1. Introduction
Usage of atrioventricular (AV) nodal blocking agents in patients with underlying kidney dysfunction can result in hyperkalemia, which in turn can result in bradyarrhythmia’s and a shock-like state. This constellation of symptoms has been termed as BRASH (Bradycardia, Renal failure, Atrioventricular-node blockers, Shock, and Hyperkalemia) Syndrome by Dr. Farkas in a blog and later as a publication [
1]. Since its description as a clinical entity, very few cases have been reported and much needs to be understood regarding the presentation of the syndrome [
2,
3,
4,
5,
6]. Here, we describe the case of a patient who presented in hypertensive urgency and congestive heart failure (CHF) and went on to develop BRASH syndrome.
2. Case Report
A 52-year-old woman presented with progressively worsening shortness of breath, leg swelling, and orthopnea for 1 week. She had a history of hypertension, insulin-dependent diabetes mellitus, and chronic kidney disease stage 3. Due to non-adherence, she was unaware of her home medications. She had no history of smoking, drinking alcohol, or substance abuse. Family history was significant for diabetes in her parents. On examination at the time of admission, her heart rate was 97/min, blood pressure was 202/112 mm of Hg, afebrile, and oxygen saturation of 94% on room air with bilateral pitting edema and S3 on cardiac auscultation. Laboratory workup showed an elevated Pro-BNP of 22,000 pg/mL (N: <125 pg/mL, creatinine of 1.78 mg/dL (N: 0.59–1.04 mg/dL), and potassium level of 4 mmol/L (N: 3.5–5.2 mmol/L). Her complete blood counts were significant for a hemoglobin of 6.5 g/dL (N: 12.5–15 g/dL). EKG showed normal sinus rhythm. Chest XR showed pulmonary congestion with mild pleural effusions. Echocardiogram displayed global hypokinesis with an ejection fraction of 40%. She was diagnosed with congestive heart failure exacerbation and was started on intravenous diuresis with furosemide. She was started on oral metoprolol tartrate 25 mg twice a day, nifedipine 60 mg daily, and intravenous labetalol as needed for the management of acute hypertension. The following day, her blood pressure remained elevated, nifedipine was increased to 90 mg/day, and metoprolol changed from 25 mg twice a day to carvedilol 12.5 mg twice daily for additive blood pressure lowering, with continued diuresis. After twelve hours of these changes, she became hypotensive (blood pressure of 67/49 mm of Hg), bradycardic (heart rate of 44/min), and had an altered mental status. EKG showed sinus bradycardia. She was resuscitated with intravenous fluid boluses with normal saline and given one dose of epinephrine along with 10 mg of intravenous glucagon (for beta-blocker toxicity) along with calcium chloride. She was transferred to the intensive care unit and her repeat labs showed a worsening creatinine of 2.2 mg/dL and a potassium of 5.3 mmol/L. She subsequently had a cardiac arrest with pulseless electrical activity. She was revived with ACLS based cardiopulmonary resuscitation and received a total of 3 doses of epinephrine prior to the return of spontaneous circulation. Her rhythm was persistently sinus bradycardia with the rate in the 40 s, and a temporary transvenous pacemaker was placed. She remained in shock and was started on dopamine, norepinephrine, epinephrine, and glucagon drips. She did not need targeted temperature monitoring as she was following commands immediately after resuscitation. Infectious etiology was ruled out. Ultimately, she was stabilized, weaned off of vasopressor agents and the transvenous pacemaker was removed. She was transferred to the medical floors, diuresis was continued, and she was discharged with guideline-directed medical therapy that included metoprolol succinate and her home diuretic. Her constellation of symptoms was most consistent with BRASH as a cause of her rapid deterioration.
3. Discussion
BRASH is characterized by bradycardia, renal failure, presence of AV nodal blockers, shock, and hyperkalemia. The proposed mechanism involves a vicious cycle in the setting of medication use, hyperkalemia, and renal failure (
Figure 1). Renal failure causes hyperkalemia and may cause the accumulation of AV node blockers. Hyperkalemia synergizes with AV nodal blocking agents to cause bradycardia and hypoperfusion. Hypoperfusion, in turn, causes worsening of renal failure. The cyclic effect is difficult to recognize but can lead to rapid clinical deterioration.
The BRASH syndrome has been observed mostly in individuals with reduced kidney function. Poor renal function in combination with dehydration or hypotension can further lead to reduced renal perfusion and worsen hyperkalemia [
4,
7]. Hyperkalemia reduces the resting membrane potential thereby prolonging the action potential resulting in reduced cardiac conduction velocity and increased repolarization [
8]. Interestingly, the degree of hyperkalemia does not always correlate with the severity of the syndrome. Several case reports have shown that mild hyperkalemia in combination with decreased renal function can lead to bradyarrhythmias [
7,
9,
10]. Additionally, mild hyperkalemia has a synergistic effect with AV nodal blockers contributing towards increased bradycardia. When reviewing the types of bradyarrhythmias in BRASH syndrome case reports have ranged from sinus bradycardia (as seen in our case) [
11] to junctional bradycardia [
12] as well as varying degrees of AV blockade including complete heart block [
7].
Among the cases of BRASH documented in the literature, beta-blockers like carvedilol and metoprolol, along with non-dihydropyridine calcium channel blockers (CCB) like verapamil and diltiazem are the most common medications that have been implicated with no previously reported association of dihydropyridine calcium channel blockers. In our case, we hypothesize that in addition to beta-blockers, nifedipine might have played a role in AV nodal suppression. Previously, there have been case reports in patients with autonomic neuropathy from diabetes, in whom nifedipine administration led to bradycardia [
13]. In animal studies with sympathetic nerve disruptions, incremental doses of nifedipine led to AV nodal blockade, which was comparable to the effects of verapamil and diltiazem, showing a dose–response effect on AV nodal suppression [
14]. It has been proposed that in conditions where the compensatory sympathetic drive is compromised like in diabetes, there can be bradycardia from nifedipine.
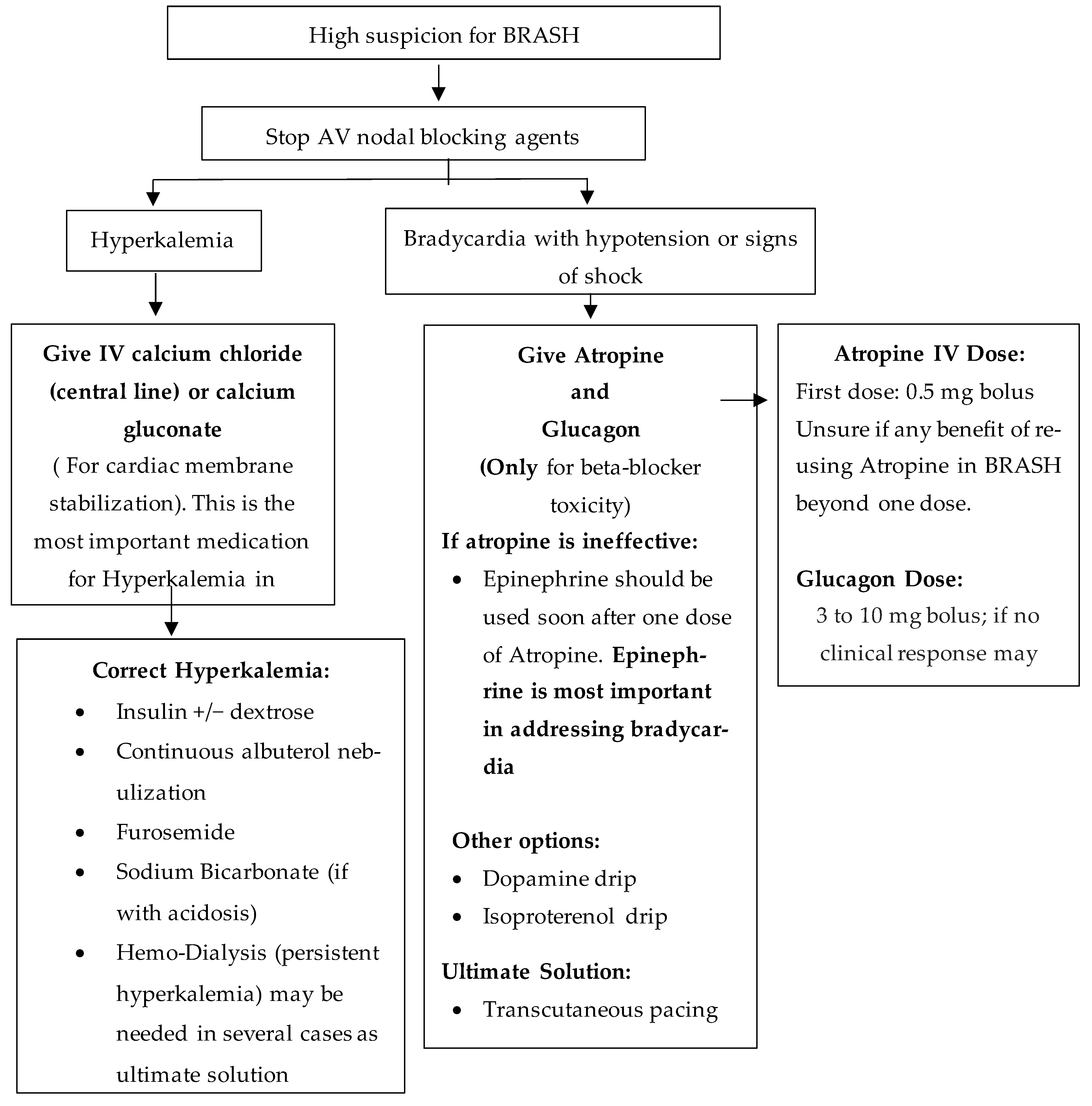
Patients with BRASH can present with different symptoms at the time of admission but can rapidly decompensate to severe bradycardia leading to cardiovascular collapse. The most important manifestation is shock, leading to poor perfusion and end-organ damage. At times, the presentation may be confusing and may not respond to initial resuscitative measures. In order to appropriately treat such patients, correction of all the underlying components leading to the syndrome in combination with aggressive hemodynamic support is the best approach (
Figure 2).
The first step is to stop all AV nodal blocking agents. In cases with severe hyperkalemia, cardiac membrane stabilization with IV calcium should be initiated, along with treatments to rapidly lower serum K+ levels like administration of insulin plus dextrose along with albuterol nebulization. In refractory cases, patients would need emergent dialysis to improve K+ levels. In patients who develop bradycardia induced shock, both dopamine and epinephrine are acceptable vasopressors. Epinephrine having both Beta-1 and Beta-2 receptor action would improve chronotropic effect and would also induce migration of K+ into the cells. In certain BRASH related cases, Isoprotenol, a non-selective Beta-agonist has been used for pressor support [
15,
16]. If the patient continues to remain bradycardic, a transvenous pacemaker can be considered. Additionally, beta-blocker toxicity should be managed with glucagon infusion. Once hemodynamic stability is achieved for more than 24 h, the overall outcome is better.
There is currently no specific data regarding the time period or need for restarting AV nodal blockers in these patients. The risks of further harm in the future are unknown. When looking at restarting beta-blockers or AV Nodal blocking agents especially in patients who need goal directed therapy (as part of heart failure management) as was the case in our patients, a shared discussion must undertake between the patient and physician. In patients with kidney disease, it is important to follow routine labs to monitor potassium levels. In our case we felt restarting a long-acting beta-blocker was appropriate in order to manage her heart failure and hypertension which was her presenting complain to the hospital. She was also given a Nephrology follow up for her chronic kidney disease.
4. Conclusions
BRASH syndrome results from the use of AV nodal blocking agents in patients with renal dysfunction and hyperkalemia which can result in severe bradycardia and shock. Beta-blockers and calcium channel blockers should be judiciously used in patients who are older adults and/or with baseline kidney disease. Slow and closely monitored up-titration of AV nodal blocking agents should be done to avoid profound bradycardia. Once identified, the management of BRASH syndrome remains aggressive hemodynamic support in a critical care setting.
Author Contributions
Conceptualization: S.S. and N.R. writing—original draft preparations: S.S., N.R., A.R.; writing—review and editing, S.S., N.R. and A.R.; visualization, S.S., N.R., A.R.; supervision: A.R., R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
All authors report no conflict of interest.
References
- Farkas, J.D.; Long, B.; Koyfman, A.; Menson, K. BRASH Syndrome: Bradycardia, Renal Failure, AV Blockade, Shock, and Hyper-kalemia. J. Emerg. Med. 2020, 59, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kemnic, T.; Hildebrandt, K.R. BRASH syndrome. BMJ Case Rep. 2020, 13, e233825. [Google Scholar] [CrossRef] [PubMed]
- Grautoff, S.; Holtz, L. Hyperkalemia and BRASH Syndrome in Emergency Medicine. Emerg. Rett. 2020, 23, 172–179. [Google Scholar] [CrossRef]
- Sohal, S.; Ramachandran, A. Syndrome of Bradycardia, Renal Failure, Atrioventricular Nodal Blockers, Shock, And Hyperkalemia (Brash Syndrome): A New Clinical Entity? Chest 2019, 156, A74. [Google Scholar] [CrossRef]
- Golchin, A.; Zhou, M.; Khan, A.H. Bradycardia, Renal Failure, AV-Nodal Blockers, Shock, and Hyperkalemia (BRASH)—A New Clinical Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 3467. [Google Scholar]
- Diribe, N.; Le, J. Trimethoprim/Sulfamethoxazole-Induced Bradycardia, Renal Failure, AV-Node Blockers, Shock and Hyper-kalemia Syndrome. Clin. Pract. Cases Emerg. Med. 2019, 3, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.F.; Javed, F.; Korniyenko, A.; Pratap, B.; Cordova, J.P.; Alviar, C.L.; Herzog, E. Mild hyperkalemia and low eGFR a tedious recipe for cardiac disaster in the elderly: An unusual reversible cause of syncope and heart block. Hear. Int. 2011, 6, e12. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, P.O.; Regan, T.J.; Oldewurtel, H.A. Hyperkalemia, cardiac conduction, and the electrocardiogram: A review. Am. Heart J. 1974, 88, 360–371. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Tan, T.L. Correlation of Iatrogenic Mild Hyperkalemia and Bradyarrhythmia: A Problem of Polypharmacy in Elderly. Med. Health-Kuala Lumpur. 2017, 12, 329–334. [Google Scholar]
- Palmisano, P.; Accogli, M.; Zaccaria, M.; Vergari, A.; Masi, G.D.L.D.; Negro, L.; De Blasi, S. Relationship between seasonal weather changes, risk of dehydration, and incidence of severe bradyarrhythmias requiring urgent temporary transvenous cardiac pacing in an elderly population. Int. J. Biometeorol. 2013, 58, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Y.; Bareeqa, S.B.; Rauf, H.; Ullah, W.; Alraies, M.C. Bradycardia, Renal Failure, Atrioventricular-nodal Blocker, Shock, and Hyperkalemia Syndrome Diagnosis and Literature Review. Cureus 2020, 12, e6985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, T.; Blazar, E. Synergistic bradycardia from beta blockers, hyperkalemia, and renal failure. J. Emerg. Med. 2019, 57, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, M.; Al-Salem, I.H. Nifedipine induced bradycardia in a patient with autonomic neuropathy. Jpn. Hear. J. 1989, 30, 679–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, N.; Narimatsu, A. Effects of nifedipine, a potent calcium antagonistic coronary vasodilator on atrioventricular conduc-tion and blood flow in the isolated atrioventricular node preparation of the dog. Arch. Pharmacol. 1975, 290, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Salomon, D.R.; Rayment, C.M.; Antman, E.M. Hypotension and sinus arrest with exercise-induced hyperkalemia and combined verapamil/propranolol therapy. Am. J. Med. 1986, 80, 1203–1204. [Google Scholar] [CrossRef]
- Váquez, C.; Huelmos, A.; Alegría, E.; Errasti, P.; Purroy, A. Verapamil deleterious effects in chronic renal failure. Nephron 1996, 72, 461–464. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).