Abstract
Intramural haematoma (IMH) of the aorta is one of the causes of acute aortic syndrome which often requires emergency or urgent life-saving surgery. In this review, we discuss the pathophysiology, epidemiology, clinical presentation, diagnostic imaging, surgery and clinical outcomes associated with IMH.
1. Introduction
Aortic intramural haematoma (IMH) represents a subtype of the acute aortic syndromes (AAS), a group of vascular emergencies known to have lethal complications. IMH was first described by Krukenburg in 1920 when he observed aortic dissection without intimal disruption during a post-mortem examination. He was then led to believe that the haemorrhage was caused by the spontaneous rupture of vasa vasorum within the aortic wall [1]. Since then, IMH has been treated as a distinct entity from the other two components of AAS, aortic dissection (AD) and penetrating aortic ulcer (PAU). These three entities, however, are clinically indistinguishable and are associated with significant morbidity and mortality [2,3]. They are often non-exclusive of each other and complications usually develop as a result of the dynamic process of their pathophysiology [4].
IMH can be subdivided into two groups based on the Stanford classification, type A, which involves the ascending aorta, and type B, which is limited to the descending aorta [5]. This anatomical divide guides treatment strategy, with surgery being advocated for the majority of type A injuries, while type B injury may be managed medically or by thoracic endovascular aortic repair (TEVAR) [2]. Although its incidence has been reported to be lower than type B injury, type A IMH has a higher tendency toward complications and adverse outcomes, including aneurysm formation, progression to dissection, rupture and death [6,7,8]. Increased frequencies of pleural/pericardial effusion and cardiac tamponade are also observed in type A IMH [9,10]. Hence, emergency surgery is usually performed in these patients.
There is, however, ongoing debate regarding the surgical management of type A IMH, with studies demonstrating successful medical management and subsequent regression [11,12,13]. Where surgery is an option, the optimum timing for aortic repair from symptom onset remains unclear, and a reliable risk stratification tool to decide if surgical management is appropriate has yet to be introduced. Furthermore, the natural history and progression of type A IMH are not well elucidated, with the suggestion that it may represent early stages of AD or thrombosis of the false lumen in dissection [14].
We aim to characterise the high-risk features of type A IMH, its surgical management and clinical outcome in order to guide clinicians in risk stratification and in deciding whether a surgical approach is appropriate.
2. Epidemiology and Risk Factors
The reported prevalence of IMH varies widely and ranges between 5% and 20% of all AAS, with some studies reporting a prevalence of up to 40% [8,15]. This wide variation may be due to the difference in reported prevalence between eastern and western regions, which may be explained by the difference in diagnostic criteria or genetic variation and susceptibility. There may also be increased diagnostic awareness among clinicians in eastern regions, resulting in the detection and inclusion of more subtle cases [16]. The International Registry of Acute Aortic Dissection (IRAD), the largest AAS study to date in North America/European regions, reported an IMH prevalence of 6.3%, a figure much lower than that of eastern regions. The authors suggested that the difference may also be attributed to the development of AD at the time of transfer to tertiary centres and the possibility of IMH being unrecognised in community hospitals [17].
Compared to the other AAS, IMH tends to occur in older age groups and significant risk factors include male gender, long-standing hypertension and Marfan syndrome [6,7,8,10,18]. Other risk factors are similar to those in AAS, which include hyperlipidaemia, coronary artery disease, peripheral vascular disease, obesity, diabetes mellitus and smoking [7]. Type A IMH is less common than type B IMH, with a reported prevalence of 40%, compared to 60% in the latter [17]. However, patients with type A IMH are at higher risk of complication, with a higher percentage progressing to dissection and rupture [19].
3. Anatomy of the Aorta, Classification Systems and Diagnostic Modalities
Aortic pathologies are defined by the aortic segment(s) involved, the layer(s) affected, aortic diameter, wall thickness and subsequent development of complications. There are three main segments of the aorta: ascending aorta, aortic arch and descending aorta. The ascending aorta spans from the aortic annulus to the brachiocephalic trunk, which marks the start of the aortic arch. This second segment ends at the left subclavian artery, where it continues to the third segment, the descending thoracic aorta. The descending thoracic aorta enters the abdominal cavity, becoming the abdominal aorta, and terminates at the bifurcation of the common iliac arteries [2].
Shifting to the microanatomy of the aortic wall, this is divided into three distinct layers: intima, media and adventitia. The tunica intima, being the innermost layer, has direct contact with luminal blood. This is the thinnest layer, consisting of endothelial cells supported by connective tissue and an elastic lamina. The medial layer or tunica media is made up of collagen, smooth muscle cells and elastic tissue, providing the aorta’s vascular tone. The tunica adventitia is composed of mainly collagen and provides the aorta’s passive structural support [2]. Vasa vasorum, translated literally to “vessel of the vessels”, are the small arteries that form the vascular supply of the aorta, entering through the adventitia and terminating in the tunica media. These arteries are believed to be the bleeding source of IMH.
The significance of this is reflected by the use of Stanford and DeBakey classification systems in categorising the AAS. The DeBakey system divides dissection based on the origin of intimal tear and extent of dissection, while the Stanford system categorises dissections into two types, regardless of their origin: type A, where IMH involves the ascending aorta, and type B, where the ascending aorta is spared [5]. Where the aortic arch is affected, this would be classified as a type A injury if the ascending aorta is involved; otherwise, it would be considered as a type B injury [20]. The Stanford classification system is more widely used, particularly in defining IMH and dictating its management strategies and prognosis.
On unenhanced computed tomography (CT) scan, the hallmark feature of IMH is a uniform, hyperattenuated (60–70 Hounsfield units), crescentic or circular thickening of the aortic wall (>7 mm), which extends longitudinally and in a non-spiral fashion. This area remains non-opacified on enhanced acquisition [2] (see Figure 1A). By combining the use of unenhanced acquisition in the first instance, followed by contrast enhancement, sensitivity of detecting IMH can reach up to 96% [2]. Magnetic resonance imaging (MRI) has the added ability of differentiating acute from chronic haematoma depending on its signal intensity due to the formation of methaemoglobin [21]. On transoesophageal echocardiography, IMH is detected by a transversely oriented crescentic structure with no visible blood flow [22]. CT remains the preferred modality as it is readily available and quick to perform.
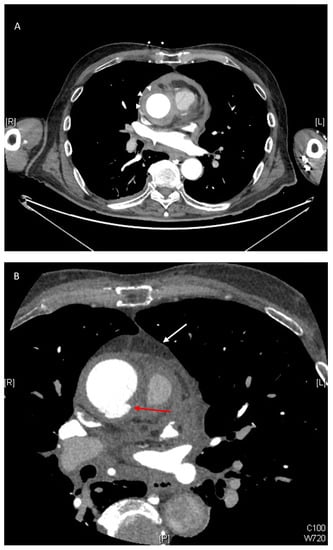
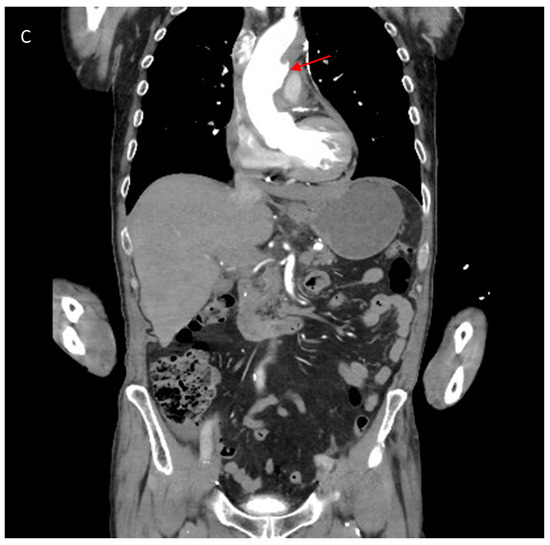
Figure 1.
(A) Contrast enhanced CT scan showing axial image of type A intramural haematoma (IMH) with a thickness measuring 11 mm (white arrows). The lesion is non-enhancing and crescentic in nature. (B) Axial view on CT scan demonstrating other high-risk features of IMH including ulcer-like projection (ULP) (red arrow) and the presence of a haemo-pericardium (white arrow). (C) Coronal view on CT scan demonstrating ulcer-like projection (red arrow).
4. Pathophysiology and Clinical Manifestations
IMH can be distinguished from AD by the absence of communication with the true aortic lumen via an intimal flap or disruption. This can be visualised with imaging modalities or at the time of surgery. The absence of direct flow communication suggests that the pathophysiology in IMH involves the spontaneous rupture of the vasa vasorum.
There is, however, increasing evidence to suggest that perhaps IMH is a precursor to the development of AD. This is supported by the detection of small intimal defects, which are also referred to as focal intimal disruption (FID) or ulcer-like projections (ULP), which increase the risk of AD [6,23,24,25,26] (see Figure 1B,C). Occasionally, small contrast accumulations within the IMH that are not in contact with the true lumen are identified and these are termed intramural blood pools (IBPs) [6,26]. The findings of PAU with type A IMH are not uncommon (though this is more frequently found in type B IMH) and equally poses a significant risk of subsequent AD, rupture and aneurysmal formation [9,25,27,28]. The pathological changes in the tunica media caused by IMH can lead to structural weakness of the aortic wall and amplify susceptibility to failure during increased loading conditions [29]. This is supported by animal models where compromise of the vasa vasorum leads to decreased aortic elasticity and increased strain on the aortic wall [30].
Presenting symptoms of IMH are not dissimilar to those of AD, with chest pain and back pain being the most common. Hence, it is impossible to differentiate the AAS based on the presenting complaint [6,7,17]. Other manifestations of the disease may include acute myocardial infarction, neurological deficit, aortic insufficiency and syncope, though these are relatively infrequent compared to the other AAS [17,24]. On imaging, higher rates of pleural and pericardial effusions (see Figure 1B) are found in type A IMH than AD and often result in cardiac tamponade and subsequent cardiogenic shock. This may be due to the relatively exterior location of the disease in the aortic wall in IMH compared to AD [31]. The mural thickening in IMH also rarely narrows the true aortic lumen sufficiently to compromise blood flow or branch vessel flow [32]. Hence, malperfusion is not commonly observed in IMH.
5. Surgery or Medical Management in Type A IMH
The management of type A IMH has been controversial, with studies demonstrating conflicting evidence between surgical and medical management outcomes. Eastern studies (mostly Japanese and Korean) have demonstrated poor emergency surgical outcomes but successful conservative management with blood pressure control, regular imaging and timely surgery and, in some cases, even achieving a high percentage of subsequent regression and disappearance of type A IMH [11,12,13]. In contrast, western studies have consistently advocated for emergency surgery in the management of type A IMH due to the observed high mortality rates in medically treated patients [8,33]. While the reason behind this remains unclear, Pelzel et al. postulate that this may be due to a difference in diagnostic criteria resulting in the inclusion of subtle, less severe cases, the influence of genetic variation and the impact of health inequalities. Additionally, type A IMH severity cannot be directly compared between eastern and western studies as some of the studies did not provide sufficient data to compare aortic diameter or wall thickness [16].
A unifying decision in the midst of these controversies is that patients who demonstrate haemodynamic instability, cardiac tamponade, vital organ malperfusion, periaortic haematoma, persistent pain and progression to aortic dissection and rupture are candidates for emergency surgery, ideally performed within 24–48 h of symptom onset [7,15,25,34]. In these patients, outcomes tend to be better and mortality rates have been shown to be relatively low [7,15,28]. Notwithstanding, the results obtained from these studies may be skewed as the majority are performed in tertiary centres where very high-risk patients who were unlikely to be candidates for surgery or patients who declined surgery at the outset would not have been included in the analysed cohorts.
Structural weakness of the tunica media from haematoma formation and mechanical stress can lead to the formation of fusiform aneurysm and potential progression to classic AD with an intimal flap [35]. Few studies include or consider ULP as part of the definition of IMH, classifying these as PAU instead—an exclusion criterion particularly in Asian studies. As a result, true evaluation of the risk of progression of IMH in the presence of small intimal defects was not possible. The presence of type A IMH in the presence of ULP or PAU has been found to be associated with a higher frequency of pericardial effusion and prevalence of life-threatening complications [9,27,28]. With the limited evidence available, the detection of ULP and PAU in type A IMH increases its risk of progression to complications [21,27,28].
While regression and disappearance have been shown, the course of type A IMH may not be as benign as portrayed in some of the Asian studies [11,12,13]. The rates of progression to complication, including aortic dissection and rupture and fusiform aneurysmal formation, are up to 33% [18,24,33,36]. This is particularly so in the medically treated cohort with a high conversion rate to surgery during hospital admission due to subsequent development of complications. Despite demonstrating good outcomes following medical management in the Japanese/Korean studies, their patients experienced significantly higher complication rates [16,18,24,36]. Pericardiocentesis was performed more frequently for pericardial effusion compared to western studies and there was a higher occurrence of progression to AD, resulting in crossover to surgery [16]. Whether there is a genetic or environmental influence on outcome resulting in successful medical management in Asian studies is unknown, but this is certainly possible. Estrera and colleagues studied a predominantly Caucasian population and demonstrated one Japanese patient with a 49 mm ascending aortic diameter who was medically managed with subsequent resolution of IMH at his 4-year follow-up scan and absence of progression in the aortic diameter [24]. On the contrary, in a population of Chinese patients, there was a survival benefit in those who underwent surgery, similar to the findings of those in western studies. They therefore disprove genetic or environmental implications and suggest that it may be due to the intrinsic differences in aortic pathology but also the lack of follow-up imaging, which led to failed delivery of timely surgery [37].
Current evidence suggests that type A IMH patients with an ascending aortic diameter >50 mm and wall thickness >11 mm at presentation are at higher risk of progression of disease and should be operated on in a timely manner [11,33,35]. An aorta with these characteristics is at the highest risk of progression to dissection and rupture within the first 8 days of symptom onset [24]. Other predictors of disease progression include large PAU on top of IMH, increasing pleural effusion, persistent and recurrent pain, absence of early beta-blockade and younger age (<55 years) [10]. In patients with connective tissue disorders such as Marfan syndrome, we recommend timely surgical repair. Although the incidence of IMH is significantly lower compared to AD in patients with Marfan syndrome, the argument for surgery in these patients stems from their predisposition to AD and rupture [25]. This is also supported by several studies that have demonstrated Marfan syndrome to be a significant risk factor for late progression to AD and rupture [9,10,38].
The long-term survival rate generally approaches 90% at 10 years for patients treated with emergency surgery [11]. Despite the eventual regression of type A IMH on surveillance scans of medically managed patients, some patients eventually develop classic AD. Unfortunately, the natural course of type A IMH remains unclear and it remains challenging to predict subsequent progression of disease. Nishigami and colleagues studied the course of IMH in 44 patients and reported that the disappearance of IMH was associated with younger age and smaller aortic diameter. During long-term follow-up, none of the patients in the disappearance group developed AD or progressive aortic dilatation [22]. In contrast, von Kodolitsch et al. reported younger age as an independent predictor of late progression [9]. Evidently, there remains a discrepancy in the predictive factors of late progression of disease.
In a small subset of patients for whom surgery may not be suitable (older patients with significant medical comorbidities) or where surgery has been declined, a conservative approach may be adopted. Kitamura et al. proposed that. in patients with an aortic diameter ≤50 mm, pain score ≤3/10 and no evidence of ascending ULP, a watch-and-wait strategy may be justified. In these patients, management involves aggressive blood pressure and pain control, with follow-up serial imaging [39]. The use of oral beta-blockers in IMH has shown better long-term outcome and reduction of risk to disease progression by reducing systolic arterial pressure and pressure changes, resulting in less strain on the aortic wall [9]. This may have beneficial effects of stabilising the extracellular matrix of the aorta in both surgically and medically managed patients [40].
Compared with medically treated patients, patients who were operated on had a significantly reduced length of hospital and intensive care unit (ICU) stay, with less frequent imaging tests performed [15]. This may have cost-benefit implications that have not been analysed previously, particularly if patients subsequently develop complications and require surgical intervention despite optimal medical management.
6. Should Type A IMH Be Treated Similarly to Type A AD?
Uchida et al. proposed the switch in definition of IMH to a thrombosed-type acute AD as they found that 78% of patients had evidence of intimal tears [14]. It is possible that, with improved imaging techniques, smaller intimal tears will be increasingly picked up, disproving the initial hypothesis of spontaneous vasa vasorum rupture as the primary cause of IMH. On the contrary, Ahn et al. argue against the separation of IMH from AD since cumulative clinical experience has demonstrated that IMH does not always progress to AD and, in some cases, even demonstrates full resorption and normalisation of the initial pathology with medical management [36]. To suggest that type A IMH should be treated similarly to type A AD would also invariably mean that all patients with type A IMH should receive prophylactic surgery to prevent progression of disease. Hence, differentiation between the two disease entities bears important clinical relevance and prognostic implications [36].
While in-hospital mortality and 30-day mortality of type A IMH have been shown to be similar to type A AD [38], patients with type A IMH may benefit from deferring their surgery by two to three days following admission if they remain haemodynamically stable. This may avoid the extensive inflammatory response encountered during emergency surgery but also allow time for thickening of the aortic wall and stabilisation of the intimal flap, facilitating aortic repair and reducing surgical mortality [24,38]. Overall long-term prognosis, however, favours type A IMH [34,38].
Patients tend to be older in the type A IMH group compared to the AD group, with a higher frequency of female patients observed [36,38]. While pleural effusion, pericardial effusion and mediastinal haemorrhage were more commonly observed in type A IMH patients, AD patients commonly develop significant aortic insufficiency, organ malperfusion, acute myocardial infarction and neurological deficits [13]. The higher incidence of pleural/pericardial effusion and periaortic haematoma may be explained by the close proximity of IMH to the tunica adventitia [17]. Although several studies have demonstrated successful pericardiocentesis in patients with pericardial effusion without further need for open surgery, they are small-scale studies whose findings require further validation [35,37,41].
The surgical approach in both type A IMH and type A AD are not dissimilar. Depending on the location of the injury, aortic repair is performed where possible, with the goal of restoring normal aortic dimension. A supracoronary tube graft is implanted in cases where the aneurysm is located distal to the aortic arch and proximally limited to the sinotubular junction, with distal anastomosis performed just below the aortic arch [2]. Where there is proximal extension of the aneurysm below the sinotubular junction and dilatation of the aortic sinuses, surgical repair depends on the extent of involvement of the aortic annulus and the aortic valve. In patients with a normal tricuspid aortic valve with no evidence of regurgitation from annular dilatation, valve-preserving techniques should be applied by using methods such as the David operation or the modified Yacoub technique. The David operation involves resuspension of the aortic valve within the tubular graft, while the modified Yacoub technique only replaces the aortic sinus and is susceptible to late annular dilatation; hence, it may require reinforcement with additional aortic annuloplasty. In cases of severe aortic regurgitation, the aortic valve should be replaced. In patients with a bicuspid aortic valve, haemodynamic flow will be affected despite repair, and aortic root replacement should be performed if a durable repair is perceived to be unlikely [2].
A total arch replacement or, more commonly, hemiarch replacement is performed where there is significant aortic arch dilatation and distal aneurysmal extension, presence of PAU or the detection of intimal tear in the distal or greater curvature of the aortic arch. In IMH, the aortic wall should also be carefully inspected, and all remaining thrombus should be evacuated before performing distal anastomosis [2,38]. The procedure will require brief antegrade cerebral perfusion and hypothermic circulatory arrest as the aortic arch is explored and partially resected in a hemiarch replacement. In aortic surgery, the risk of paraplegia depends on the speed of repair and cross-clamp duration [2]. Patients with evidence of coronary artery disease may also have concomitant coronary artery bypass graft (CABG) surgery performed.
Reoperation rates of IMH and AD should be taken into consideration when planning aortic surgeries. Indications for reoperation may include aortic rupture or an impending rupture, rapid progressive dilatation of the aorta, graft infection and severe aortic valve insufficiency. Matsushita et al. reported a 10% risk of reoperation in patients with IMH or AD, and some studies report late reoperation rates of up to 40% [34,42]. Mortality rates after reoperation are also not negligible and risk factors include graft infection, concomitant CABG, higher body mass index, combined open arch and descending procedures, lower distal anastomosis and longer time between reinterventions [42]. Long-term periodical follow-up with early reintervention may, therefore, be necessary to improve outcome in these patients [34].
Generally, surgery for type A IMH is less complex compared to type A AD, mainly because aortic valve insufficiency and organ malperfusion are less common or severe. Moreover, patients tend to be more stable, with less complications preoperatively, leading to better operative outcomes [34]. On the other hand, post-operative complications are more common in AD, owing to longer operative times and duration of cardiopulmonary bypass, with stroke being a common postoperative complication [38]. Compiling the evidence available to date, type A IMH should remain as a distinct entity from type A AD, since the pathophysiology, risk factors, operative approach, pre- and post-operative complications and overall long-term prognosis are different.
7. Future Research
While a randomised study would be of great use, it would be challenging to perform such a study design considering its ethical implications. At best, a large multicentre study with ethnic variation and uniform IMH diagnostic criteria may be feasible. It would be important to re-evaluate and update current diagnostic criteria and imaging characteristics of IMH considering the ever-changing and improvement in imaging modalities. This is of particular importance in patients with evidence of ULP or PAU, considering that these were features excluded for analysis in many IMH studies. Patient inclusion and selection criteria should also be specified, particularly when studies are performed in tertiary referral centres where time for transfer and patients who were declined at the outset are important factors that may affect the results of a study.
The introduction of a risk stratification tool and its validation in clinical practice may be helpful in deciding the appropriateness of surgical intervention in type A IMH. This may include the use of D-dimer levels since several studies have suggested their diagnostic role in AAS [6,20]. Where a watch-and-wait strategy is decided, the question arises regarding time interval between serial imaging. More studies are needed in order to validate the sensitivity of scans at different time intervals. We suggest that factors such as cost-benefit analysis, hospital or ICU length of stay and the impact on quality of life should also be taken into consideration when designing future studies. Finally, while studies report subsequent regression of type A IMH, the predictive factors remain unknown and it would be interesting to study this cohort of patients to evaluate the natural course of the disease.
8. Conclusions
IMH of the proximal aorta is a potentially lethal condition with a significant risk of progression to AD, rupture and aneurysm formation. Emergency surgery for type A IMH should be performed within 24 to 48 h of symptom onset for patients with evidence of haemodynamic compromise, vital organ malperfusion, periaortic haematoma, persistent pain, cardiac tamponade and where there is evidence of progression to AD and rupture. Patients at high risk of progression to AD include those with an ascending aortic diameter >50 mm, aortic wall thickness >11 mm and evidence of intimal disruption from ULP or PAU. Timely surgery should be performed in these patients. Medical management and regular surveillance imaging may be an option for patients without these high-risk features or for patients who are not suitable for surgery, with beta-blockade conferring a clear survival benefit.
Author Contributions
All authors contributed to the design and implementation of the research and to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Krukenberg, E. Beitrage zur Frage des Aneurysma dissecans. Beitr. Pathol. Anat. Allg. Pathol. 1920, 67, 329–351. [Google Scholar]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart. J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Evangelista, A. Progress in the acute aortic syndrome. Rev. Española Cardiol. 2007, 60, 428–439. [Google Scholar]
- Chou, A.S.; Ziganshin, B.A.; Charilaou, P.; Tranquilli, M.; Rizzo, J.A.; Elefteriades, J.A. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J. Thorac. Cardiovasc. Surg. 2016, 151, 361–373.e1. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.; Clough, R.E. Management of acute aortic dissection. Lancet 2015, 385, 800–811. [Google Scholar] [CrossRef]
- Ferrera, C.; Vilacosta, I.; Gómez-Polo, J.C.; Villanueva-Medina, S.; Cabeza, B.; Ortega, L.; Cañadas, V.; Alcázar, M.C.; Martínez-López, I.; Maroto-Castellanos, L.; et al. Evolution and prognosis of intramural aortic hematoma. Insights from a midterm cohort study. Int. J. Cardiol. 2017, 249, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Schönhoff, F.; Zanchin, C.; Czerny, M.; Makaloski, V.; Gahl, B.; Carrel, T.; Schmidli, J. Aorta Related and All-cause Mortality in Patients with Aortic Intramural Haematoma. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 447–453. [Google Scholar] [CrossRef]
- Maraj, R.; Rerkpattanapipat, P.; Jacobs, L.E.; Makornwattana, P.; Kotler, M.N. Meta-analysis of 143 reported cases of aortic intramural hematoma. Am. J. Cardiol. 2000, 86, 664–668. [Google Scholar] [CrossRef]
- von Kodolitsch, Y.; Csösz, S.K.; Koschyk, D.H.; Schalwat, I.; Loose, R.; Karck, M.; Dieckmann, C.; Fattori, R.; Haverich, A.; Berger, J.; et al. Intramural hematoma of the aorta: Predictors of progression to dissection and rupture. Circulation 2003, 107, 1158–1163. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Von Kodolitsch, Y.; Petersen, B.; Loose, R.; Helmchen, U.; Haverich, A.; Spielmann, R.P. Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic Implications. Circulation 1995, 92, 1465–1472. [Google Scholar] [CrossRef]
- Kitai, T.; Kaji, S.; Yamamuro, A.; Tani, T.; Tamita, K.; Kinoshita, M.; Ehara, N.; Kobori, A.; Nasu, M.; Okada, Y.; et al. Clinical outcomes of medical therapy and timely operation in initially diagnosed type a aortic intramural hematoma: A 20-year experience. Circulation 2009, 120, S292–S298. [Google Scholar] [CrossRef] [PubMed]
- Moizumi, Y.; Komatsu, T.; Motoyoshi, N.; Tabayashi, K. Management of patients with intramural hematoma involving the ascending aorta. J. Thorac. Cardiovasc. Surg. 2002, 124, 918–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, J.-K.; Kim, H.-S.; Kang, D.-H.; Lim, T.-H.; Song, M.-G.; Park, S.-W.; Park, S.-J. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J. Am. Coll. Cardiol. 2001, 37, 1604–1610. [Google Scholar] [CrossRef]
- Uchida, K.; Imoto, K.; Karube, N.; Minami, T.; Cho, T.; Goda, M.; Suzuki, S.-I.; Masuda, M. Intramural haematoma should be referred to as thrombosed-type aortic dissection†. Eur. J. Cardio Thorac. Surg. 2013, 44, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Hata, H.; Sezai, A.; Yoshitake, I.; Wakui, S.; Shiono, M. Optimal treatment strategy for type A acute aortic dissection with intramural hematoma. J. Thorac. Cardiovasc. Surg. 2014, 147, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Pelzel, J.M.; Braverman, A.C.; Hirsch, A.T.; Harris, K.M. International heterogeneity in diagnostic frequency and clinical outcomes of ascending aortic intramural hematoma. J. Am. Soc. Echocardiogr. 2007, 20, 1260–1268. [Google Scholar] [CrossRef]
- Harris, K.M.; Braverman, A.C.; Eagle, K.A.; Woznicki, E.M.; Pyeritz, R.E.; Myrmel, T.; Peterson, M.D.; Voehringer, M.; Fattori, R.; Januzzi, J.L.; et al. Acute aortic intramural hematoma: An analysis from the international registry of acute aortic dissection. Circulation 2012, 126 (Suppl. 1), S91–S96. [Google Scholar] [CrossRef]
- Evangelista, A.; Dominguez, R.; Sebastia, C.; Salas, A.; Permanyer-Miralda, G.; Avegliano, G.; Gomez-Bosh, Z.; Gonzalez-Alujas, T.; Del Castillo, H.G.; Soler-Soler, J. Prognostic value of clinical and morphologic findings in short-term evolution of aortic intramural haematoma. Therapeutic implications. Eur. Hear. J. 2004, 25, 81–87. [Google Scholar] [CrossRef]
- von Kodolitsch, Y.; Nienaber, C.A. Intramural hemorrhage of the thoracic aorta: Diagnosis, therapy and prognosis of 209 in vivo diagnosed cases. Z. Kardiol. 1998, 87, 797–807. [Google Scholar] [CrossRef]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Hear. J. 2017, 39, 739–749d. [Google Scholar] [CrossRef]
- Nienaber, C. The role of imaging in acute aortic syndromes. Eur. Hear. J. Cardiovasc. Imaging 2012, 14, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Nishigami, K.; Tsuchiya, T.; Shono, H.; Horibata, Y.; Honda, T. Disappearance of aortic intramural hematoma and its significance to the prognosis. Circulation 2000, 102 (Suppl. 3), Iii-243–Iii-247. [Google Scholar] [CrossRef]
- Park, K.-H.; Lim, C.; Choi, J.H.; Sung, K.; Kim, K.; Lee, Y.T.; Park, P.W. Prevalence of aortic intimal defect in surgically treated acute type a intramural hematoma. Ann. Thorac. Surg. 2008, 86, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Estrera, A.L.; Miller, C.; Lee, T.-Y.; De Rango, P.; Abdullah, S.; Walkes, J.-C.; Milewicz, D.; Safi, H. Acute type A intramural hematoma: Analysis of current management strategy. Circulation 2009, 120 (Suppl. 1), S287–S291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Svensson, L.G.; Labib, S.B.; Eisenhauer, A.C.; Butterly, J.R. Intimal tear without hematoma: An important variant of aortic dissection that can elude current imaging techniques. Circulation 1999, 99, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.J.; Johnson, P.T.; Fishman, E.K.; Zimmerman, S. Aortic intramural hematoma: Review of high-risk imaging features. J. Cardiovasc. Comput. Tomogr. 2013, 7, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ganaha, F.; Miller, D.C.; Sugimoto, K.; Do, Y.S.; Minamiguchi, H.; Saito, H.; Mitchell, R.S.; Dake, M.D. The prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: A clinical and radiological analysis. Circulation 2002, 106, 342–348. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yen, H.-T.; Lo, C.-M.; Wu, C.-C.; Huang, D.K.-R.; Sheu, J.-J. Natural courses and long-term results of type A acute aortic intramural haematoma and retrograde thrombosed type A acute aortic dissection: A single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2019, 30, 113–120. [Google Scholar] [CrossRef]
- Okamoto, R.J.; Xu, H.; Kouchoukos, N.T.; Moon, M.R.; Sundt, T.M. The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. J. Thorac. Cardiovasc. Surg. 2003, 126, 842–850. [Google Scholar] [CrossRef]
- Stefanadis, C.I.; Karayannacos, P.E.; Boudoulas, H.K.; Stratos, C.G.; Vlachopoulos, C.V.; Dontas, I.A.; Toutouzas, P.K. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc. Res. 1993, 27, 951–956. [Google Scholar] [CrossRef]
- Goldberg, J.B.; Kim, J.B.; Sundt, T.M. Current understandings and approach to the management of aortic intramural hematomas. Semin. Thorac. Cardiovasc. Surg. 2014, 26, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Maslow, A.; Atalay, M.K.; Sodha, N. Intramural Hematoma. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1341–1362. [Google Scholar] [CrossRef] [PubMed]
- Attia, R.Q.; Young, C.; Fallouh, H.B.; Scarci, M. In patients with acute aortic intramural haematoma is open surgical repair superior to conservative management? Interact. Cardiovasc. Thorac. Surg. 2009, 9, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.; Fukui, T.; Tabata, M.; Sato, Y.; Takanashi, S. Preoperative characteristics and surgical outcomes of acute intramural hematoma involving the ascending aorta: A propensity score–matched analysis. J. Thorac. Cardiovasc. Surg. 2016, 151, 351–358. [Google Scholar] [CrossRef]
- Kaji, S.; Nishigami, K.; Akasaka, T.; Hozumi, T.; Takagi, T.; Kawamoto, T.; Okura, H.; Shono, H.; Horibata, Y.; Honda, T.; et al. Prediction of progression or regression of type A aortic intramural hematoma by computed tomography. Circulation 1999, 100 (Suppl. 2), II-281–Ii-286. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-M.; Kim, H.; Kwon, O.; Om, S.Y.; Heo, R.; Lee, S.; Kim, D.-H.; Kim, H.J.; Kim, J.B.; Jung, S.H.; et al. Differential clinical features and long-term prognosis of acute aortic syndrome according to disease entity. Eur. Hear. J. 2019, 40, 2727–2736. [Google Scholar] [CrossRef]
- Ho, H.H.; Cheung, C.W.; Jim, M.H.; Miu, K.M.; Siu, C.W.; Lam, Y.M.; Chan, H.W.; Lee, W.L.; Tse, H.-F. Type A aortic intramural hematoma: Clinical features and outcomes in chinese patients. Clin. Cardiol. 2011, 34, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.K.; Tanaka, A.; Charlton-Ouw, K.M.; Afifi, R.O.; Iii, C.C.M.; Safi, H.J.; Estrera, A.L.; Miller, C.C. Outcomes and management of type A intramural hematoma. Ann. Cardiothorac. Surg. 2016, 5, 317–327. [Google Scholar] [CrossRef]
- Kitamura, T.; Torii, S.; Miyamoto, T.; Mishima, T.; Ohkubo, H.; Fujioka, S.; Yakuwa, K.; Araki, H.; Kondo, S.; Tamura, Y.; et al. Watch-and-wait strategy for type A intramural haematoma and acute aortic dissection with thrombosed false lumen of the ascending aorta: A Japanese single-centre experience. Eur. J. Cardiothorac. Surg. 2020. [Google Scholar] [CrossRef]
- Shores, J.; Berger, K.R.; Murphy, E.A.; Pyeritz, R.E. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan syndrome. N. Engl. J. Med. 1994, 330, 1335–1341. [Google Scholar] [CrossRef]
- Shimizu, H.; Yoshino, H.; Udagawa, H.; Watanuki, A.; Yano, K.; Ide, H.; Sudo, K.; Ishikawa, K. Prognosis of aortic intramural hemorrhage compared with classic aortic dissection. Am. J. Cardiol. 2000, 85, 792–795. [Google Scholar] [CrossRef]
- Roselli, E.E.; Loor, G.; He, J.; Rafael, A.E.; Rajeswaran, J.; Houghtaling, P.L.; Svensson, L.G.; Blackstone, E.H.; Lytle, B.W. Distal aortic interventions after repair of ascending dissection: The argument for a more aggressive approach. J. Thorac. Cardiovasc. Surg. 2015, 149, S117–S124.e3. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).