Early Graft Failure after Coronary Artery Bypass Surgery: A Case of Anastomosis Detachment Due to Fibromuscular Dysplasia
Abstract
1. Introduction
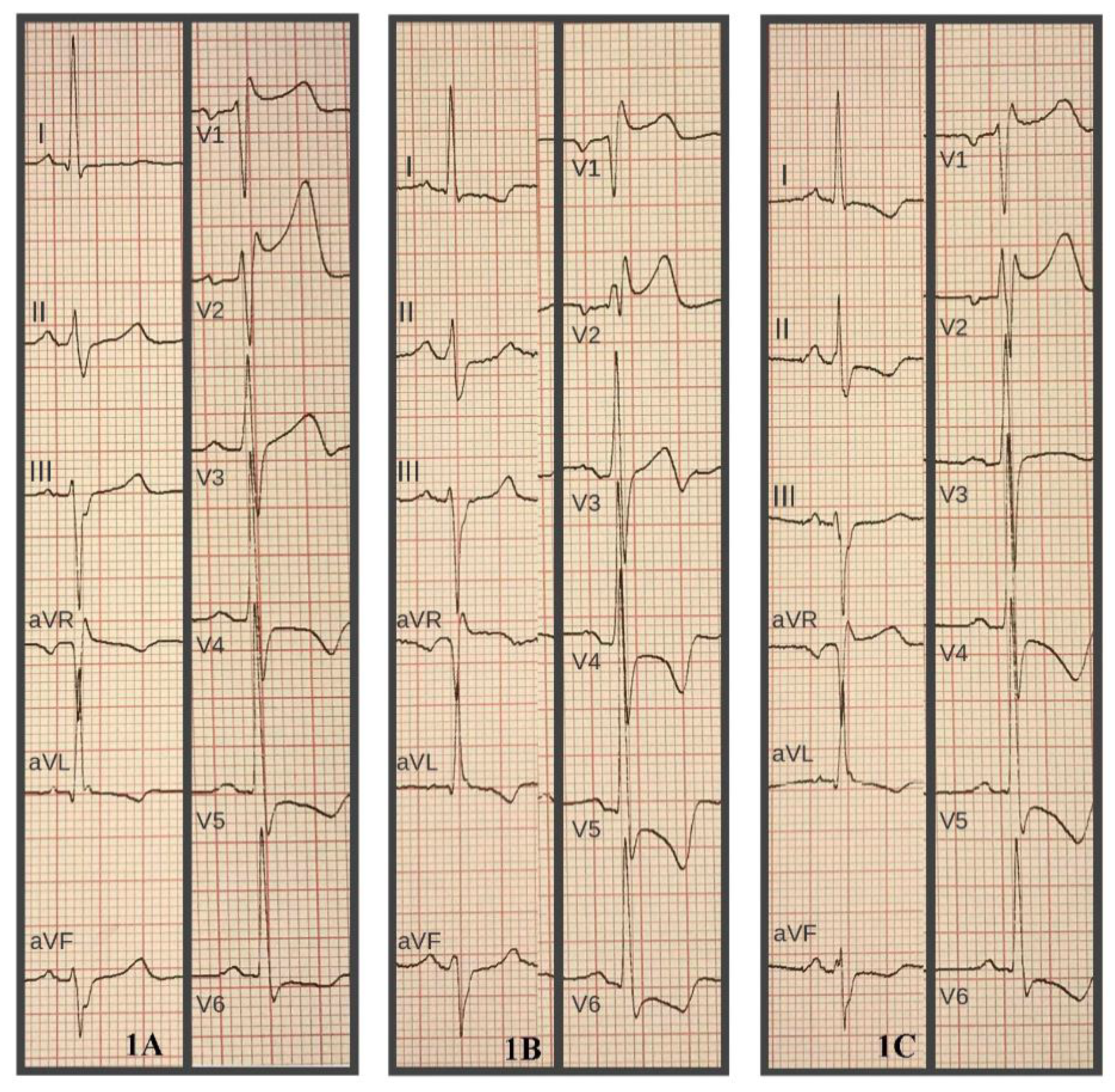
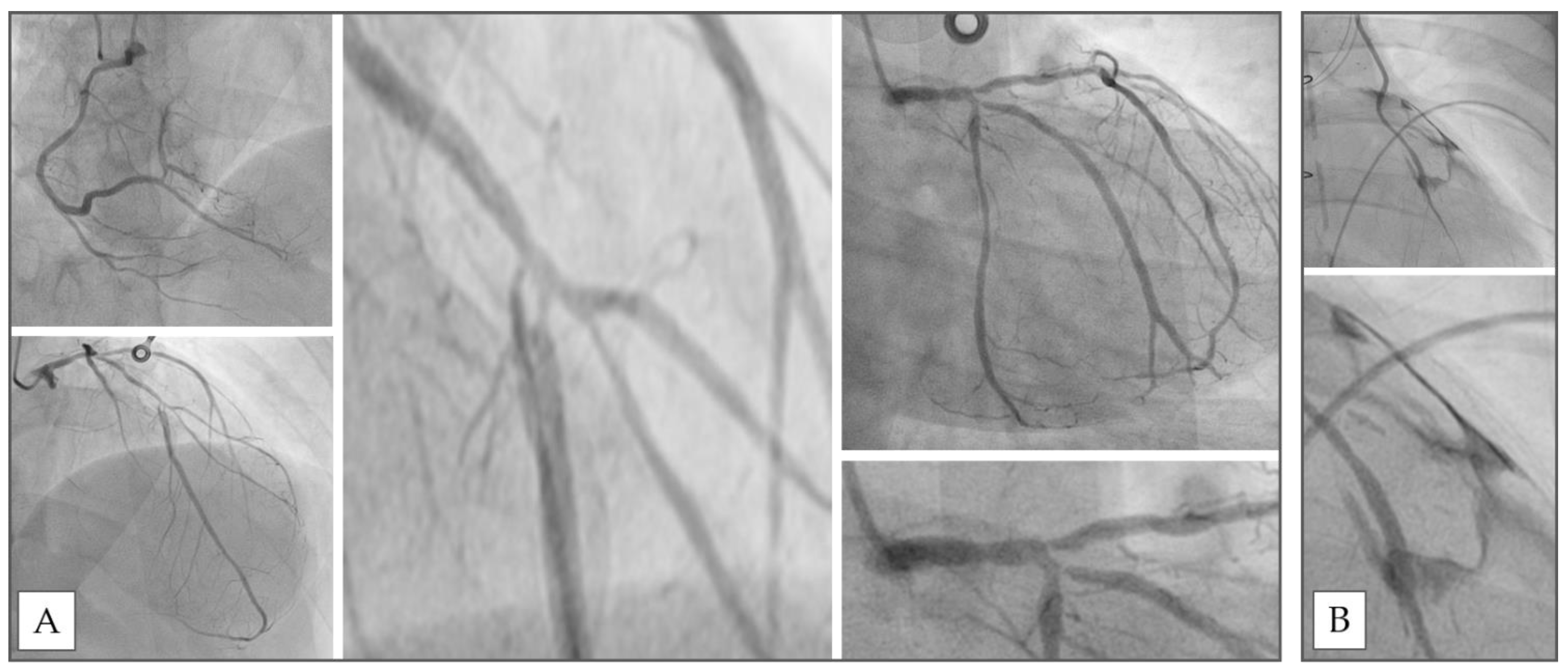
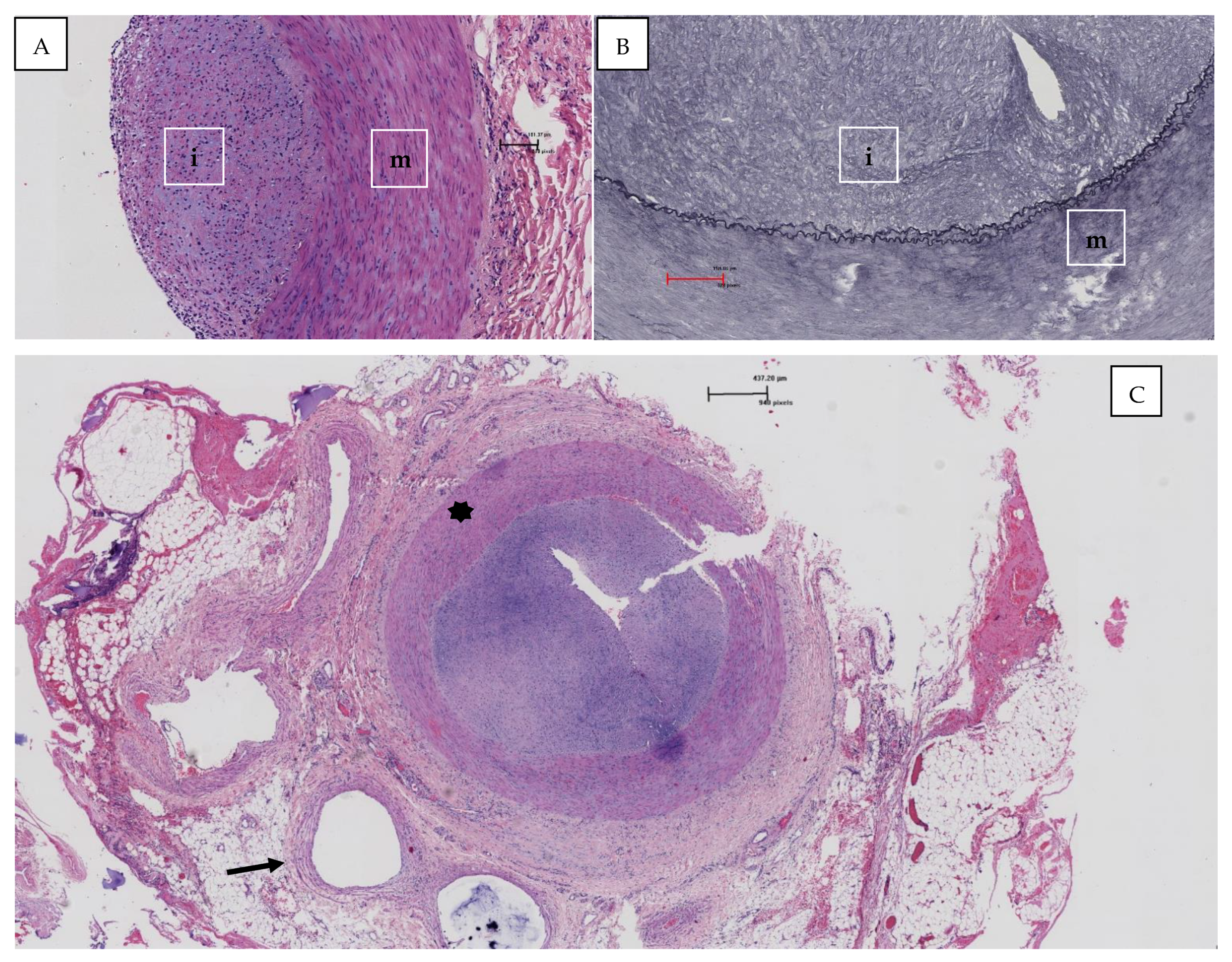
2. Clinical Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olin, J.W.; Froehlich, J.; Gu, X.; Bacharach, J.M.; Eagle, K.; Gray, B.H.; Jaff, M.R.; Kim, E.S.; Mace, P.; Matsumoto, A.H.; et al. The United States Registry for Fibromuscular Dysplasia: Results in the first 447 patients. Circulation 2012, 125, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Michelis, K.C.; Olin, J.W.; Kadian-Dodov, D.; d’Escamard, V.; Kovacic, J.C. Coronary artery manifestations of fibromuscular dysplasia. J. Am. Coll. Cardiol. 2014, 64, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Brinza, E.; Grabinski, V.; Durga, S.; O’Connor, S.; Yesenko, S.L.; Kim, E.S.H.; Gornik, H.L. Lower Extremity Fibromuscular Dysplasia: Clinical Manifestations, Diagnostic Testing, and Approach to Management. Angiology 2017, 68, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, I.; Knowles, M.; Wirtz, E.; Pascarella, L. An Unusual Case of Bilateral Upper Extremity Ischemia Caused by Forearm Vessel Fibromuscular Dysplasia. Ann. Vasc. Surg. 2019, 56, 353.e7–353.e11. [Google Scholar] [CrossRef] [PubMed]
- Bachi, K.; Mani, V.; Jeyachandran, D.; Fayad, Z.A.; Goldstein, R.Z.; Alia-Klein, N. Vascular disease in cocaine addiction. Atherosclerosis 2017, 262, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Pannier-Moreau, I.; Grimbert, P.; Fiquet-Kempf, B.; Vuagnat, A.; Jeunemaitre, X.; Corvol, P.; Plouin, P.F. Possible familial origin of multifocal renal artery fibromuscular dysplasia. J. Hypertens. 1997, 15 Pt 2, 1797–1801. [Google Scholar] [CrossRef]
- Suzuki, H.; Daida, H.; Sakurai, H.; Yamaguchi, H. Familial fibromuscular dysplasia of bilateral brachial arteries. Heart 1999, 82, 251–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, E.G., Jr.; McCormack, L.J. Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin. Proc. 1971, 46, 161–167. [Google Scholar]
- Stanley, J.C.; Gewertz, B.L.; Bove, E.L.; Sottiurai, V.; Fry, W.J. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch. Surg. 1975, 110, 561–566. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Lie, J.T.; Stanson, A.W.; Houser, O.W.; Hollier, L.H.; Sheps, S.G. Arterial fibromuscular dysplasia. Mayo Clin. Proc. 1987, 62, 931–952. [Google Scholar] [CrossRef]
- Lin, W.W.; McGee, G.S.; Patterson, B.K.; Yao, J.S.; Pearce, W.H. Fibromuscular dysplasia of the brachial artery: A case report and review of the literature. J. Vasc. Surg. 1992, 16, 66–70. [Google Scholar] [CrossRef][Green Version]
- Zucker, M.I.; Craven, J.D.; Eisenman, J.I.; Lynch, K. A problem in hemodialysis management: Fibromuscular dysplasia of radial and ulnar arteries—Report of a case. J. Urol. 1976, 115, 606–607. [Google Scholar] [CrossRef]
- Khatri, V.P.; Gaulin, J.C.; Amin, A.K. Fibromuscular dysplasia of distal radial and ulnar arteries: Uncommon cause of digital ischemia. Ann. Plast Surg. 1994, 33, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Gimenez-Roqueplo, A.P.; Fine, E.; Laloux, B.; Fiquet-Kempf, B.; Plouin, P.F.; Jeunemaitre, X.; Laurent, S. Evidence for carotid and radial artery wall subclinical lesions in renal fibromuscular dysplasia. J. Hypertens. 2003, 21, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Gharib, B.; Ghareh Zadeh Shirazi, A.; Moradi, E.; Yaghmaei, B.; Ziaee, V. A rare disease with a rare presentation: Hemi-atrophy caused by fibromuscular dysplasia in a 27 month old girl. Reumatismo 2019, 71, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kesler, W.W., 3rd; Maleszewski, J.J.; Payatakes, A.H. Spontaneous radial artery pseudoaneurysm in an infant due to idiopathic medial hypoplasia—A case report. Case Rep. Plast Surg. Hand Surg. 2019, 6, 69–73. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchi, A.L.; Maragliano, R.; Sessa, F.; Beghi, C.; De Ponti, R.; Castiglioni, B. Early Graft Failure after Coronary Artery Bypass Surgery: A Case of Anastomosis Detachment Due to Fibromuscular Dysplasia. Hearts 2021, 2, 379-383. https://doi.org/10.3390/hearts2030030
Vecchi AL, Maragliano R, Sessa F, Beghi C, De Ponti R, Castiglioni B. Early Graft Failure after Coronary Artery Bypass Surgery: A Case of Anastomosis Detachment Due to Fibromuscular Dysplasia. Hearts. 2021; 2(3):379-383. https://doi.org/10.3390/hearts2030030
Chicago/Turabian StyleVecchi, Andrea Lorenzo, Roberta Maragliano, Fausto Sessa, Cesare Beghi, Roberto De Ponti, and Battistina Castiglioni. 2021. "Early Graft Failure after Coronary Artery Bypass Surgery: A Case of Anastomosis Detachment Due to Fibromuscular Dysplasia" Hearts 2, no. 3: 379-383. https://doi.org/10.3390/hearts2030030
APA StyleVecchi, A. L., Maragliano, R., Sessa, F., Beghi, C., De Ponti, R., & Castiglioni, B. (2021). Early Graft Failure after Coronary Artery Bypass Surgery: A Case of Anastomosis Detachment Due to Fibromuscular Dysplasia. Hearts, 2(3), 379-383. https://doi.org/10.3390/hearts2030030






