Usefulness of Nanoparticles in the Fight Against Esophageal Cancer: A Comprehensive Review of Their Therapeutic Potential
Abstract
1. Introduction
2. Methodology
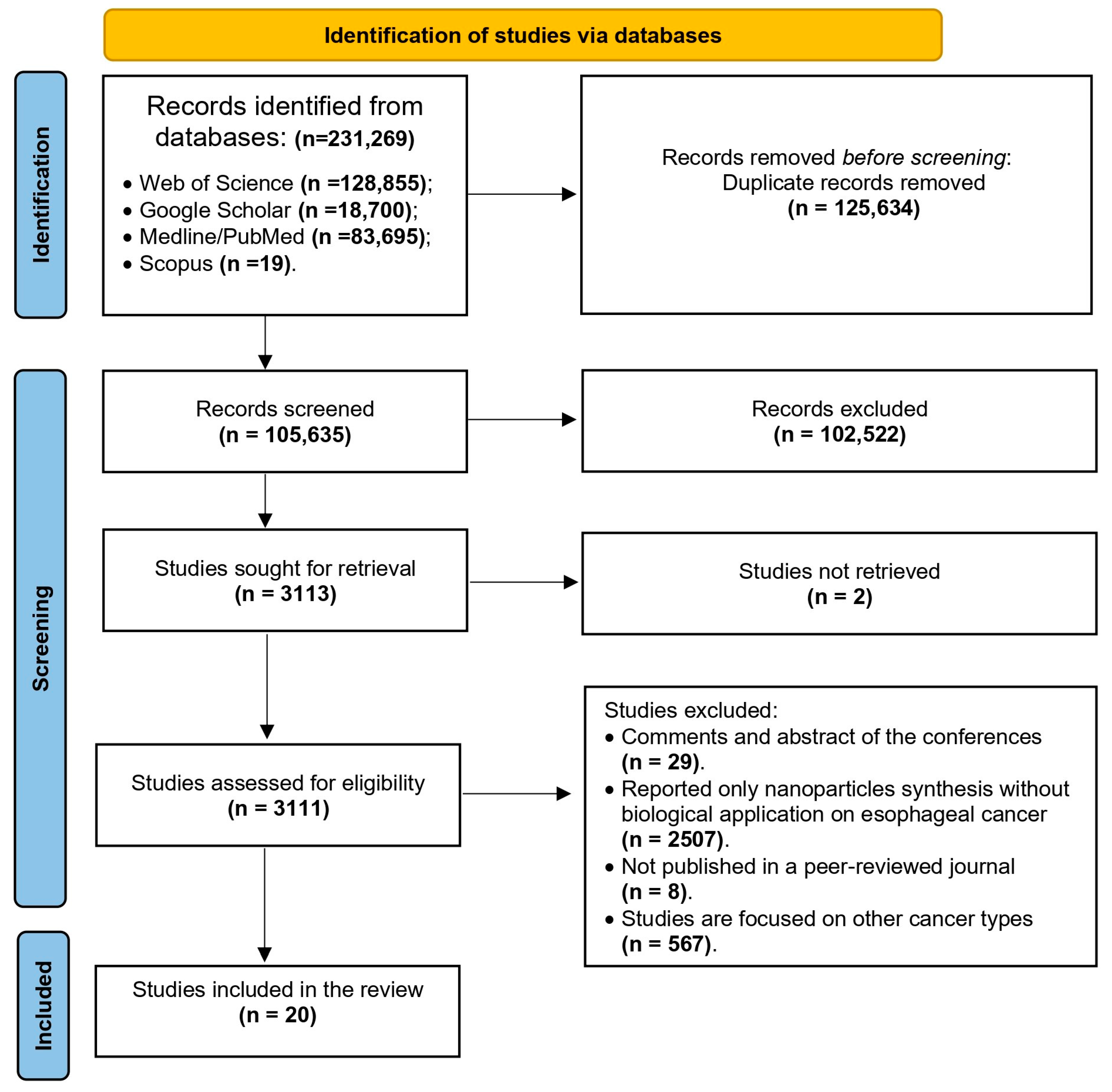
2.1. Data Sources, Search Strategy, and Eligibility Criteria
2.2. Selection Process and Data Collection
2.3. Risk of Bias and Certainty Assessment
2.4. Synthesis Methods
3. Results
3.1. Search Outcomes and Studies Characteristics
| Type of Nanoparticles | Types of Activities | Cancer Lines | Cell Seeded Density | Biological Activities | (Country) References |
|---|---|---|---|---|---|
| Copper oxide nanoparticles (CuO NPs) synthesized using viable cells, cell lysate supernatant, and protein extracts of Vibrio sp. VLC | Cytotoxicity activity by the MTT assay | KYSE30 | 10,000 cells/well (KYSE30) and 8000 cells/well (HDF) | IC50 = 13.96 mg/L | (Iran) [16] |
| Gold nanoparticles synthesized using Moringa oleifera leaf extract | Cytotoxicity activity by the MTT assay | SNO cells | 20,000 cells/well | IC50 = 92.01 µg/mL | (South Africa) [17] |
| Cu (II) nanoparticles synthesized using 1,3,5-benzenetricarboxylic acid (H3btc) | Cytotoxicity activity by the MTT assay | KYSE150, EC109, and EC8712 | 2 × 105 cells/well | KYSE150 (IC50 = 86 μM), EC109 (IC50 = 73 μM), and EC8712 (IC50 = 78 μM) | (China) [27] |
| Cu (II) nanoparticles synthesized using 1,2,4-triazole (Htrz) | 2 × 105 cells/well | KYSE150 (IC50 = 48 μM), EC109 (IC50 = 42 μM), and EC8712 (IC50 = 34 μM) | |||
| Nanoparticle colloidal dispersion synthesized using curcumin (nano-curcumin) | Cell proliferation was measured using a BrdU incorporation assay | OE33, and OE19 | 5 × 105 cells/mL | OE33 (20%) and OE19 (17%) when treated with 50 mM | (The Netherlands) [18] |
| Copper nanoparticles (Cu NPs) synthesized using Mentha piperita aqueous extract | Cytotoxicity activity by the MTT assay | KYSE-270, OE33, and ESO26 | 5 × 104 cells (per square centimeter) | IC50 of the Cu NPs were 241, 278, and 240 mg/mL against KYSE-270, OE33, and ESO26 | (China) [19] |
| Nickel nanoparticles (NiONPs) synthesized using Calendula officinalis leaf aqueous extract | Cytotoxicity activity by the MTT assay | FLO-1, ESO26, OE33, and KYSE-270 normal esophageal cell line HUVEC | 7 × 103 | FLO-1 (IC50 = 380 µg/mL), ESO26 (IC50 = 263 µg/mL), OE33 (IC50 = 229 µg/mL), and KYSE-270 (IC50 = 251 µg/mL) | (China) [20] |
| Silver nanoparticles synthesized using pomegranate peel extract | Cytotoxicity activity by the MTT assay | KYSE-30, KYSE-50, KYSE-70, KYSE-110, KYSE-270, OE33, ESO26, and FLO-1 | / | KYSE-30 (IC50 = 487 μg/mL), KYSE-50 (IC50 = 500 μg/mL), KYSE-70 (IC50 = 435 μg/mL), KYSE-110 (IC50 = 461 μg/mL), KYSE-270 (IC50 = 285 μg/mL), OE33 (IC50 = 338 μg/mL), ESO26 (IC50 = 253 μg/mL), and FLO-1 (IC50 = 288 μg/mL) | (Iran) [21] |
| Silver nanoparticles synthesized using aqueous Photinia glabra fruit extract (PG-Ag NPs) | Cytotoxicity activity by the MTT assay | Eca-109 | / | IC50 less than 20 µg/mL | (China) [22] |
| Gold nanoparticles green-synthesized by Rhus coriaria L. fruit aqueous extract | Cytotoxicity activity by the MTT assay | FLO-1, ESO26, OE33, and KYSE-270 Normal esophageal cell line HUVEC | 1 × 103 cell | KYSE-270 (IC50 = 226 mg/mL), OE33(IC50 = 213 mg/mL), ESO26(IC50 = 267 mg/mL), and FLO-1(IC50 = 294 mg/mL) | (China) [23] |
| Multifunctional nanoparticles co-loaded with Adriamycin | Cell viability and Cell cycle and apoptosis | KYSE510 and Adriamycin-resistant KYSE510 (KYSE510K) | 1 × 104 | Inhibit the growth of cancer cells and tumor development by reducing drug efflux by ESCC cells and promoting apoptosis in mice | (China) [34] |
| Gel-nano systems | Cytotoxicity activity by the cell counting kit-8 (CCK8) assay | TE-1 and KYSE-150 | 3000 cells/well for CCK8 assay and 10,000 cells/well for apoptosis | Boost T-cell immunity and restore p53 activity in mice | (China) [28] |
| Cerium oxide nanoparticles | Cell Viability Assay by Resazurin assay | YM1 | 25,000 cells/well | IC50s = 630 μM after 48 h | (Iran) [29] |
| Gold nano-immuno-conjugate (NIC) | Cell viability Cytotoxicity activity by the MTT assay | HKESC-1 cell line | 5 × 105 | 2.333 µM ≤ IC50 ≤ 2.998 µM | (South Africa) [32] |
| Copper (Cu) nanoparticles | Cytotoxicity activity by the MTT assay | OE33, KYSE-270, and ESO26 | / | OE33 (IC50 = 241 mg/mL), ESO26 (IC50 = 278 mg/mL), and KYSE-270 (IC50 = 240 mg/mL) | (China) [30] |
| Gold nanoparticles mediated by potato starch | Cytotoxicity activity by the MTT assay | (KYSE-30 and FLO-1) and normal cells (HUVEC) | 3 × 103 cells | KYSE-30 (IC50 = 125 μg/mL) and FLO-1 (IC50 = 176 μg/mL) | (India) [31] |
| Silver nanoparticles synthesized using peel of pomegranate | Cytotoxicity activity by the MTT assay | KYSE-30, KYSE-50, KYSE-70, KYSE-110, KYSE-270, OE33, ESO26 and FLO-1 cell and normal (HUVEC) | 410 × 3 cells in 100 microliters | KYSE-30 (IC50 = 487 µg/mL), KYSE-50 (IC50 = 500 µg/mL), KYSE-70 (IC50 = 435 µg/mL), KYSE-110 (IC50 = 461 µg/mL), KYSE-270 (IC50 = 285 µg/mL), OE33 (IC50 = 338 µg/mL), ESO26 (IC50 = 253 µg/mL), and FLO-1 (IC50 = 288 µg/mL) | (China) [24] |
| Hypericum perforatum-loaded nanoparticles | Cytotoxicity activity by the MTT assay | KYSE-30 (Cat No: 94072011) | 5000 cells/mL | Dox NPs (IC50 = ~0.04–0.06 mg/mL) and HP-NPs (IC50 = ~0.6–0.7 mg/mL) | (Iran) [25] |
| Silver-nanoparticles-enhanced doxorubicin | Cytotoxicity activity by the MTT assay | OE33 | / | IC50 = 2.399 ±1.39 μM | (Egypt) [33] |
| Au NPs decorated over sodium lignosulfonate (NaLS) by using Cydonia oblonga extract | Cytotoxicity activity by the MTT assay | FLO-1, ESO26, OE33, and KYSE-270 | 105 | FLO-1 (IC50 = 181 µg/mL), ESO26 (IC50 = 130 µg/mL), OE33 (IC50 = 205 µg/mL), and KYSE-270 (IC50 = 133 µg/mL) | (China) [26] |
3.2. Anti-Esophageal Cancer Activities of Green-Synthesized Nanoparticles
3.2.1. Copper Oxide Nanoparticles (CuO NPs) Synthesized from Vibrio Luminescent Strain C (Vibrio sp. VLC)
3.2.2. Gold Nanoparticles Synthesized from Moringa oleifera
3.2.3. Colloidal Nanoparticle Dispersion Synthesized with Curcumin (Nano-Curcumin)
3.2.4. Copper Nanoparticles (Cu NPs) Synthesized from Mentha piperita
3.2.5. Nickel Nanoparticles (NiONPs) Synthesized from Calendula officinalis
3.2.6. Silver Nanoparticles Derived from Pomegranate Peel Extract
3.2.7. Silver Nanoparticles from Photinia glabra (PG) Fruit Extract
3.2.8. Gold Nanoparticles Green-Synthesized from Rhus coriaria L. Fruit Extract
3.2.9. Gold Nanoparticles Mediated by Potato Starch
3.2.10. Sodium Lignosulfonate (NaLS) Nanoparticles from Cydonia oblonga
3.3. Anti-Esophageal Cancer Activities of Chemically Synthesized NPs
3.3.1. Cerium Oxide Nanoparticles (CeO2 NPs)
3.3.2. Cu2(btc)(trz)3-NPs and Cu5(Hbtc)4(trz)2(H2O)4-NPs
3.4. Anti-Esophageal Cancer Activities of Multifunctional and Combined System Nanoparticles
3.4.1. Gold Nano-Immuno-Conjugates (NIC)
3.4.2. Silver Nanoparticles Enhanced Doxorubicin
4. Discussion
5. Limitations and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac. Cancer 2023, 1, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal Carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.C.; Cunningham, D.; Lagergren, P. Oesophageal Cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Ammar, M.M.; Ali, R.; Abd Elaziz, N.A.; Habib, H.; Abbas, F.M.; Yassin, M.T.; Maniah, K.; Abdelaziz, R. Nanotechnology in Oncology: Advances in Biosynthesis, Drug Delivery, and Theranostics. Discov. Onc. 2025, 16, 1172. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yue, G.; Guo, L.; Hu, Y.; Cui, Q.; Wang, J.; Tang, J.; Liu, H. Nanodrug systems for therapy and diagnosis of esophageal cancer. Front. Bioeng. Biotechnol. 2023, 11, 1233476. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2018, 13, 482. [Google Scholar] [CrossRef]
- Bondarenko, O.; Mortimer, M.; Kahru, A.; Feliu, N.; Javed, I.; Kakinen, A.; Lin, S.; Xia, T.; Song, Y.; Davis, T.P.; et al. Nanotoxicology and Nanomedicine: The Yin and Yang of Nano-Bio Interactions for the New Decade. Nano Today 2021, 39, 101184. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ndebia, E.J.; Kamsu, G.T. Harnessing the Power of Natural Terpenoid Compounds against Esophageal Squamous Cell Carcinoma: A Systematic Review. Future Pharmacol. 2025, 5, 21. [Google Scholar] [CrossRef]
- Kamsu, G.T.; Ndebia, E.J. Uncovering Risks Associated with Smoking Types and Intensities in Esophageal Cancer within High-Prevalence Regions in Africa: A Comprehensive Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2024, 33, 874–883. [Google Scholar] [CrossRef]
- Nakhaeepour, Z.; Mashreghi, M.; Matin, M.M.; NakhaeiPour, A.; Housaindokht, M.R. Multifunctional CuO Nanoparticles with Cytotoxic Effects on KYSE30 Esophageal Cancer Cells, Antimicrobial and Heavy Metal Sensing Activities. Life Sci. 2019, 234, 116758. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in A549 cells. J. Cell. Biochem. 2015, 117, 2302–2314. [Google Scholar] [CrossRef]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-Curcumin Inhibits Proliferation of Esophageal Adenocarcinoma Cells and Enhances the T Cell Mediated Immune Response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef]
- Zhuang, X.; Kang, Y.; Zhao, L.; Guo, S. Design and Synthesis of Copper Nanoparticles for the Treatment of Human Esophageal Cancer: Introducing a Novel Chemotherapeutic Supplement. J. Exp. Nanosci. 2022, 17, 274–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Mahdavi, B.; Mohammadhosseini, M.; Rezaei-Seresht, E.; Paydarfard, S.; Qorbani, M.; Karimian, M.; Abbasi, N.; Ghaneialvar, H.; Karimi, E. Green Synthesis of NiO Nanoparticles Using Calendula officinalis Extract: Chemical Characterization, Antioxidant, Cytotoxicity, and Anti-Esophageal Carcinoma Properties. Arab. J. Chem. 2021, 14, 103105. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Almasi, M. Pomegranate Peel Extract-Mediated Green Synthesis of Silver Nanoparticles: Evaluation of Cytotoxicity, Antioxidant, and Anti-Esophageal Cancer Effects. ChemistrySelect 2023, 8, e202204841. [Google Scholar] [CrossRef]
- Namulinda, T.; Bao, L.L.; Kwetegyeka, J.; Gumula, I.; Yan, Y.J.; Chen, Z.L. Antibacterial and Anticancer Activities of Green-Synthesized Silver Nanoparticles Using Photinia glabra Fruit Extract. Nanomedicine 2023, 18, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zangeneh, A.; Zangeneh, M.M.; Guo, B. Antioxidant, Cytotoxicity, Anti-Human Esophageal Squamous Cell Carcinoma, Anti-Human Caucasian Esophageal Carcinoma, Anti-Adenocarcinoma of the Gastroesophageal Junction, and Anti-Distal Esophageal Adenocarcinoma Properties of Gold Nanoparticles Green Synthesized by Rhus coriaria L. Fruit Aqueous Extract. J. Exp. Nanosci. 2020, 15, 202–216. [Google Scholar] [CrossRef]
- Fan, P. Green Formulation, Chemical Characterization, Anti-Esophageal Cancer, Cytotoxicity and Antioxidant Potentials of Silver Nanoparticles Containing Plant Extract. SSRN Electron. J. 2022, 19. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4238345 (accessed on 10 July 2025).
- Amjadi, I.; Mohajeri, M.; Borisov, A.; Hosseini, M.S. Antiproliferative Effects of Free and Encapsulated Hypericum perforatum L. Extract and Its Potential Interaction with Doxorubicin for Esophageal Squamous Cell Carcinoma. J. Pharmacopunct. 2019, 22, 102–108. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Pan, J.; Li, M.; Wang, J.; Fang, G.; Shang, Y. Chemical Characterization, Antioxidant and Anti-Esophageal Cancer Properties of Au NPs over Sodium Lignosulfonate Formulated by Cydonia oblonga. Inorg. Chem. Commun. 2024, 160, 111985. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, L.; Jin, X.; Qu, C. Two Cu(II) Coordination Polymers Based on Benzene-1,3,5-Tricarboxylate and 1,2,4-Triazolide Ligands: Their Crystal Structures and Application of Nanoparticles in Anti-Esophageal Cancer Activity Evaluation. Struct. Chem. 2019, 30, 1485–1494. [Google Scholar] [CrossRef]
- Gong, K.; Lin, J.; Chen, X.; Duan, Y.; Zhang, J.; Yu, J.; Wang, J.; Sun, R.; Li, J.; Duan, Y. Thermosensitive Gel-Nano System against Esophageal Cancer via Restoring p53 Activity and Boosting T-Cell Immunity. J. Control. Release 2024, 371, 111–125. [Google Scholar] [CrossRef]
- Javid, H.; Hashemy, S.I.; Heidari, M.F.; Esparham, A.; Gorgani-Firuzjaee, S. The Anticancer Role of Cerium Oxide Nanoparticles by Inducing Antioxidant Activity in Esophageal Cancer and Cancer Stem-Like ESCC Spheres. Biomed. Res. Int. 2022, 2022, 3268197. [Google Scholar] [CrossRef]
- Zang, K. Nanotechnology-Enhanced Chemoradiotherapy Using Copper and Gold Nanoparticles for Esophageal Cancer. J. Angiother. 2024, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, M.; Xue, X.; Karmakar, B.; Eltantawy, W.; El-Kott, A.F.; El Nashar, E.M.; Abd-Ella, E.M. Sonochemical Synthesis of Gold Nanoparticles Mediated by Potato Starch: Its Performance in the Treatment of Esophageal Cancer. Open Chem. 2024, 22, 20230193. [Google Scholar] [CrossRef]
- Didamson, O.C.; Chandran, R.; Abrahamse, H. Synthesis, Characterisation, and Anti-Tumour Activity of Nano-Immuno-Conjugates for Enhanced Photodynamic Therapy of Oesophageal Cancer Stem Cells. Biomed. Pharmacother. 2024, 181, 117693. [Google Scholar] [CrossRef] [PubMed]
- Moawad, M.; Youssef, A.M.; Elsherbeni, S.A.E.; Fahmy, A.M.; El-Ghannam, G. Silver Nanoparticles Enhanced Doxorubicin Treatment for Improving Their Efficacy against Esophageal Cancer Cells. Egypt. J. Chem. 2024, 67, 505–512. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Feng, J.; Qin, B.; Zhang, C.; Zhu, C.; Liu, W.; Wang, Y.; Liu, W.; Huang, L.; et al. Multifunctional Nanoparticles Co-Loaded with Adriamycin and MDR-Targeting siRNAs for Treatment of Chemotherapy-Resistant Esophageal Cancer. J. Nanobiotechnol. 2022, 20, 166. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Mehr, F.P.; Poor, A.K. Extracellular Synthesis of Copper Nanoparticles Using Culture Supernatants of Salmonella typhimurium. Orient J. Chem. 2015, 31, 527–529. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of Antioxidant and Anticancer Activity of Copper Oxide Nanoparticles Synthesized Using Medicinally Important Plant Extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Devkota, H.P.; Gaire, B.P.; Hori, K.; Subedi, L.; Adhikari-Devkota, A.; Belwal, T.; Paudel, K.R.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; et al. The Science of Matcha: Bioactive Compounds, Analytical Techniques and Biological Properties. Trends Food Sci. Technol. 2021, 118, 735–743. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Balusamy, S.R.; Sukweenadhi, J.; Nag, S.; MubarakAli, D.; Farh, M.E.-A.; Vijay, H.; Rahimi, S. A Comprehensive Review on Moringa oleifera Nanoparticles: Importance of Polyphenols in Nanoparticle Synthesis, Nanoparticle Efficacy and Their Applications. J. Nanobiotechnol. 2024, 22, 71. [Google Scholar] [CrossRef]
- Belliraj, T.S.; Nanda, A.; Ragunathan, R. In-Vitro Hepatoprotective Activity of Moringa oleifera Mediated Synthesis of Gold Nanoparticles. J. Chem. Pharm. Res. 2015, 7, 781–788. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of Curcumin: An Emerging Paradigm for Improved Remedial Application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef]
- Morsy, M.K.; Al-Dalain, S.Y.; Haddad, M.A.; Diab, M.M.E.; Abdeen, A.; Ibrahim, S.F.; Shukry, M.; Fericean, L.; Ghamry, H.I.; Abdelaziz, M.; et al. Curcumin Nanoparticles as a Natural Antioxidant and Antimicrobial Preservative against Foodborne Pathogens in Processed Chicken Fingers. Front. Sustain. Food Syst. 2023, 7, 1267075. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita Phytochemicals in Agriculture, Food Industry and Medicine: Features and Applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A Review of the Bioactivity and Potential Health Benefits of Peppermint Tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Valencia, G.A.; Vercik, L.C.O.; Ferreira, L.G.; Llanos, J.H.R.; Vercik, A. Synthesis and Characterisation of Gold Nanoparticles Using Mentha piperita Leaf Extract: A Green, Non-Toxic and Rapid Method. Int. J. Nano Biomater. 2015, 5, 181–192. [Google Scholar] [CrossRef]
- Hussain, Z.; Jahangeer, M.; Sarwar, A.; Ullah, N.; Aziz, T.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Synthesis and Characterization of Silver Nanoparticles Mediated by the Mentha piperita Leaves Extract and Exploration of Its Antimicrobial Activities. J. Chil. Chem. Soc. 2023, 68, 5865–5870. [Google Scholar] [CrossRef]
- Aftab, R.; Akbar, F.; Afroz, A.; Asif, A.; Khan, M.R.; Rehman, N.; Zeeshan, N. Mentha piperita Silver Nanoparticle-Loaded Hydrocolloid Film for Enhanced Diabetic Wound Healing in Rats. J. Wound Care 2024, 33, xlviii–lx. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, R.; Zain, M.; Ijaz, U. Green Synthesis and Evaluation of Mentha piperita Mediated Iron Nanoparticles for In Vitro Antibacterial Activity. J. Xi’an Shiyou Univ. 2024, 20, 653–663. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4970247 (accessed on 12 July 2025).
- American College of Toxicology (ACT). Final Report on the Safety Assessment of Mentha piperita (Peppermint) Oil, Mentha piperita (Peppermint) Leaf Extract, Mentha piperita (Peppermint) Leaf, and Mentha piperita (Peppermint) Leaf Water. Int. J. Toxicol. 2001, 20 (Suppl. 3), 61–73. [Google Scholar] [CrossRef]
- Olfati, A.; Kahrizi, D.; Balaky, S.T.J.; Sharifi, R.; Tahir, M.B.; Darvishi, E. Green Synthesis of Nanoparticles Using Calendula officinalis Extract from Silver Sulfate and Their Antibacterial Effects on Pectobacterium carotovorum. Inorg. Chem. Commun. 2021, 125, 108439. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; El-kott, A.E.; Ahmed, A.E.; Khames, A. Calendula officinalis-Based Green Synthesis of Titanium Nanoparticle: Fabrication, Characterization, and Evaluation of Human Colorectal Carcinoma. J. Saudi Chem. Soc. 2021, 25, 101343. [Google Scholar] [CrossRef]
- Xu, Y.; Mahdavi, B.; Zangeneh, M.M.; Zangeneh, A.; Qorbani, M.; Paydarfard, S. Calendula officinalis Green-Mediated Silver Nanoparticles: Formulation, Characterization and Assessment of Colorectal Cancer Activities. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- International Journal of Toxicology (IJT). Final Report on the Safety Assessment of Calendula officinalis Extract and Calendula officinalis. Int. J. Toxicol. 2001, 20 (Suppl. 2), 13–20. [Google Scholar] [CrossRef] [PubMed]
- Alnehia, A.; Al-Odayni, B.; Al-Sharabi, A.; Al-Hammadi, A.H.; Saeed, W.S. Pomegranate Peel Extract-Mediated Green Synthesis of ZnO-NPs: Extract Concentration-Dependent Structure, Optical, and Antibacterial Activity. J. Chem. 2022, 2022, 9647793. [Google Scholar] [CrossRef]
- Azmat, F.; Safdar, M.; Ahmad, H.; Khan, M.R.J.; Abid, J.; Naseer, M.S.; Aggarwal, S.; Imran, A.; Khalid, U.; Zahra, S.M.; et al. Phytochemical Profile, Nutritional Composition of Pomegranate Peel and Peel Extract as a Potential Source of Nutraceutical: A Comprehensive Review. Food Sci. Nutr. 2024, 12, 661–674. [Google Scholar] [CrossRef]
- Plants For A Future (PFAF). Photinia glabra-(Thunb.) Franch. & Sav; Plants For A Future: Dawlish, UK, 2025; Available online: https://pfaf.org/user/Plant.aspx?LatinName=Photinia+glabra (accessed on 8 July 2025).
- Abu-Reidah, I.M.; Jamous, R.M.; Ali-Shtayeh, M.S. Phytochemistry, Pharmacological Properties and Industrial Applications of Rhus coriaria L. (Sumac). Jordan J. Biol. Sci. 2014, 7, 233–244. [Google Scholar] [CrossRef]
- Gur, T. Green Synthesis, Characterizations of Silver Nanoparticles Using Sumac (Rhus coriaria L.) Plant Extract and Their Antimicrobial and DNA Damage Protective Effects. Front. Chem. 2022, 10, 968280. [Google Scholar] [CrossRef]
- Rahimzadeh, C.Y.; Barzinjy, A.A.; Mohammed, A.S.; Hamad, S.M. Green Synthesis of SiO2 Nanoparticles from Rhus coriaria L. Extract: Comparison with Chemically Synthesized SiO2 Nanoparticles. PLoS ONE 2022, 17, e0268184. [Google Scholar] [CrossRef] [PubMed]
- Mongy, Y.; Shalaby, T. Green Synthesis of Zinc Oxide Nanoparticles Using Rhus coriaria Extract and Their Anticancer Activity against Triple-Negative Breast Cancer Cells. Sci. Rep. 2024, 14, 13470. [Google Scholar] [CrossRef]
- Janik, M.; Khachatryan, K.; Khachatryan, G.; Krystyjan, M.; Oszczęda, Z. Comparison of Physicochemical Properties of Silver and Gold Nanocomposites Based on Potato Starch in Distilled and Cold Plasma-Treated Water. Int. J. Mol. Sci. 2023, 24, 2200. [Google Scholar] [CrossRef]
- Li, M.; Shi, P.; Xu, C.; Ren, J.; Qu, X. Cerium Oxide Caged Metal Chelator: Anti-Aggregation and Anti-Oxidation Integrated H2O2-Responsive Controlled Drug Release for Potential Alzheimer’s Disease Treatment. Chem. Sci. 2013, 4, 2536–2542. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Li, J.; Feizipour, S.; Delirezh, N.; Sheikhzadeh, S.; Hobbenaghi, R.; Amraii, S.A.; Khorasani, F.; Hemmati, S.; Joshani, Z.; Kamangar, S.A.; et al. Green Synthesis of Gold Nanoparticles Using Potato Starch as a Phytochemical Template, Green Reductant and Stabilizing Agent and Investigating Its Cytotoxicity, Antioxidant and Anti-Ovarian Cancer Effects. Inorg. Chem. Commun. 2023, 155, 111002. [Google Scholar] [CrossRef]
- Tisi, A.; Pulcini, F.; Carozza, G.; Mattei, V.; Flati, V.; Passacantando, M.; Antognelli, C.; Maccarone, R.; Delle Monache, S. Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo. Antioxidants 2022, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sohail; Raja, N.I.; Asad, M.J.; Mashwani, Z.-U.-R. Antioxidant and Hypoglycemic Potential of Phytogenic Cerium Oxide Nanoparticles. Sci. Rep. 2023, 13, 4514. [Google Scholar] [CrossRef]
- Corsi, F.; Deidda Tarquini, G.; Urbani, M.; Bejarano, I.; Traversa, E.; Ghibelli, L. The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More Than Redox? Nanomaterials 2023, 13, 2803. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, A.; Ghosh, I.; Moriyasu, Y.; Mukherjee, A.; Bandyopadhyay, M. Role of cerium oxide nanoparticle-induced autophagy as a safeguard to exogenous H2O2-mediated DNA damage in tobacco by-2 cells. Mutagenesis 2018, 33, 161–177. [Google Scholar] [CrossRef] [PubMed]
- El-Seidy, A.M.A.; Elbaset, M.A.; Ibrahim, F.A.A.; Abdelmottaleb Moussa, S.A.; Bashandy, S.A. Nano Cerium Oxide and Cerium/Zinc Nanocomposites Characterization and Therapeutic Role in Combating Obesity via Controlling Oxidative Stress and Insulin Resistance in Rat Model. J. Trace Elem. Med. Biol. 2023, 80, 127312. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium Oxide Nanoparticles in Neuroprotection and Considerations for Efficacy and Safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Gunasekaran, N.K.; Nazario Bayon, N.; Tumkur, P.P.; Prabhakaran, K.; Hall, J.C.; Ramesh, G.T. Anticancer Activity of Cerium Oxide Nanoparticles Towards Human Lung Cancer Cells. Nanomanufacturing 2025, 5, 6. [Google Scholar] [CrossRef]
- García, A.; Espinosa, R.; Delgado, L.; Casals, E.; González, E.; Puntes, V.; Barata, C.; Font, X.; Sánchez, A. Acute Toxicity of Cerium Oxide, Titanium Oxide and Iron Oxide Nanoparticles Using Standardized Tests. Desalination 2011, 269, 136–141. [Google Scholar] [CrossRef]
- Khorrami, M.B.; Sadeghnia, H.R.; Pasdar, A.; Ghayour-Mobarhan, M.; Riahi-Zanjani, B.; Hashemzadeh, A.; Zare, M.; Darroudi, M. Antioxidant and Toxicity Studies of Biosynthesized Cerium Oxide Nanoparticles in Rats. Int. J. Nanomed. 2019, 14, 2915–2926. [Google Scholar] [CrossRef]
- Crous, A.; Abrahamse, H. Effective Gold Nanoparticle-Antibody-Mediated Drug Delivery for Photodynamic Therapy of Lung Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 3742. [Google Scholar] [CrossRef]
- Didamson, O.C.; Chandran, R.; Abrahamse, H. A Gold Nanoparticle Bioconjugate Delivery System for Active Targeted Photodynamic Therapy of Cancer and Cancer Stem Cells. Cancers 2022, 14, 4558. [Google Scholar] [CrossRef] [PubMed]
- Prakashan, D.; Shrikrishna, N.S.; Byakodi, M.; Nagamani, K.; Gandhi, S. Gold Nanoparticle Conjugate-Based Lateral Flow Immunoassay (LFIA) for Rapid Detection of RBD Antigen of SARS-CoV-2 in Clinical Samples Using a Smartphone-Based Application. J. Med. Virol. 2023, 95, e28416. [Google Scholar] [CrossRef]
- Saeidi, J.; Dolatabadi, S.; Esfahani, M.B.; Saeidi, M.; Mohtashami, M.; Mokhtari, K.; Ghasemi, A. Anticancer Potential of Doxorubicin in Combination with Green-Synthesized Silver Nanoparticle and Its Cytotoxicity Effects on Cardio-Myoblast Normal Cells. Anti-Cancer Agents Med. Chem. 2021, 21, 1842–1849. [Google Scholar] [CrossRef]
- Gołuński, G.; Konkel, K.; Bełdzińska, P.; Bury, K.; Zakrzewski, M.; Butowska, K.; Sądej, R.; Piosik, J. Influence of Silver Nanoparticles’ Size on Their Direct Interactions with Doxorubicin and Its Biological Effects. Sci. Rep. 2024, 14, 18544. [Google Scholar] [CrossRef]
- Le-Thi, P.; Trung-Nguyen, D.; An-Nguyen-Huu, T.; Tran, Q.; Truong, M.; Thanh-Hang, N.; Quyen-Tran, N.; Dong-Park, K. Hyaluronic Acid-Coated Silver Nanoparticles Releasing Doxorubicin for Combinatorial Antitumor Therapy. J. Ind. Eng. Chem. 2025, 142, 431–440. [Google Scholar] [CrossRef]
- Kamsu, G.T.; Ndebia, E.J. Usefulness of Natural Phenolic Compounds in the Fight against Esophageal Cancer: A Systematic Review. Future Pharmacol. 2024, 4, 626–650. [Google Scholar] [CrossRef]
- Devaraji, M.; Thanikachalam, P.V.; Elumalai, K. The Potential of Copper Oxide Nanoparticles in Nanomedicine: A Comprehensive Review. Biotechnol. Notes 2024, 5, 80–99. [Google Scholar] [CrossRef]
- Peng, L.; Gao, Z.; Liang, Y.; Guo, X.; Zhang, Q.; Cui, D. Nanoparticle-Based Drug Delivery Systems: Opportunities and Challenges in the Treatment of Esophageal Squamous Cell Carcinoma (ESCC). Nanoscale 2025, 17, 8270–8288. [Google Scholar] [CrossRef]
- Udekwu, K.I.; Parrish, N.; Ankomah, P.; Baquero, F.; Levin, B.R. Functional Relationship between Bacterial Cell Density and the Efficacy of Antibiotics. J. Antimicrob. Chemother. 2009, 63, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, Y.; Niu, X.; Pei, J.; Yan, R.; Xu, F.; Ma, J.; Ma, X.; Jia, S.; Ma, W. Silver Nanoparticles Induce Endothelial Cytotoxicity through ROS-Mediated Mitochondria-Lysosome Damage and Autophagy Perturbation: The Protective Role of N-Acetylcysteine. Toxicology 2024, 502, 153734. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef]
| Nanoparticles | Origin/Synthesis | EC Cell Lines Tested | IC50 (µg/mL or µM) | Mechanism of Action | Toxicity (Normal Cells or Organism) |
|---|---|---|---|---|---|
| CuO NPs | Vibrio sp. VLC | KYSE30 | 13.96 mg/L | / | HDF: IC50 = 48.88 mg/L) |
| Au NPs | Moringa oleifera | SNO | 92.01 µg/mL | Apoptosis (↑Caspases, p53, ↓Bcl-2, ATP…) | Hepatoprotection effect (in vitro) |
| NaLS-Au NPs | Cydonia oblonga + NaLS | FLO-1, ESO26, OE33, KYSE-270 | 181, 130, 205, 133 µg/mL | / | IC50 > 1000 µg/mL on HUVEC cells |
| Nano-curcumin | Curcuma longa | OE33, OE19 | 20% and 17% inhibition at 50 mM | / | Non-toxic on HET-1A cells |
| Cu NPs | Mentha piperita | KYSE-270, OE33, ESO26 | 241, 278, 240 mg/mL | / | / |
| NiO NPs | Calendula officinalis | FLO-1, ESO26, OE33, KYSE-270 | 380, 263, 229, 251 µg/mL | / | Non-toxic on HUVEC cells |
| Ag NPs | Pomegranate peel | KYSE30-270, OE33, ESO26, FLO-1 | 253–500 µg/mL | / | / |
| Ag NPs | Photinia glabra | Eca-109 | <20 µg/mL | / | / |
| Au NPs | Rhus coriaria | FLO-1, ESO26, OE33, KYSE-270 | 226–294 mg/mL | Apoptosis | / |
| CeO2 NPs | Chemical synthesis | YM1 | 630 µM | Oxidative stress | Highly toxic in vitro; low toxicity in vivo |
| Cu2(btc)(trz)3-NPs | Chemical synthesis | KYSE150, EC109, EC8712 | 48, 42, 34 µM | / | / |
| Cu5(Hbtc)4(trz)2(H2O)4-NPs | Chemical synthesis | 86, 73, 78 µM | / | / | |
| Au NPs | Potato starch | KYSE-30, FLO-1 | 125, 176 µg/mL | / | IC50 > 1000 µg/mL on HUVEC cells |
| Gold NIC | AuNPs + antibody CD271 | HKESC-1 | 2.3–2.9 µM | / | / |
| Ag-Dox NPs | AgNPs + doxorubicin | OE33 | 2.399 µM | ↑Dox efficacy, ↓migration | No harmful effect on H9c2 cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamsu, G.T.; Ndebia, E.J. Usefulness of Nanoparticles in the Fight Against Esophageal Cancer: A Comprehensive Review of Their Therapeutic Potential. Appl. Nano 2025, 6, 18. https://doi.org/10.3390/applnano6030018
Kamsu GT, Ndebia EJ. Usefulness of Nanoparticles in the Fight Against Esophageal Cancer: A Comprehensive Review of Their Therapeutic Potential. Applied Nano. 2025; 6(3):18. https://doi.org/10.3390/applnano6030018
Chicago/Turabian StyleKamsu, Gabriel Tchuente, and Eugene Jamot Ndebia. 2025. "Usefulness of Nanoparticles in the Fight Against Esophageal Cancer: A Comprehensive Review of Their Therapeutic Potential" Applied Nano 6, no. 3: 18. https://doi.org/10.3390/applnano6030018
APA StyleKamsu, G. T., & Ndebia, E. J. (2025). Usefulness of Nanoparticles in the Fight Against Esophageal Cancer: A Comprehensive Review of Their Therapeutic Potential. Applied Nano, 6(3), 18. https://doi.org/10.3390/applnano6030018







