An Analysis of Frontoethmoid Cell Types According to the International Frontal Sinus Anatomy Classification in the Korean Population and Their Relation to Frontal Sinusitis
Abstract
1. Introduction
2. Material and Methods
CT Scan Protocol
3. Results
3.1. Demographic Data
3.2. Prevalence of Frontal Cell Variants
3.3. Association of Frontal Cell Variants with Frontal Sinus Involvement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valdes, C.J.; Bogado, M.; Samaha, M. Causes of failure in endoscopic frontal sinus surgery in chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2014, 4, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.A. Chronic frontal sinusitis: The endoscopic frontal recess approach. Oper. Tech. Otolaryngol. Head Neck Surg. 1996, 7, 222–229. [Google Scholar] [CrossRef]
- Wormald, P.J.; Hoseman, W.; Callejas, C.; Weber, R.K.; Kennedy, D.W.; Citardi, M.J.; Senior, B.A.; Smith, T.L.; Hwang, P.H.; Orlandi, R.R.; et al. The International Frontal Sinus Anatomy Classification (IFAC) and Classification of the Extent of Endoscopic Frontal Sinus Surgery (EFSS). Int. Forum Allergy Rhinol. 2016, 6, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, N.E.A.; Lazim, N.M.; Aziz, M.E.; Mohammad, Z.W.; Abdullah, B. The prevalence of frontal cell variants according to the International Frontal Sinus Anatomy Classification and their associations with frontal sinusitis. Eur. Arch. Otorhinolaryngol. 2022, 279, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.K.; Tran, T.T.; Nguyen, T.V.; Thai, T.T. Multiplanar Computed Tomographic Analysis of Frontal Cells According to International Frontal Sinus Anatomy Classification and Their Relation to Frontal Sinusitis. Rep. Med. Imaging. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Nofal, A.A.B.; El-Anwar, M.W. Frontal Recess Cells in International Frontal Sinus Anatomy Classification (IFAC); Prevalence, Infection Incidence, and Relation to Frontal Sinus Infection in Chronic Sinusitis Patients. Indian J. Otolaryngol. Head Neck Surg. 2022, 74 (Suppl. 3), 4748–4755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- DelGaudio, J.M.; Hudgins, P.A.; Venkatraman, G.; Beningfield, A. Multiplanar Computed Tomographic Analysis of Frontal Recess Cells: Effect on Frontal Isthmus Size and Frontal Sinusitis. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 230–235. [Google Scholar] [CrossRef]
- Villarreal, R.; Wrobel, B.B.; Macias-Valle, L.F.; Davis, G.E.; Prihoda, T.J.; Luong, A.U.; McMains, K.C.; Weitzel, E.K.; Yao, W.C.; Brunworth, J.; et al. International assessment of inter- and intrarater reliability of the International Frontal Sinus Anatomy Classification system. Int. Forum Allergy Rhinol. 2019, 9, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.V.; Ngo, N.H.; Psaltis, A.J. A radiological study assessing the prevalence of frontal recess cells and the most common frontal sinus drainage pathways. Am. J. Rhinol. Allergy 2019, 33, 323–330. [Google Scholar] [CrossRef]
- Seth, N.; Kumar, J.; Garg, A.; Singh, I.; Meher, R. Computed tomographic analysis of the prevalence of International Frontal Sinus Anatomy Classification cells and their association with frontal sinusitis. J. Laryngol. Otol. 2020, 134, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Başer, E.; Sarioglu, O.; Bulut, C.; Arslan, I.B.; Çukurova, I. Frequency of frontal cells according to the International Frontal Sinus Anatomy Classification. Turk. J. Ear Nose Throat 2020, 30, 33–40. [Google Scholar] [CrossRef]
- Som, P.M.; Lawson, W. The frontal intersinus septal air cell: A new hypothesis of its origin. Am. J. Neuroradiol. 2008, 29, 1215–1217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miłoński, J.; Pietkiewicz, P.; Urbaniak, J.; Kuśmierczyk, K.; Olszewski, J. Inflammation of the frontal intersinus septal air cell as a cause of headaches. Int. J. Surg. Case Rep. 2014, 5, 1292–1294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Joo, Y.H. Two Cases of Isolated Frontal Septal Cell Inflammation Treated with the Draf IIb Procedure. J. Clin. Otolaryngol. Head Neck Surg. 2021, 32, 85–89. [Google Scholar] [CrossRef]
- Makihara, S.; Kariya, S.; Okano, M.; Naito, T.; Uraguchi, K.; Matsumoto, J.; Noda, Y.; Nishizaki, K. The Relationship Between the Width of the Frontal Recess and the Frontal Recess Cells in Japanese Patients. Clin. Med. Insights Ear Nose Throat 2019, 12, 1179550619884946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, J.H.; Citardi, M.J.; Lee, W.T.; Sautter, N.B.; Lee, H.M.; Yoon, J.H.; Hong, S.C.; Kim, J.K. Comparison of frontal pneumatization patterns between Koreans and Caucasians. Otolaryngol. Head Neck Surg. 2006, 135, 780–786. [Google Scholar] [CrossRef] [PubMed]


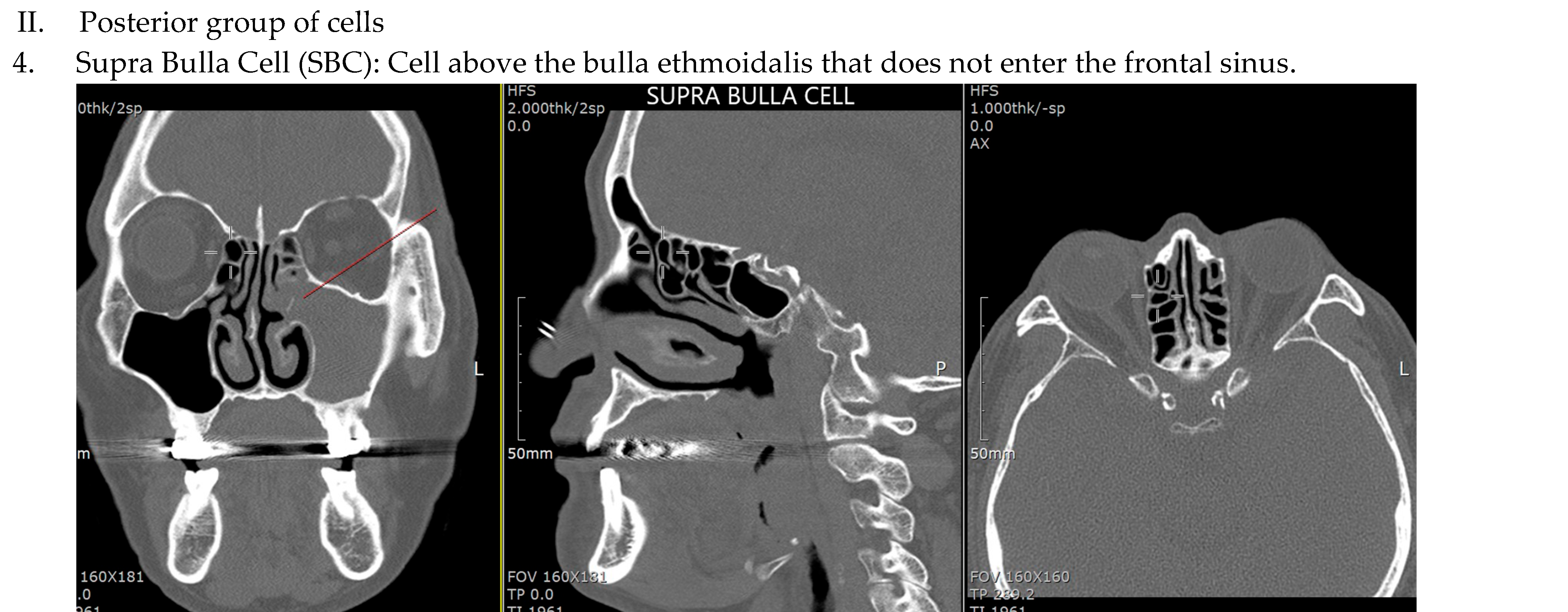
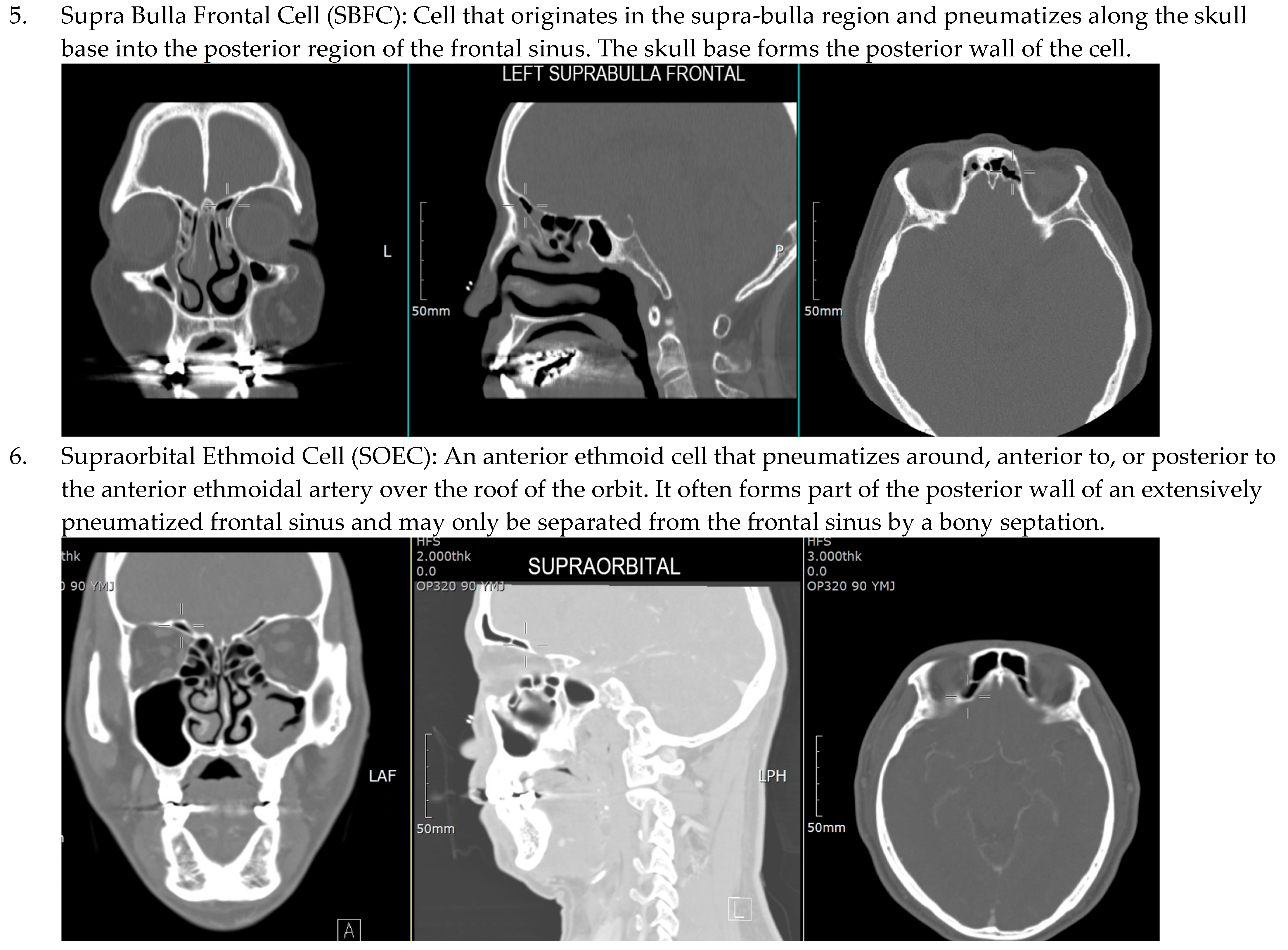
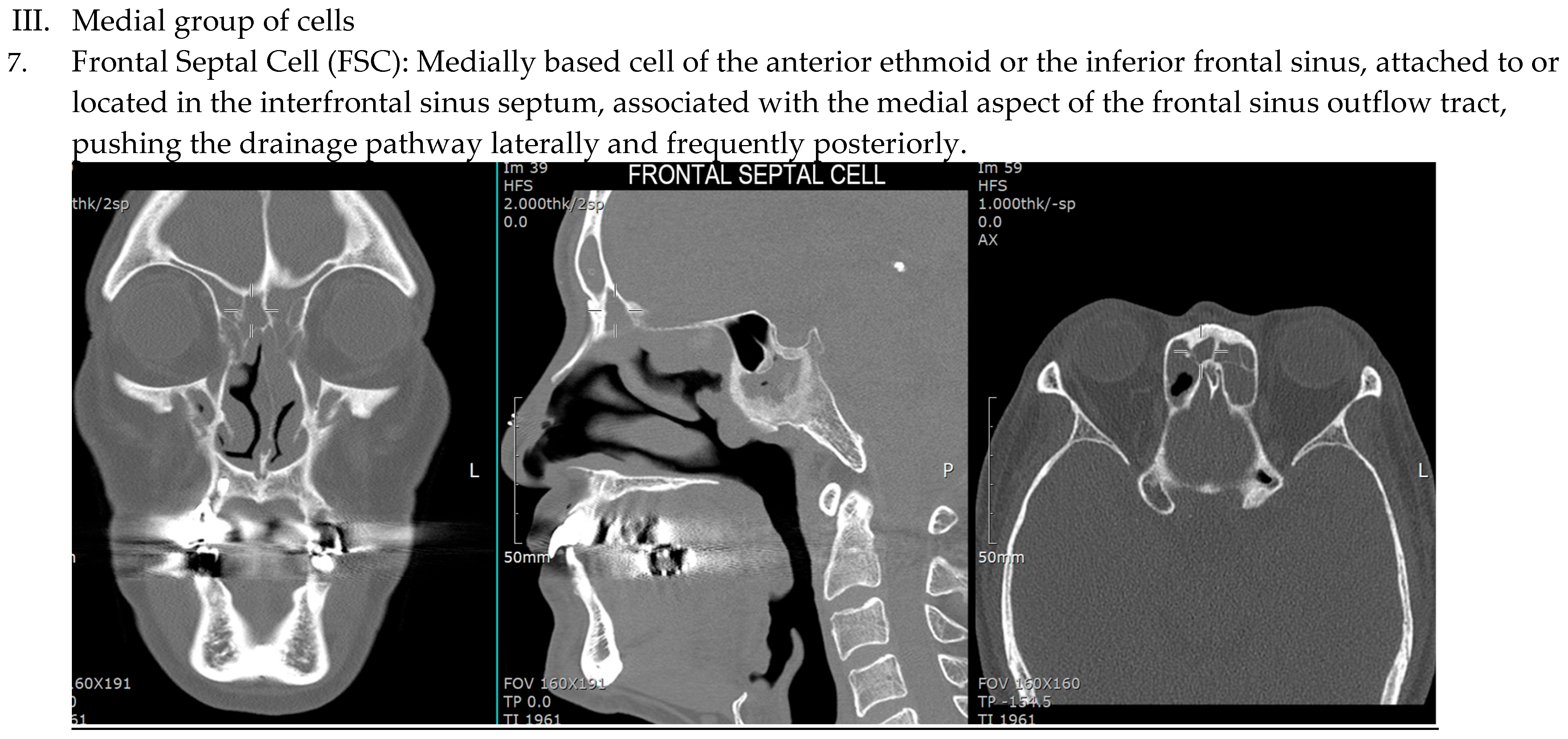

| IFAC Cell Type | X2 | df | p-Value | r (Effect Size) |
|---|---|---|---|---|
| ANC | 1.262 | 1 | 0.261 | |
| SAC | 3.282 | 1 | 0.07 | |
| SBC | 2.191 | 1 | 0.139 | |
| SOEC | 1.982 | 1 | 1.59 | |
| FSC | 0 | 1 | 0.987 | |
| SAFC | 10.537 | 1 | 0.001 | 0.1 |
| SBFC | 64.038 | 1 | 0 | 0.249 |
| Frontal Cell Type | Odds Ratio | Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| ANC | 1.83 | 0.535 | 6.291 | 0.335 |
| SAC | 0.73 | 0.51 | 1.044 | 0.85 |
| SAFC | 1.646 | 1.022 | 2.652 | 0.04 |
| SBC | 1.324 | 0.885 | 1.982 | 0.172 |
| SBFC | 4.483 | 2.983 | 6.738 | 0 |
| SOEC | 1.271 | 0.868 | 1.859 | 0.218 |
| FSC | 1.168 | 0.763 | 1.79 | 0.474 |
| Author | Year | Country | Sample | Results (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size | ANC | SAC | SAFC | SBC | SBF | SOEC | FSC | |||
| Villareal | 2019 | North America | 200 | 96.5 | 30 | 20 | 72 | 5.5 | 28.5 | 30 |
| Tran LV | 2019 | German | 498 | 95 | 49 | 25 | 89 | 27 | 9 | 28 |
| Seth | 2020 | India | 180 | 95.5 | 33.3 | 22.2 | 36.1 | 21.1 | 39.4 | 21.1 |
| Baser | 2020 | Turkey | 300 | 94.3 | 40 | 14.7 | 59.7 | 7.3 | 7.3 | 29.3 |
| Pham | 2021 | Vietnam | 1006 | 91.6 | 28.7 | 15.8 | 59.7 | 25.8 | 6.9 | 14.3 |
| Fawzi | 2021 | Malaysia | 400 | 95.5 | 50 | 36 | 60.8 | 53 | 5.5 | 8.3 |
| Nofal | 2022 | Egypt | 200 | 97 | 48 | 11 | 72 | 23 | 42 | 21 |
| Kho | 2024 | Korea | 1033 | 97.1 | 38.1 | 10.7 | 73.8 | 16.3 | 23.3 | 19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kho, J.P.Y.; Mohammad, S.; Rhee, C.-S. An Analysis of Frontoethmoid Cell Types According to the International Frontal Sinus Anatomy Classification in the Korean Population and Their Relation to Frontal Sinusitis. Sinusitis 2025, 9, 14. https://doi.org/10.3390/sinusitis9020014
Kho JPY, Mohammad S, Rhee C-S. An Analysis of Frontoethmoid Cell Types According to the International Frontal Sinus Anatomy Classification in the Korean Population and Their Relation to Frontal Sinusitis. Sinusitis. 2025; 9(2):14. https://doi.org/10.3390/sinusitis9020014
Chicago/Turabian StyleKho, Jasmine Pei Ying, Sakinah Mohammad, and Chae-Seo Rhee. 2025. "An Analysis of Frontoethmoid Cell Types According to the International Frontal Sinus Anatomy Classification in the Korean Population and Their Relation to Frontal Sinusitis" Sinusitis 9, no. 2: 14. https://doi.org/10.3390/sinusitis9020014
APA StyleKho, J. P. Y., Mohammad, S., & Rhee, C.-S. (2025). An Analysis of Frontoethmoid Cell Types According to the International Frontal Sinus Anatomy Classification in the Korean Population and Their Relation to Frontal Sinusitis. Sinusitis, 9(2), 14. https://doi.org/10.3390/sinusitis9020014







