already existed in the previous versions of SCP-ECG and, although they have been significantly updated, they remain almost backwards compatible with SCP-ECG V1.x and V2.x, except for non-UTF-8 text strings encoding and beat subtraction or bimodal compression schemes, which are no longer supported. Starting with SCP-ECG V3.0, only lossless compression (difference and Huffman encoding) of the long-term rhythm data (Section 6) and of the reference beat type 0 data (Section 5) are now allowed. In addition, to simplify encoding, the present standard recommends storing all ECG signal data uncompressed as a series of fixed length, signed integers, and to reserve difference data calculations and Huffman encoding for mobile and/or wearable devices, when they are intended to be used in poorly served areas with limited wireless connectivity, such as GPRS, where lossless data compression strategies are still relevant. Converting legacy SCP-ECG V1.x and V2.x files into SCP-ECG V3.0 compliant files would, thus, only require (i) converting non-UTF-8 text strings into UTF-8; (ii) store ECG signal data, if any, uncompressed. Sections 12 to 18, which are new, were introduced to support the storage of continuous, long-term ECG recordings, of selected sequences of stress tests, drug trials, and protocol-based ECG recordings, and the related metadata, measurements, and annotations.
All over the document, emphasis was put on cross-referencing, whenever possible, all SCP-ECG terms, measurements, annotations, diagnosis statements, and metadata with the REFIDs, specified by the ISO/IEEE 11073-10102 annotated ECG (aECG) [80] and 11073-10101 nomenclature (vital signs) [81] standards, and on removing the ambiguities and inaccuracies of some of these, other than SCP-ECG standards [96].
Additional changes to Sections 0 to 11 are shortlisted in 4.2 and new Sections 12 to 18 are described in 4.3.
4.2. Main Updates to Sections 0 to 11 Existing in SCP-ECG V2.3
The revisions to sections 0 to 11 of SCP-ECG V2.3 are the following:
- Update and harmonization of the description of the various terms, measurements, annotations, diagnosis statements, and metadata to be compliant with the existing health informatics norms (e.g., ISO/IEEE 11073, CDISC, DICOM, HL7, IEC, etc.), the recommendations from cardiology societies (e.g., AHA, ACC, ESC, etc.), and the scientific literature;
- Precise definition of the semantics inherent to the various terms, measurements, annotations, diagnosis statements, and metadata [96];
- Renaming of Section 1: header information—patient data/ECG metadata;
- Update of SCP-ECG drug codes and move of the drug codes lists to new Annex A;
- Update of the medical history codes;
- Amendment of the definitions of the electrode configuration codes;
- Introduction of two new tags to support the description of implanted cardiac devices and the storage of the patient’s drugs, according to the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) classification system [97];
- Introduction of a new “Global, virtual lead” in Section 2, to support the coding of per-lead measurements with suffix _LEAD_CONFIG;
- Deprecation of Section 4 (now reserved for legacy SCP-ECG versions);
- Introduction of three new fields in the header of Section 5: a Huffman-encoding specifier (HES), the number of samples per lead, and the location of a fiducial point (QRS trigger point) in the reference beat type 0. These changes are intended to simplify the implementation of the SCP-ECG protocol in case no (or only) default Huffman tables are used;
- Deprecation of the bimodal compression scheme in Sections 5 and 6;
- Substantial amendment and extension of the content of Section 7 “Global ECG measurements” and Section 10 “Per-lead ECG measurements” in order to be compliant with ISO/IEEE 11073-10101 and 11073-10102, and with the latest recommendations of the scientific and medical societies [96];
- Introduction of the possibility to store the local time zone in sections 8 and 11;
- Extension of the content of Section 11 [96] by introducing the possibility to store interpretive statements, encoded according to the categorized AHA statement codes [92] and CDISC code specifications [95].
4.3. New Sections in SCP-ECG V3.0
4.3.1. Long-Term and Protocol-Based ECG Recordings—Sections 12 to 14
Starting with version V3.0, in addition to the short duration resting ECG (Section 6) and the corresponding type 0 reference beat (Section 5), the standard now provides means for storing long-term ECG rhythm data in Section 12, e.g., up to 40 days of continuous recording of 3-lead ECG signals sampled at 200 samples/sec, with a 16 bit resolution, in Section 14, several selected short- to medium-duration ECG sequences, and, in Section 13, the related metadata and reference beats (or pointers to selected reference beats). These two additional sections were included to support protocol-based ECG recordings, namely stress tests and drug trials procedures.
Section 14 may also be used to store high resolution, large bandwidth ECG recordings, with sampling rates beyond 150,000 samples/s for improved pacemaker spikes detection and analysis [98,99], while simultaneously storing a standard ECG in Sections 6 or 12.
The format of Section 12 is very similar to the International Society for Holter and Noninvasive Electrocardiology (ISHNE) format [100,101]. In order to preserve random access to the record’s segments, no compression or encoding is allowed in this section.
4.3.2. Beat-by-Beat ECG Measurements and Annotations—Section 15
In addition to the full set of global measurements (Section 7), and the per-lead measurements (Section 10) of the type 0 reference beat, starting with version V3.0, the standard now allows storing, in this section, several pre-defined global and per-lead beat measurements and annotations, for the reference beats stored in or pinpointed by Section 13, and for all (or for only some) selected beats of the long-term and/or short-term ECGs stored in Sections 12 and 14 and/or in Section 6. The beats may have been selected one-by-one by a physician or by a beat typing algorithm (reference beats of different types, etc.), or may refer to the entire set of beats from one or more selected time windows within the long-term ECG stored in Section 12 or in the long-term ECGs stored in Sections 6 or 14. The data format is designed to support a large number of use cases, such as selecting and daily analyzing a set of 10 min duration time windows from a continuous long-term ECG recording, for example, for time windows starting at 2 a.m. and 2 p.m., and then storing (P-on, P-off, QRS-on, QRS-off, T-off), and some additional useful annotations to assess day and night differences and day-to-day variabilities of the selected measurements.
In another scenario, e.g., performing thorough QT studies, one may select and store the measurements and annotations for K preselected, not necessarily consecutive beats, in as much measurement blocks (MB) as there are selected beats. To facilitate the comparison of reference beat measurements, the standard also allows saving, in separate MBs, the measurements and annotations performed on the reference beats stored in Sections 5 and 13.
4.3.3. Additional ECG Beat and Spike Measurements, and ECG Annotations—Sections 16–18
Although Section 15 already provides means for storing several pre-defined global and per-lead beat measurements and annotations for different subsets of computed or selected (reference) beats of the analyzed signals, there are various scenarios which, for example, require storage of a few measurements and annotations for all beats of the rhythm signals, and a larger set of measurements and annotations for a much smaller number of beats, i.e., for some selected or computed reference beats. One solution would be to extend the number of (optional) additional measurements in Section 15 in order to include the additional measurements used to quantify the selected or computed reference beats, but this could introduce huge overheads, as all measurement and annotation arrays in Section 15 do have the same MB length, which would require the storage of void measurement values for the non-selected beats, even if not computed.
Section 16 thus provides a solution for storing a different set of measurements and annotations than those stored in Section 15 and is therefore complementary to Section 15. Its structure and format are much the same as for Section 15, except that there is no provision for specifying analysis time windows and that there are no reserved fields for systematically storing the PP and RR intervals (the latter can nevertheless be stored, if need be, as optional additional measurements).
Section 16 is the preferred section for storing selected ECG beat measurements and annotations, if no beat-by-beat measurements and annotations are required (i.e., if Section 15 is not present).
Section 17 was designed to include support for pre-defining and storing (much like the way used for storing beat-by-beat ECG measurements in Section 15) large sets of global, and/or per-lead spike measurements and annotations, spike-by-spike, in one or more spike measurement array(s), one measurement array per analyzed ECG sequence (full long-term ECG record, selected ECG sequence), or reference beat.
The main objective of Section 18 “Additional ECG annotations” is to provide a solution for storing any type of manually or automatically produced annotation, which has not been stored in a systematic way in Sections 7, 8, 10, 11, and 15 to 17, such as the onset (and end) of a bigeminal rhythm or atrial fibrillation, the identification of a pacemaker spike that was not listed in Section 17, measurements that were not foreseen in Sections 15 and 16 (or a few measurements, such as QT intervals in drug studies, in case neither Section 15 nor Section 16 were implemented), manual annotation of complex cases with different types of aberrant QRS complexes (LBBB aberrancy, etc.), and P waves (AV dissociation, etc.), noise annotations in a given lead, etc.
5. Discussion
The ECG, non-invasive, easy-to-use (anywhere, anytime), and inexpensive, has been (and still is) the main source of information for the early detection of cardiovascular diseases and rhythm disorders, which are the leading causes of death in the world, excluding pandemics. The ECG, the first physiological signal ever to be analyzed by computer, benefited from unprecedented international cooperative research, bringing together clinicians, engineers, and scientists from academia and industry.
Digital ECG analyses, in different modalities at rest, during exercise, and on an outpatient basis, have been the subject of intensive and pioneering methodological research for the improvement of the quality of the signals, thanks to advanced and specific filtering techniques, for the recognition, identification, characterization, and delineation of waveforms, and for medical decision support. All known theories in signal processing and data analysis have been applied, compared, and evaluated on the ECG. Although deep learning was not yet mature some decades ago (however, in 1970, neuron weights were already adjusted by means of backpropagation algorithms, based on linear programming [102]), it is on the ECG that the most sophisticated wavelet and artificial intelligence techniques, for example, have been tested at a large-scale, thanks to the advent of databases. This proliferation of results on the ECG was a pioneering asset in the medical field, and explains why (and how) the CSE concerted research action was initiated, in order to objectively, independently, and consensually compare the results of the different methods used. One piece of fundamental knowledge produced and proven by the CSE concerted action from the end of the 1970s, and then during the 1980s, was the importance of inter-subject, inter-observer, as well as intra-observer variability in digital and diagnostic medicine. Medicine is not an exact science as laymen in the field have just discovered in these times of the COVID-19 pandemic.
Once again, in a pioneering way, and since the 1970s, the main world actors in digital and quantitative electrocardiology research developed the concept of personalized medicine to improve the performance of ECG analysis and interpretation methods, by creating the so-called serial analysis approach. Since the patient’s ECG is like a fingerprint and the patient himself becomes his own reference, any suspicion of abnormal changes in the ECG descriptors, over time, is a cardiac risk factor.
Thus, while the CSE concerted action was almost over, J.L.W. launched, in 1989, a new collaborative research project aimed at bringing together the entire R&D community in the field of quantitative electrocardiology at the international level, and to propose a standard for the exchange of digital ECG signals and data, in order to retrieve (for any patient) a previous self-documenting and re-analyzable reference ECG, and to compare it with any of the just-recorded ECGs by a so-called serial analysis.
SCP-ECG was born, bringing together more than 80% of the electrocardiograph manufacturers from all over the world and the main European research teams in the field of quantitative electrocardiology, thanks to the support of DG XIII of the European Commission.
Millions of ECGs are recorded every day, and in most cases, they need to be transmitted and/or archived for reviewing and/or for serial ECG analysis. However, although all ECGs today are digitally acquired, most so-called rest ECGs are immediately printed and the printouts are either stored as a paper record or are scanned and sent as a pdf file to be included in the patient’s EHR. Very often, and for different aims, these hard copies were also sent by fax, which was considered, until the mid-2010s, to be “more secure”! According to Badilini et al. [103], one reason for this situation is because many companies have never widely implemented a universal ECG storage and exchange standard format such as SCP-ECG, and still use proprietary formats, mainly to protect intellectual property and technology; thus, inciting users to adopt printed/scanned solutions for ECG transmission and archival.
Paradoxically, while we are already in the digital era, in several use cases, these “ECG images” need to be processed shortly after being printed, to recover the “voltage-versus-time” nature of the signals [104,105,106] (this was the subject of P.R.’s PhD thesis in the late 1960s!), either for research purposes, for submitting annotated digital ECGs to the FDA for drug approval [104], or for computing new ECG measurements. Some authors claim that by using this “reverse-printing” process they can achieve (only) up to 97% digitization accuracy [105], a figure that does not guarantee that the reconstructed signal will allow detecting small Q waves as defined in [13]. Other AI researchers further argue that the original ECG signals are not really needed as AI-based ECG interpretation may be performed by directly processing the “ECG images”, just as any medical image record [103,106,107]. However, these new approaches have not yet been assessed against well-documented databases such as the CSE Diagnostic database. Knowing that, because of the high slew rate of the QRS wave, especially in pediatric ECGs, the digitalization of a standard ECG with a 5 µV resolution requires that the uncertainty in the sampling time (i.e., the maximum allowable sample-to-sample variation in the encoding process) of the acquiring device is less than 12.5 µs (which represents less than 0.015 pixels for an 25 mm/s ECG printout on a 1200 dpi printer); it is difficult to imagine that any “reverse printing” process would ever achieve this performance. It is P.R.’s belief that, because the electrocardiogram is a spatiotemporal image, e.g., (8D+time) for the standard 12-lead ECG, none of the methods cited above will allow recovery, with enough accuracy, of one of the most important features of the original digital ECG signals, the phase relationship between the different leads [28]. Image processing researchers are working on pixel-based images, but that is the only thing they have. Why should researchers in quantitative electrocardiology and cardiologists turn their ECG signals into pixels and lose spatiotemporal information (intra-beat, beat-to-beat, intra-day, day-to-day, month-to-month, etc.) when information age technologies already allow retrieval of the original signals, anywhere, anytime, using a universal ECG storage and exchange format such as SCP-ECG?
Several other arguments are also in favor of SCP-ECG, which allows, if need be, retrieval of the original, unfiltered, high fidelity multilead ECG signals for reprocessing and/or interpretation by another ECG analysis program, for serial comparison, for pacemaker spike analysis, clustering, and identification [98,99], for operator-guided retrospective relabeling, or sometimes just for displaying the ECG in a different way than the one used on the standard printout. Among the different types of presentations used, the most common options are: filtered vs. unfiltered ECG, 25 vs. 50 mm/sec, panoramic display according to the Cabrera sequence [91], 24-lead ECG display for enhanced recognition of STEMI-equivalent patterns in the 12-lead ECG [108], display of the VCG computed by using an inverse Dower-like reconstruction method [109], etc. SCP-ECG also provides easy access to additional measurements, clinical information, and ECG metadata already embedded in the SCP-ECG record—information that is usually not printed on the paper copy, but that is very important for decision-making.
SCP-ECG V3.0 was designed to accommodate almost any use case, from the simplest to the most complex, e.g., storing in Section 17 spike measurements performed with a standard spike detection program, which analyzed the standard 12-lead rest ECG stored in Section 6 or in Section 12, and then storing the spike measurements performed with a high-resolution ECG analysis program on a simultaneously-recorded high-bandwidth ECG stored in Section 14. The expected benefit is to empower the monitoring of the functioning of implanted pacing devices, improve computerized interpretation or human over-reading of paced rhythms, and allow correlations with clinical patient outcomes.
Various authors have recently compared and provided extensive reviews on complementary standards dealing with ECG formats (DICOM, EDF, HL7 aECG, MFER, PDF-ECG, etc.) and on converters between different ECG formats, e.g., SCP-ECG to DICOM, HL7 aECG, MFER, and vice-versa [73,74,75,77,78,79,103,110,111,112]. European data format (EDF) and MFER are intended to only store the raw signals. HL7 aECG is intensively used for drug trials annotations, but to overcome the problem of excessive message size inherent to the use of XML, the HL7 aECG standard recommends that the ECG signals are stored in a separate file, which may be an issue for integrity preservation, whereas SCP-ECG V3.0 may store the HL7 aECG annotations in Section 18. DICOM 3.0 (supplement 30) is mainly used to store the ECG signals that have been used for synchronizing cardiovascular magnetic resonance imaging (MRI) image acquisition with heart motion. PDF-ECG is another approach that allows encapsulating, in the same PDF/A-3u file, both the standard graphical report of a rest ECG in the pdf format and the ECG signal data in any of the existing formats/standards, such as HL7 or DICOM, and possibly SCP-ECG [103], but it needs ad-hoc tools to extract the embedded files, and it is not guaranteed that the standard graphical report can be displayed by any PDF viewer, whereas SCP-ECG V3.0 could store PDF reports as single annotations in Section 18.
SCP-ECG V3.0 is the latest, most comprehensive, and universal ECG storage and ECG exchange standard today. It has been significantly amended, with the objective to provide means to support the storage and interchange of almost all existing ECG recording modalities, processing results, annotations and diagnoses, and to alleviate some implementation burdens for manufacturers by removing the little-used lossy compression methods that were supported in previous versions of the standard, and that are no longer allowed in version 3.0, as the need for compression is significantly lower compared to 30 years ago. All terms, measurements, and metadata are precisely defined, enabling the harmonization with other standards in health informatics.
The information conveyed by SCP-ECG is structured in a coherent way with regard to the different fundamental stages of the processing of the ECG signal, and has been selected to supply the knowledge useful for the exploitation and interpretation of the transmitted data. However, the option chosen in SCP-ECG is not to transmit another complete patient medical record encapsulating information, which is redundant with other standards, but rather to provide an ECG-centric data and metadata record. The size of an SCP-ECG file is thus reduced to its minimum, and allows its transmission via a smartphone in case of emergency. Binary encoding of the stored information not only reduces the size of the SCP-ECG files, but also secures the exchange of medical data. In addition, the exploitation of SCP-ECG files does not require expensive computer equipment, but only basic PCs and it is expected that the use of the SCP-ECG standard will be all the more widespread, as automatic decoding tools and viewers of SCP-ECG files will be inexpensive or open access.
Hence, only the syntax of the language used for the exchanges and the semantics of the conveyed data are standardized, and not the logic for obtaining these data nor the intelligence embedded in the devices, which must nevertheless be documented to be exploitable. Indeed, like universal thought, which can only result from the application of a rule known as majority voting, especially in medicine, and because no method of reasoning can be privileged and standardized, only the fusion of the results of these methods allows to approach the supposed truth, in agreement with the Central Limit Theorem. This is why SCP-ECG was designed to be a self-documented standard and to be open to all numerical methods used to compute, analyze, and interpret ECG descriptors, and to the different modalities of ECG signal acquisition techniques. Except for the patient characteristics that are essential to link the ECG data to the persons on whom the stored ECGs have been recorded, and the specification of the ECG recording modalities that are useful for the spatiotemporal exploitation of the conveyed signal data, all other information is optional, thus facilitating the opening of the ECG devices market.
SCP-ECG V3.0 seems to be complex, but in reality, it is not. Only Sections 0, 1, and one of the signal sections (e.g., Section 6) are compulsory, all other sections are optional. Moreover, only some information stored in Section 1 is mandatory, most tagged fields are optional. An original SCP-ECG record, storing only the raw signals, may then be completed step-by-step, either automatically by a (remote) ECG device/software and/or annotated by an over-reading physician. All amendments to the SCP-ECG record are time-stamped. Most sections are self-contained, i.e., if, for example, one wants to exploit the data content of one of the measurement data sections, and only exchange lead-by-lead, beat-by-beat, or spike-by-spike measurements and annotations, without exchanging signals, no other information than the patient data stored in Section 1 is needed.
SCP-ECG is an open, efficient, and flexible standard, with a small footprint that may be easily extended to accommodate other types of vital signs and related context-aware and patient-centric data, as recently demonstrated by Mandellos et al. [76,113], who used sections 200 to 212 to store saturation of peripheral oxygen (SPO2), accelerometer, gyroscopic and magnetometer signals, and metadata not yet covered by other standards.
Should SCP-ECG be further extended to several other signal modalities? This was already experimented with in the mid-1990s within the frame of the European AIM A2050 Biosignal Representation, Integration, and Telecommunication services in Rehabilitation (BRITER), 1993–1996 project, which attempted to define a general communication protocol based on ASN.1, including different signals, such as ECG, EEG, EMG, EOG, movement analysis signals, MRI, etc. [59]. The project was technically successful, but evidenced that a “universal” protocol, which encompasses so many different signal modalities would be very difficult to maintain, and that the only solution is to have and maintain interchange protocols that are optimized to the clinical application domain. However, the building concepts of SCP-ECG may be reused as a reference for other areas of medical applications; thus, easing data interoperability. If need be, data and signal fusion (used for designing integrated clinical workstations, storage in databases, epidemiology studies, etc.) could then be achieved by converting each of the signal specific data formats into an intermediate-, ASN.1-, or XML-based format, which would be part of a mediation meta model [62,63].
Henceforth, one future challenge of the SCP-ECG standard is the widespread use of its powerful capabilities, to empower clinical applications and the development of novel approaches, e.g., self-improving ECG interpretation programs [114] and ambient intelligence solutions [29,30,115], for the enhancement of ECG-based quality of care and the early prevention of cardiovascular diseases.
6. Conclusions
The consensual objective of the initiators of SCP-ECG, which makes SCP-ECG an exemplary standard, was to exploit the wealth of knowledge and experience acquired in the field of quantitative electrocardiography, in an open manner, and independent of particular interests, in order to allow secure and low-cost data exchange, to improve timely medical decision-making and for the benefit of citizens. This approach was followed throughout the years, allowing the various versions (up to the most recent one, SCP-ECG V3.0) to continuously improve.
The SCP-ECG standard was designed to support practically any use case, in clinical practice, research and evaluation trials, of raw and computerized digital ECG data storage in a stand-alone file, for electronic data interchange and archiving, for display, reviewing, reprocessing, converting into another format, or for distributed storage within a medical information system.
Numerous researchers, engineers, and stakeholders from the scientific and medical computerized ECG community and industry, have made significant contributions toward systematic progress in the development of the SCP-ECG standard. We should all be grateful to them for their accomplished efforts in developing such an exemplary standard, and highly encourage all cardiologists and general practitioners to promote its use in order to enhance ECG diagnosis accuracy, for the benefit of patients, and to reduce societal costs.
Author Contributions
P.R. and J.F. wrote the original draft. All of the authors are major contributors to the development of the SCP-ECG standard—J.F., P.W.M., and P.R. (since 1989), A.S. and A.V. (since the late 1990s), and D.P. (since 2013). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank the large number of researchers, engineers, cardiologists, and clinicians from academia, industry, and normalization organizations who contributed to the development and testing of the SCP-ECG standards.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rautaharju, P.M. Eyewitness to history: Landmarks in the development of computerized electrocardiography. J. Electrocardiol. 2016, 49, 1–6. [Google Scholar] [CrossRef]
- Rubel, P.; Bailly, G.; Giard, M.H. Microprocessor based biomedical instrumentation. A modular approach. In Digest of Papers, Proceedings of the 11th International Conference on Medical and Biological Engineering, Ottawa, ON, Canada, 2–6 August 1976; Durie, N.D., Swail, E.I., Eds.; National Research Council: Ottawa, ON, Canada, 1976; pp. 560–561. [Google Scholar]
- Rubel, P.; Varrot, M.; Morlet, D.; Arnaud, P.; Forlini, M.C.; Bailly, G. Architecture and performance of digital ECG acquisition systems. In Optimization of Computer ECG Processing; Wolf, H.K., Macfarlane, P.W., Eds.; North Holland Publ. Comp.: Amsterdam, The Netherlands, 1980; pp. 41–53. [Google Scholar]
- Bailey, J.J.; Horton, M.; Itscoitz, S.B. A Method for Evaluating Computer Programs for Electrocardiographic Interpretation. III. Reproducibility Testing and the Sources of Program Errors. Circulation 1974, 50, 88–93. [Google Scholar] [CrossRef][Green Version]
- Willems, J.L.; Paerdens, J. Differences in measurement results obtained by four different ECG computer programs. In Proceedings of the Computers in Cardiology, Rotterdam, The Netherlands, 29 September–1 October 1977; Ostrow, H.G., Ripley, K.L., Eds.; IEEE: Long Beach, CA, USA, 1977; pp. 115–121. [Google Scholar]
- Rautaharju, P.M.; Ariet, M.; Pryor, T.A.; Arzbaecher, R.C.; Bailey, J.J.; Bonner, R.; Goetowski, C.R.; Hooper, J.K.; Klein, V.; Millar, C.K.; et al. The quest for optimal electrocardiography. Task Force III: Computers in diagnostic electrocardiography. Am. J. Cardiol. 1978, 41, 158–170. [Google Scholar] [CrossRef]
- Willems, J.L. A plea for common for common standards in computer aided analysis. Comput. Biomed. Res. 1980, 13, 120–131. [Google Scholar] [CrossRef]
- Willems, J.L. A review of computer ECG analysis: Time to evaluate and standardize. CRC Crit. Rev. Med. Inform. 1986, 1, 165–207. [Google Scholar]
- Brohet, C.; Willems, J.L.; Derwael, D.; De Schreye, D.; Fesler, R.; Pardaens, J. Impact of measurement precision on diagnostic ECG computer classification. In Computer ECG Analysis: Towards Standardization; Willems, J.L., van Bemmel, J.H., Zywietz, C., Eds.; North-Holland Publ.: Amsterdam, The Netherlands, 1986; pp. 171–176. [Google Scholar]
- Willems, J.L.; Abreu-Lima, C.; Arnaud, P.; Brohet, C.R.; Denis, B.; Gehring, J.; Graham, I.; van Herpen, G.; Machado, H.; Michaelis, J.; et al. Evaluation of ECG Interpretation Results Obtained by Computer and Cardiologists. Methods Inf. Med. 1990, 4, 308–316. [Google Scholar]
- The CSE European Working Party; Willems, J.L.; Arnaud, P.; van Bemmel, J.H.; Bourdillon, P.J.; Brohet, C.; Dalla Volta, S.; Degani, R.; Denis, B.; Demeester, M.; et al. Common Standards for Quantitative Electrocardiography: The CSE Pilot Study. In Medical Informatics Europe 81. Lecture Notes in Medical Informatics; Grémy, F., Degoulet, P., Barber, B., Salamon, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; Volume 11, pp. 319–326. [Google Scholar] [CrossRef]
- Willems, J.L. Common standards for quantitative electrocardiography. J. Med. Eng. Technol. 1985, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]
- The CSE Working Party. Recommendations for measurement standards in quantitative electrocardiography. Eur. Heart J. 1985, 6, 815–825. [Google Scholar]
- Willems, J.L.; Arnaud, P.; van Bemmel, J.H.; Bourdillon, P.; Degani, R.; Denis, B.; Graham, I.; Harms, F.M.A.; Macfarlane, P.W.; Mazzocca, G.; et al. A reference data base for multi-lead electrocardiographic computer measurement programs. J. Am. Coll. Cardiol. 1987, 10, 1313–1321. [Google Scholar] [CrossRef]
- Morlet, D.; Rubel, P.; Arnaud, P.; Willems, J.L. An improved method to evaluate the precision of computer ECG measurement programs. Int. J. Bio-Med. Comput. 1988, 22, 199–216. [Google Scholar] [CrossRef]
- Morlet, D.; Rubel, P.; Willems, J.L. Value of Scatter-Graphs for the Assessment of ECG Computer Measurement Results. Methods Inf. Med. 1990, 29, 413–423. [Google Scholar] [CrossRef]
- Willems, J.L.; Abreu-Lima, C.; Arnaud, P.; van Bemmel, J.H.; Brohet, C.; Degani, R.; Denis, B.; Graham, I.; van Herpen, G.; Macfarlane, P.W. Effect of combining electrocardiographic interpretation results on diagnostic accuracy. Eur. Heart J. 1988, 9, 1348–1355. [Google Scholar] [CrossRef]
- Van Bemmel, J.H.; Willems, J.L. Standardization and validation of medical support-systems: The CSE Project. Methods Inf. Med. 1990, 29, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.; Arnaud, P.; Van Bemmel, J.; Degani, R.; Macfarlane, P.; Zywietz, C. Common standards for quantitative electrocardiography: Goals and main results. Methods Inf. Med. 1990, 29, 263–271. [Google Scholar]
- Willems, J.L.; Abreu-Lima, C.; Arnaud, P.; van Bemmel, J.H.; Brohet, C.; Degani, R.; Denis, B.; Gehring, J.; Graham, I.; van Herpen, G.; et al. The diagnostic performance of computer programs for the interpretation of the electrocardiogram. N. Engl. J. Med. 1991, 325, 1767–1773. [Google Scholar] [CrossRef]
- Willems, J.L. Assessment of diagnostic ECG results using information and decision theory: Results from the CSE diagnostic study. J. Electrocardiol. 1992, 25, 120–125. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P.; Macfarlane, P.W. Can the lessons learned from the assessment of automated electrocardiogram analysis in the Common Standards for quantitative Electrocardiography study benefit measurement of delayed contrast-enhanced magnetic resonance images? J. Electrocardiol. 2007, 40, 246–250. [Google Scholar] [CrossRef]
- International Electrotechnical Commission. IEC 60601-2-25:2011, Medical Electrical Equipment—Part 2-25: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographs, 2nd ed.; IEC: Geneva, Switzerland, 19 October 2011; pp. 1–196. ISBN 978-2-88912-719-1. [Google Scholar]
- International Electrotechnical Commission. IEC 60601-2-51:2003, Medical Electrical Equipment—Part 2-51: Particular Requirements for Safety, Including Essential Performance, of Recording and Analysing Single Channel and Multichannel Electrocardiographs, 1st ed.; IEC: Geneva, Switzerland, 27 February 2003; pp. 1–175. [Google Scholar]
- Schlant, R.C.; Adolph, R.J.; DiMarco, J.P.; Dreifus, L.S.; Dunn, M.I.; Fisch, C.; Garson, A.; Haywood, L.J.; Levine, H.J.; Murray, J.A.; et al. Guidelines for electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Electrocardiography). Circulation 1992, 85, 1221–1228. [Google Scholar] [CrossRef]
- Kadish, A.H.; Buxton, A.E.; Kennedy, H.L.; Knight, B.P.; Mason, J.W.; Schuger, C.D.; Tracy, C.M.; Winters, W.L.; Boone, A.W.; Elnicki, M.; et al. ACC/AHA clinical competence statement on electrocardiography and ambulatory electrocardiography: A report of the ACC/AHA/ACP-ASIM task force on clinical competence (ACC/AHA Committee to develop a clinical competence statement on electrocardiography and ambulatory electrocardiography) endorsed by the International Society for Holter and noninvasive electrocardiology. Circulation 2001, 104, 3169–3178. [Google Scholar]
- Rubel, P.; Willems, J.L.; Zywietz, C.; Fayn, J. Towards open data interchange and processing of serial ECGs. In Proceedings of the Computers in Cardiology, Durham, NC, USA, 11–14 October 1992; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1992; pp. 79–82. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P.; Pahlm, O.; Wagner, G.S. Improvement of the detection of myocardial ischemia thanks to information technologies. Int. J. Cardiol. 2007, 120, 172–180. [Google Scholar] [CrossRef]
- Fayn, J. A Classification Tree Approach for Cardiac Ischemia Detection Using Spatiotemporal Information from Three Standard ECG Leads. IEEE Trans. Biomed. Eng. 2011, 58, 95–102. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P. Toward a personal health society in cardiology. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 401–409. [Google Scholar] [CrossRef]
- Atoui, H.; Fayn, J.; Gueyffier, F.; Rubel, P. Cardiovascular Risk Stratification in Decision Support Systems: A Probabilistic Approach. Application to pHealth. In Proceedings of the Computers in Cardiology, Valencia, Spain, 17–20 September 2006; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2006; pp. 281–284. Available online: https://www.cinc.org/archives/2006/pdf/0281.pdf (accessed on 15 August 2021).
- Willems, J.L.; Zywietz, C.; Rubel, P.; Degani, R.; Macfarlane, P.W.; van Bemmel, J.H. A standard communications protocol for computerized electrocardiography. J. Electrocardiol. 1991, 24, 173–178. [Google Scholar] [CrossRef]
- Willems, J.L.; Zywietz, C.; Rubel, P.; Degani, R.; Macfarlane, P.W.; van Bemmel, J.H. Development of a standard communications protocol for computerized electrocardiography. In Telematics in Medicine; Duisterhout, J.S., Hasman, A., Salamon, R., Eds.; Elsevier Science Publ., North Holland: Amsterdam, The Netherlands, 1991; pp. 299–311. [Google Scholar]
- Rubel, P.; Willems, J.L.; Zywietz, C.; Degani, R.; Macfarlane, P.W.; van Bemmel, J.H. Development of a standard communications protocol for the exchange and the storage of digital ECGs. In Proceedings of the 13th Annual International Conference of the IEEE Eng. Med. Biol. Soc., Orlando, FL, USA, 31 October–3 November 1991; Nagel, J.H., Smith, W.M., Eds.; IEEE Press: New York, NY, USA, 1991; pp. 572–573. [Google Scholar] [CrossRef]
- Willems, J.L.; Zywietz, C.; Rubel, P.; Degani, R.; Macfarlane, P.W.; van Bemmel, J.H. SCP-ECG: A standard communications protocol for computerized electrocardiography. In Advances in Medical Informatics. Results for the AIM Exploratory Action; Noothoven van Goor, J., Christensen, J.P., Eds.; IOS Press: Amsterdam, The Netherlands, 1992; pp. 325–332. [Google Scholar] [CrossRef]
- Willems, J.L.; Rubel, P.; Zywietz, C. Standard Interchange for Computerized Electrocardiography. In Progress in Standardization in Health Care Informatics; De Moor, G.J.E., McDonald, C.L., Noothoven van Goor, J., Eds.; IOS Publisher: Amsterdam, The Netherlands, 1993; pp. 185–194. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 1064:2005+A1:2007, Health Informatics—Standard Communication Protocol—Computer-Assisted Electrocardiography; CEN: Brussels, Belgium, 2007; pp. 1–188. [Google Scholar]
- International Organization for Standardization. ISO 11073 91064:2009, Health Informatics—Standard Communication Protocol—Part 91064: Computer-Assisted Electrocardiography; ISO: Geneva, Switzerland, 2009; pp. 1–159. [Google Scholar]
- European Committee for Standardization. EN 1064:2020, Health Informatics—Standard Communication Protocol—Computer-Assisted Electrocardiography; CEN: Brussels, Belgium, 2020; pp. 1–240. [Google Scholar]
- Willems, J.L. Standard Communications Protocol for Computerized Electrocardiography: Final Specifications and Recommendations; ACCO Publ.: Leuven, Belgium, 1991; ISBN 90-73402-01-7. [Google Scholar]
- Marquette Electronics, Inc. Internal Document: Definition of the Data Contents within a Universal ECG Protocol; GE Healthcare: Milwaukee, WI, USA, 1987; pp. 1–24. [Google Scholar]
- Zywietz, C.; Joseph, G.; Fischer, R.; Degani, R.; Willems, J.L. Compression and encoding of ECG data within the European standard communications protocol. In Proceedings of the Computers in Cardiology, Venice, Italy, 23–26 September 1991; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1991; pp. 105–108. [Google Scholar] [CrossRef]
- Rubel, P.; Fayn, J.; Macfarlane, P.W.; Willems, J.L. Development of a conceptual reference model for digital ECG data storage. In Proceedings of the Computers in Cardiology, Venice, Italy, 23–26 September 1991; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1992; pp. 109–112. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P.; Girard, P.; Ravel, C.; Forlini, M.C.; Boissel, J.P. CAVIAR, a Serial ECG Processing and Management System for Pharmacological Drug Trials. In Proceedings of the Computers in Cardiology, Venice, Italy, 23–26 September 1991; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1992; pp. 301–304. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P.; Willems, J.L. Management of serial ECGs and control strategies for the comparison process. Meth. Inform. Med. 1994, 33, 148–152. [Google Scholar] [PubMed]
- Rubel, P.; Willems, J.L.; Zywietz, C.; Fayn, J.; Assanelli, D.; Malossi, C.; Todd, S. OEDIPE: Open European Data Interchange and Processing for Electrocardiography. In Health in the New Communications Age; Laires, M.F., Ladeira, M.J., Christensen, J.P., Eds.; IOS Press: Amsterdam, The Netherlands, 1995; pp. 313–321. [Google Scholar]
- Rubel, P.; Fayn, J.; Zywietz, C.; Willems, J.L.; Assanelli, D.; Malossi, C.; Florin, C. Open European electrocardiological data interchange. In Health in the Information Society, Proceedings of the Health Telematics ’95, Laco Ameno Ischia, Naples, Italy, 2–6 July 1995; Bracale, M., Denoth, F., Eds.; CNR: Pisa, Italy, 1995; pp. 381–386. ISBN 88-7958-003-5. [Google Scholar]
- Fayn, J.; Rubel, P.; Willems, J.L.; Conti, L.; Reniers, R. Design and implementation strategies of a core database model for the storage and retrieval of serial ECG data. In Proceedings of the Computers in Cardiology, Bethesda, MD, USA, 25–28 September 1994; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1994; pp. 109–112. [Google Scholar] [CrossRef]
- Rubel, P.; Fayn, J.; Willems, J.L.; Zywietz, C. New trends in serial ECG analysis. J. Electrocardiol. 1993, 26, 122–128. [Google Scholar]
- Pfirsch, F.; Reniers, R.; Rubel, P.; Willems, J.L. A Generic Interface between Communication Protocols and Relational Databases. In Communication and Integration in Healthcare Informatics, Proceedings of the Medical Informatics Conference M.I.C. ’93, Gent, Belgium, 11–12 November 1993; De Moor, G.J.E., Ed.; Acco Publ.: Leuven, Belgium, 1993; pp. 23–32. [Google Scholar]
- Al-Ahmad, W.; Rubel, P.; Willems, J.L. OSI standards for ECG data interchange: Use of ASN.1 in the OEDIPE project. In Communication and Integration in Healthcare Informatics, Proceedings of the Medical Informatics Conference M.I.C. ’93, Gent, Belgium, 11–12 November 1993; De Moor, G.J.E., Ed.; Acco Publ.: Leuven, Belgium, 1993; pp. 115–122. [Google Scholar]
- Mertins, V.; Fischer, R.; Joseph, G.; Zywietz, C.; Malossi, C.; Wack, K. Implementation of the SCP-ECG Protocol into stand-alone Electrocardiographs. In Proceedings of the Computers in Cardiology, Durham, NC, USA, 11–14 October 1992; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1992; pp. 83–86. [Google Scholar] [CrossRef]
- Zywietz, C.; Mertins, V.; Assanelli, D.; Malossi, C. Digital ECG Transmission from Ambulance Cars with Application of the European Standard Communications Protocol SCP-ECG. In Proceedings of the Computers in Cardiology, Bethesda, MD, USA, 25–28 September 1994; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1994; pp. 341–344. [Google Scholar] [CrossRef]
- Al-Ahmad, W.; Willems, J.L.; Rubel, P. ECG Data Interchange within the Framework of the SCP-ECG and the OEDIPE Projects. In Proceedings of the Computers in Cardiology, Bethesda, MD, USA, 25–28 September 1994; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1994; pp. 337–340. [Google Scholar] [CrossRef]
- Badr, A.; Al-Ahmad, W.; Rubel, P.; Willems, J.L. Protection of Data Privacy for Open ECG Data Interchange. In Trends in Medical Informatics, Proceedings of the Medical Informatics Conference M.I.C. ’94, Velthoven, The Netherlands, 25–26 November 1994; De Moor, G.J.E., Ed.; Acco Publ.: Leuven, Belgium, 1994; pp. 145–153. [Google Scholar]
- Ron, J.L.; Fayn, J.; Rubel, P. Towards a generic domain information model in cardiology. Experiences from OEDIPE. In Proceedings of the Computers in Cardiology, Indianapolis, IN, USA, 8–11 September 1996; Murray, A., Arzbaecher, R., Eds.; IEEE: Piscataway, NJ, USA, 1996; pp. 301–304. [Google Scholar] [CrossRef]
- Fayn, J.; Conti, L.; Fareh, S.; Maison-Blanche, P.; Nony, P.; Rubel, P. Interactive and Dynamic ECG Analysis. Is it Just an IDEA or a clinically relevant approach? J. Electrocardiol. 1996, 29 (Suppl. S1), 21–25. [Google Scholar] [CrossRef]
- Ferrari, G.; Zywietz, C.; Willems, J.L.; Fayn, J.; Poeta, M.; Assanelli, D. User interface database for digital SCP-ECG and epidemiological information. In Proceedings of the Computers in Cardiology, Bethesda, MD, USA, 25–28 September 1994; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1994; pp. 177–180. [Google Scholar] [CrossRef]
- Loukil, A.; Fayn, J.; Conti, L.; Bortone, S.; Fioretti, S.; Bedini, R.; Nentidis, G.; Leo, T.; Rubel, P.; Talbot, A. A system for accessing and handling heterogeneous sources of patient data. Application to the rehabilitation domain. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; IEEE: Piscataway, NJ, USA, 1996; Volume 18, pp. 1240–1241. [Google Scholar] [CrossRef]
- Willems, J.L.; Zywietz, C. Conformance Testing for Computerized Electrocardiography. In Proceedings of the European Conference on Conformance Testing and Certification in Information Technology and Telecommunications, Brussels, Belgium, 13–15 June 1990; CEN/CENELEC&ETSI, Ed.; IOS Press: Amsterdam, The Netherlands, 1991; pp. 307–319. ISBN 9051990383. [Google Scholar]
- Chronaki, C.E.; Chiarugi, F.; Lees, P.J.; Bruun-Rasmussen, M.; Conforti, F.; Ruiz Fernandez, R.; Zywietz, C. Open ECG: A European Project to Promote the SCP-ECG Standard, a Further Step Towards Interoperability in Electrocardiography. In Proceedings of the Computers in Cardiology, Memphis, TN, USA, 22–25 September 2002; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2002; pp. 285–288. [Google Scholar] [CrossRef]
- Jumaa, H.; Fayn, J.; Rubel, P. XML based mediation for automating the storage of SCP-ECG data into relational databases. In Proceedings of the Computers in Cardiology, Bologna, Italy, 14–17 September 2008; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2008; pp. 445–448. [Google Scholar] [CrossRef]
- Jumaa, H.A. XML and Relational Databases Mediation Automation. Ph.D. Thesis, National Institute of Applied Sciences of Lyon (INSA-Lyon), Lyon, France, 16 December 2010. Available online: http://theses.insa-lyon.fr/publication/2010ISAL0120/these.pdf (accessed on 12 July 2021).
- Zywietz, C.; Willems, J.L. European Conformance Testing Services for computerized electrocardiography. New procedures and standards. J. Electrocardiol. 1993, 26, 137–146. [Google Scholar]
- Zywietz, C.; Alraun, W.; Mertins, V. Quality Assurance in Electrocardiography: Inappropriate Performance Standards, ECG Characteristics and a New Test Database. In Proceedings of the Computers in Cardiology, Bethesda, MD, USA, 25–28 September 1994; Murray, A., Arzbaecher, R., Eds.; IEEE Computer Society Press: Los Alamitos, CA, USA, 1994; pp. 333–336. [Google Scholar] [CrossRef]
- Zywietz, C.; Alraun, W.; Fischer, R. Quality assurance in biosignal processing—Procedures and recommendations for evaluation for electrocardiological analysis systems. In Proceedings of the Computers in Cardiology, Rotterdam, The Netherlands, 23–26 September 2001; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2001; pp. 201–204. [Google Scholar] [CrossRef]
- Chronaki, C.E.; Chiarugi, F.; Lees, P.J.; Macerata, A.; Conforti, F.; Bruun-Rasmussen, M.; Ruiz Fernandez, R.; Zywietz, C. A Year in the Life of the OpenECG Network. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2003; pp. 17–20. [Google Scholar] [CrossRef]
- Lees, P.J.; Chronaki, C.E.; Chiarugi, F.; The OpenECG Consortium. Standards and Interoperability in Digital Electrocardiography. The OpenECG Project. Hell. J. Cardiol. 2004, 45, 364–369. [Google Scholar]
- Chronaki, C.E.; Chiarugi, F.; Macerata, A.; Conforti, F.; Voss, H.; Johansen, I.; Ruiz-Fernandez, R.; Zywietz, C. Interoperability in digital electrocardiography after the OpenECG project. In Proceedings of the Computers in Cardiology, Chicago, IL, USA, 19–22 September 2004; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2004; pp. 49–52. [Google Scholar] [CrossRef]
- Chronaki, C.E.; Chiarugi, F.; Sfakianakis, S.; Zywietz, C. A Web Service for Conformance Testing of ECG Records to the SCP-ECG Standard. In Proceedings of the Computers in Cardiology, Lyon, France, 25–28 September 2005; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2005; pp. 961–964. [Google Scholar] [CrossRef]
- Chronaki, C.E.; Chiarugi, F.; Fischer, R. OpenECG: Medical Device Interoperability as a Quality Label for eHealth Services. In Proceedings of the Computers in Cardiology, Valencia, Spain, 17–20 September 2006; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2006; pp. 465–468. [Google Scholar]
- Chiarugi, F.; Spanakis, M.; Lees, P.J.; Chronaki, C.E.; Tsiknakis, M.; Orphanoudakis, S.C. ECG in Your Hands: A Multi-Vendor ECG Viewer for Personal Digital Assistants. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2003; pp. 359–362. [Google Scholar] [CrossRef]
- Schloegl, A.; Chiarugi, F.; Cervesato, E.; Apostolopoulos, E.; Chronaki, C.E. Two-way converter between the HL7 aECG and SCP-ECG data formats using BioSig. In Proceedings of the Computers in Cardiology, Durham, NC, USA, 30 September–3 October 2007; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2007; pp. 253–256. [Google Scholar] [CrossRef]
- Sakkalis, V.; Chiarugi, F.; Kostomanolakis, S.; Chronaki, C.E.; Tsiknakis, M.; Orphanoudakis, S.C. A gateway between the SCP-ECG and the DICOM supplement 30 waveform standard. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2003; pp. 25–28. [Google Scholar] [CrossRef]
- Schlögl, A. An overview on data formats for biomedical signals. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; Dössel, O., Schlegel, W.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 25/4, pp. 1557–1560. [Google Scholar] [CrossRef]
- Mandellos, G.J.; Koukias, M.N.; Styliadis, I.S.; Lymberopoulos, D.K. e-SCP-ECG+ Protocol: An Expansion on SCP-ECG Protocol for Health Telemonitoring—Pilot Implementation. Int. J. Telemed. Appl. 2010, 2010, 137201. [Google Scholar] [CrossRef]
- Gonçalves, B.; Guizzardi, G.; Pereira Filho, J.G. Using an ECG reference ontology for semantic interoperability of ECG data. J. Biomed. Inf. 2011, 44, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.R.; Finlay, D.D.; Nugent, C.D.; Moore, G. A review of ECG storage formats. Int. J. Med. Inform. 2011, 80, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.D.; Alesanco, A.; Martinez, I.; Garcia, J. A Review on Digital ECG Formats and the Relationships Between Them. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 432–444. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO/IEEE 11073-10102:2014, Health Informatics—Point-of-Care Medical Device Communication—Part 10102: Nomenclature—Annotated ECG, 1st ed.; ISO: Geneva, Switzerland, 2014; pp. 1–177. [Google Scholar]
- International Organization for Standardization. ISO/IEEE 11073-10101:2020, Health informatics—Point-of-Care Medical Device Communication—Part 10101: Nomenclature, 2nd ed.; ISO: Geneva, Switzerland, 2020; pp. 1–1040. [Google Scholar]
- Healh Level Seven. ANSI/HL7 V3 ECG, R1-2004 (R2019), HL7 Version 3 Standard: Regulated Studies—Annotated ECG, Release 1; HL7 International: Ann Arbor, MI, USA; Available online: https://www.hl7.org/implement/standards/product_brief.cfm?product_id=70 (accessed on 15 August 2021).
- Healh Level Seven. HL7 V3 IG ECGR1 R2, HL7 Version 3 Implementation Guide: Regulated Studies; Annotated ECG R1, Release 2; HL7 International: Ann Arbor, MI, USA, 2015; pp. 1–111. Available online: https://www.hl7.org/implement/standards/product_brief.cfm?product_id=102 (accessed on 15 August 2021).
- Healh Level Seven. HL7 Annotated ECG Implementation Guide. Continuous Waveforms Supplement; Brown, B., Ed.; HL7 International: Ann Arbor, MI, USA, 2014; pp. 1–25. Available online: https://www.hl7.org/documentcenter/public/wg/healthcaredevices/HL7_aECG_Continuous_ECG_Update%20-%20Brown.zip (accessed on 15 August 2021).
- DICOM Standards Committee, Working Group 1-Cardiac and Vascular Information. Digital Imaging and Communications in Medicine (DICOM) Supplement 30: Waveform Interchange; DICOM Standard Committee: Arlington, VA, USA, 2000; pp. 1–77. Available online: https://www.dicomstandard.org/News-dir/ftsup/docs/sups/sup30.pdf (accessed on 15 August 2021).
- International Organization for Standardization. ISO 22077-1:2015, Health Informatics—Medical Waveform Format—Part 1: Encoding Rules, 1st ed.; ISO: Geneva, Switzerland, 2015; pp. 1–42. [Google Scholar]
- International Organization for Standardization. ISO 22077-2:2015, Health Informatics—Medical Waveform Format—Part 2: Electrocardiography, 1st ed.; ISO: Geneva, Switzerland, 2015; pp. 1–38. [Google Scholar]
- International Organization for Standardization. ISO/TS 22077-3:2015, Health Informatics—Medical Waveform Format—Part 3: Long Term Electrocardiography, 1st ed.; ISO: Geneva, Switzerland, 2015; pp. 1–29. [Google Scholar]
- International Organization for Standardization. ISO/TS 22077-4:2019, Health Informatics—Medical Waveform Format—Part 4: Stress Test Electrocardiography, 1st ed.; ISO: Geneva, Switzerland, 2019; pp. 1–30. [Google Scholar]
- Bailey, J.J.; Berson, A.S.; Garson, A.; Horan, L.G.; Macfarlane, P.W.; Mortara, D.W.; Zywietz, C. Recommendations for standardization and specifications in automated electrocardiography: Bandwidth and digital signal processing. A report for health professionals by an ad hoc writing group of the Committee on Electrocardiography and Cardiac Electrophysiology of the Council on Clinical Cardiology, American Heart Association. Circulation 1990, 81, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the standardization and interpretation of the electrocardiogram. Part I: The electrocardiogram and its technology. A Scientific Statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, the American College of Cardiology Foundation, and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2007, 49, 1109–1127. [Google Scholar] [CrossRef]
- Mason, J.W.; Hancock, E.W.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Josephson, M.; Kligfield, P.; Kors, J.A.; Macfarlane, P.; et al. Recommendations for the standardization and interpretation of the electrocardiogram. Part II: Electrocardiography diagnostic statement list. J. Am. Coll. Cardiol. 2007, 49, 1128–1135. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST segment, T and U waves, and the QT interval. A Scientific Statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.S.; Macfarlane, P.; Wellens, H.; Josephson, M.; Gorgels, A.; Mirvis, D.M.; Pahlm, O.; Surawicz, B.; Kligfield, P.; Childers, R.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part VI: Acute ischemia/infarction: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 1003–1111. [Google Scholar] [CrossRef] [PubMed]
- CDISC Controlled Terminology. Available online: http://www.cdisc.org/terminology (accessed on 15 August 2021).
- Rubel, P.; Pani, D.; Schloegl, A.; Fayn, J.; Badilini, F.; Macfarlane, P.W.; Varri, A. SCP-ECG V3.0: An enhanced standard communication protocol for computer-assisted electrocardiography. In Proceedings of the Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; Murray, A., Ed.; IEEE: Piscataway, NJ, USA, 2016; pp. 309–312. [Google Scholar]
- The Anatomical Therapeutic Chemical (ATC) Classification System and the Defined Daily Dose (DDD). WHO Collaborating Centre for Drug Statistics Methodology. Available online: http://www.whocc.no/ (accessed on 11 July 2021).
- Ricke, A.D.; Swiryn, S.; Bauernfeind, R.A.; Conner, J.A.; Young, B.; Rowlandson, G.I. Improved pacemaker pulse detection: Clinical evaluation of a new high-bandwidth electrocardiographic system. J. Electrocardiol. 2011, 44, 265–274. [Google Scholar] [CrossRef]
- Luo, S.; Johnston, P.; Macfarlane, P.W. Implanted cardiac pacemaker pulses as recorded from the body surface. J. Electrocardiol. 2012, 45, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zareba, W.; Locati, E.H.; Maison Blanche, P. The ISHNE Holter Standard Output File Format: A Step Toward Compatibility of Holter Systems. Ann. Noninvasive Electrocardiol. 1998, 3, 261–262. [Google Scholar] [CrossRef]
- Badilini, F. The ISHNE Holter Standard Output File Format. Ann. Noninvasive Electrocardiol. 1998, 3, 263–266. [Google Scholar] [CrossRef]
- Ibaraki, T.; Muroga, S. Adaptive Linear Classifier by Linear Programming. IEEE Trans. Syst. Sci. Cybern. 1970, 6, 53–62. [Google Scholar] [CrossRef]
- Badilini, F.; Young, B.; Brown, B.; Vaglio, M. Archiving and exchange of digital ECGs: A review of existing data formats. J. Electrocardiol. 2018, 51, S113–S115. [Google Scholar] [CrossRef]
- Badilini, F.; Erdem, T.; Zareba, W.; Moss, A.J. ECGScan: A method for conversion of paper electrocardiographic printouts to digital electrocardiographic files. J. Electrocardiol. 2005, 38, 310–318. [Google Scholar] [CrossRef]
- Li, Y.; Qu, Q.; Wang, M.; Yu, L.; Wang, J.; Shen, L.; He, K. Deep learning for digitizing highly noisy paper-based ECG records. Comput. Biol. Med. 2020, 127, 104077. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Khatwani, G.; Patil, R.; Sapariya, D.; Shah, V.; Parmar, D.; Dinesh, S.; Daphal, P. ECG Paper Record Digitization and Diagnosis Using Deep Learning. J. Med. Biol. Eng. 2021, 41, 422–432. [Google Scholar] [CrossRef]
- Loresco, P.J.M.; Africa, A.D. ECG Print-Out Features Extraction Using Spatial-Oriented Image Processing Techniques. JTEC 2018, 10, 15–20. [Google Scholar]
- Pahlm, U.; Pahlm, O.; Wagner, G.S. The 24-lead ECG display for enhanced recognition of STEMI-equivalent patterns in the 12-lead ECG. J. Electrocardiol. 2014, 47, 425–429. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P. An ECG web services portal for standard and serial ECG analysis with enhanced 3D graphical capabilities. In Proceedings of the Computing in Cardiology, Rennes, France, 24–27 September 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Husain, K.; Zahid, M.S.M.; Ul Hassan, S.; Hasbullah, S.; Mandala, S. Advances of ECG Sensors from Hardware, Software and Format Interoperability Perspectives. Electronics 2021, 10, 105. [Google Scholar] [CrossRef]
- Zentara, T.; Murawski, K. ECG signal coding methods in digital systems. In Communication Papers of the 2018 Federated Conference on Computer Science and Information Systems FedCSIS, Poznań, Poland, 9–12 September 2018; Ganzha, M., Maciaszek, L., Paprzycki, M., Eds.; ACSIS: New Jersey, NJ, USA, 2018; Volume 17, pp. 95–102. [Google Scholar] [CrossRef]
- Trigo, J.D.; Chiarugi, F.; Alesanco, A.; Martinez-Espronceda, M.; Serrano, L.; Chronaki, C.E.; Escayola, J.; Martinez, I.; Garcia, J. Interoperability in Digital Electrocardiography: Harmonization of ISO/IEEE x73-PHD and SCP-ECG. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Mandellos, G.J.; Papaioannou, M.; Panagiotakopoulos, T.; Lymberopoulos, D.K. e-SCP-ECG+v2 Protocol: Expanding the e-SCP-ECG+ Protocol. In Broadband Communications, Networks, and Systems BROADNETS 2018, Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Sucasas, V., Mantas, G., Althunibat, S., Eds.; Springer: Cham, Switzerland, 2019; Volume 263, pp. 125–135. [Google Scholar] [CrossRef]
- Behlouli, H.; Miquel, M.; Fayn, J.; Rubel, P. Towards Self-improving NN based ECG Classifiers. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; IEEE: Piscataway, NJ, USA, 1996; Volume 18, pp. 927–928. [Google Scholar] [CrossRef]
- Fayn, J.; Rubel, P. False Alarm Reduction in Self-Care by Personalized Automatic Detection of ECG Electrode Cable Interchanges. Int. J. Telemed. Appl. 2020, 9175673. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).