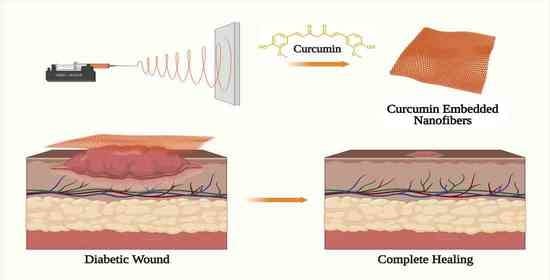
Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing
Abstract
1. Introduction
| S. No | Wound Dressing Materials | Curcumin with Composition | Method of Formulation | Outcomes | Ref |
|---|---|---|---|---|---|
| 1 | Nanofibrous mats | Gelatin, Trifluoroethanol, Glutaraldehyde. | Electrospinning method | Curcumin has a prolonged release profile from the formulation. Curcumin/gelatin blended nanofibrous mats promoted faster and more effective wound healing in Sprague–Dawley rats. Compared to the control group, the epidermis layers in the group that had significant reepithelialization and differentiation were well-developed. | [26] March 2017 |
| 2 | Nanofibers | PCL | Electrospinning method | It has the potential to be biocompatible and cytoprotective, according to in vitro investigations. On the third day, a release analysis showed that fibres containing 3% and 17% curcumin released 35 mg and 20 mg of curcumin over an extended period. Studies on in vivo wound healing have shown significant wound closure capacity in addition to antioxidant and anti-inflammatory action. | [27] December 2009 |
| 3 | Nanocrystal scaffolds containing curcumin-loaded microspheres | Bovine gelatin, Collagen | Emulsion solvent evaporation method | Curcumin release profile over time enhanced dermal regeneration and successfully reduced local inflammation in a rat full-thickness burn infection model. | [28] December 2017 |
| 4 | Nanocomposite hydrogel | MPEG-PCL copolymer, Oxidized alginate, Chitosan | Thin-film evaporation method | Nanocomposite hydrogel regulates and sustains the release profile of curcumin. On day 14, an in vivo examination showed that the wound had fully healed. Improved collagen deposition, reepithelization, and granulation tissue development | [29] November 2012 |
| 5 | Hydrogel film | Sacran, 2-hydroxypropyl g-cyclodextrin | Solvent evaporation method | Curcumin release is slow and persistent. Enhanced curcumin antioxidant activity Faster healing of wounds relative to other groups | [30] May 2017 |
| 6 | Hydrogel system containing micellar curcumin | PEG-PCL micellar curcumin, PEG-PCL-PEG copolymer hydrogel | Curcumin micelle by solid dispersion method and hydrogel by crosslinked methods | Wound dressing exhibited more significant cutaneous wound healing, increased collagen content, improved granulation, and increased wound maturity. 60% sustained release of curcumin during 14 days | [31] September 2013 |
| 7 | Collagen films | Collagen from bovine achilles tendon | Crosslinking | The in vitro release kinetics demonstrated more than 60% curcumin release after 12 days of investigation. High expression of collagen and granulation tissue development with the application of collagen films containing curcumin | [32] May 2004 |
| 8 | Chitosan–alginate sponge | Curcumin, Alginate, Chitosan | Ionic interaction and crosslinking | The in vitro studies demonstrated enhanced water absorption and biodegradability. 40% to 80% sustained release of curcumin in vitro for up to 20 days. In vivo wound healing tests showed superior healing efficacy due to fast wound contraction and collagen deposition. | [33] September 2009 |
| 9 | Nanostructured lipid carriers | Curcumin, Glyceryl monostearate, Stearic acid, Caprylic/capric triglyceride, Soya lecithin | Emulsion evaporation– solidification method | Significant skin permeability ability in comparison to standard formulations. Significant anti-inflammatory efficacy accelerated skin regeneration and enhanced skin thickness. | [34] October 2016 |
| 10 | Polymeric bandage | Curcumin, Oleic acid, Alginate, Chitosan | Ionic interaction and crosslinking | For a protracted period of 10 days, there was a release of curcumin that was more than 40%. 10 days after application, control, empty bandage, and curcumin bandage-treated wounds contracted 70%, 80%, and 94%. | [35] October 2012 |
| 11 | Nanoparticle/hydrogel | Curcumin, Polyethylene glycol, Polyvinyl alcohol, PLA–10R5–PLA copolymer | w/o/w double emulsion solvent evaporation method | In vitro drug release behaviour with low cytotoxicity with an increase in granulation tissue development, collagen deposition, and angiogenesis demonstrated good wound healing efficacy in vivo. | [36] August 2016 |
| 12 | Curcumin nanoparticles | Curcumin, Chitosan, Tetramethyl orthosilicate, Polyethylene glycol 400 | Sol–gel-based | Curcumin releases slowly over time. Significantly improved collagen deposition, granulation tissue development, re-epithelization, and tissue regeneration | [37] January 2015 |
| 13 | Polymeric bioadhesive emulsion | Neem and turmeric extract, Shellac, Casein and Polyvinyl alcohol and Maleic anhydride | Emulsion method | It has antibacterial qualities, is harmless, and degrades naturally. | [38] December 2005 |
| 14 | Methoxy poly(ethylene glycol)-graft-chitosan composite film containing curcumin nanoformulation | Curcumin, Poly (e-caprolactone)-Poly (ethylene glycol) methyl ether (MPEG-PCL) copolymer, Linoleic acid, Tween1 20, Chitosan | Casting/solvent evaporation method | 8.4% of the curcumin was released early on day 1 and continued throughout the next five days. When the wound area was less than 10% at day 14, an in vivo wound healing research showed quicker healing. Rapid reepithelialization, collagen synthesis, and wound healing were seen after administration. | [39] March 2012 |
| 15 | Hyalurosomes, a nanovesicle and liposomes | Curcumin, Soy Phosphatidylcholine, Sodium hyaluronate, ultrasonic disintegrator | Sonication | Human keratinocytes in vitro were shielded from oxidative stress damage by biocompatible materials. Compared to other groups, in vivo data demonstrated improved skin restoration activity in terms of decreased edema, myeloperoxidase activity, and early skin reepithelization. | [40] December 2015 |
| 16 | Gel-core hyalurosome (nanovesicle) | Curcumin, Lipoid1 S100, Tween1 80, Hyaluronic acid | Film hydration technique | After two hours of in vitro testing, there was a 50% release of curcumin. At day 10, the wound had healed properly and early with no scars. Compared to other groups, improved granulation tissue development, collagen fibre deposition, re-epithelization, and tissue regeneration | [41] May 2015 |
| 17 | Nanovesicles | Curcumin, Lipoid1 S75, PEG400, Oramix1 | Sonication method | It is spherical, multi- or oligolamellar, compact, and biocompatible. Application on skin injured by tissue plasminogen activator (TPA) revealed decreased oxidative inflammation. Data from histology showed significant re-epithelization with several thick epidermal layers. | [42] March 2014 |
| 18 | Curcumin-loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles | Curcumin, Poly (lactic-glycolic acid), Polyvinyl alcohol | Oil/water emulsion– solvent evaporation technique | Over the period of eight days, there was a steady release of curcumin, from 40.5% to 75.7%. Angiogenesis and wound healing were enhanced by lactate produced from PLGA. Studies using histology and RT-PCR showed that PLGA-curcumin had more potential for reepithelialization, granulation tissue development, and anti-inflammatory effects. | [43] October 2013 |
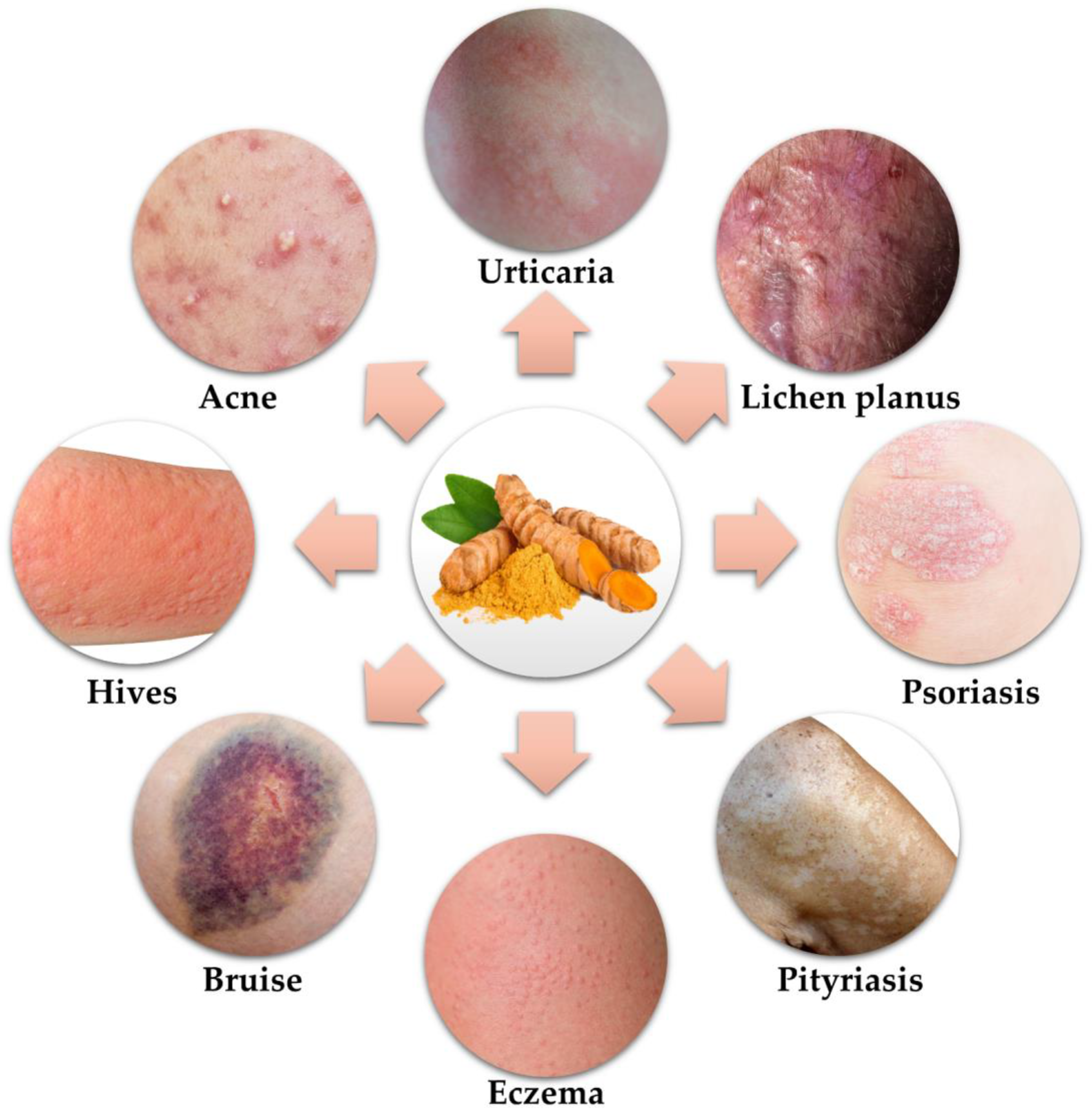
2. Potential of Curcumin in Skin Disorders
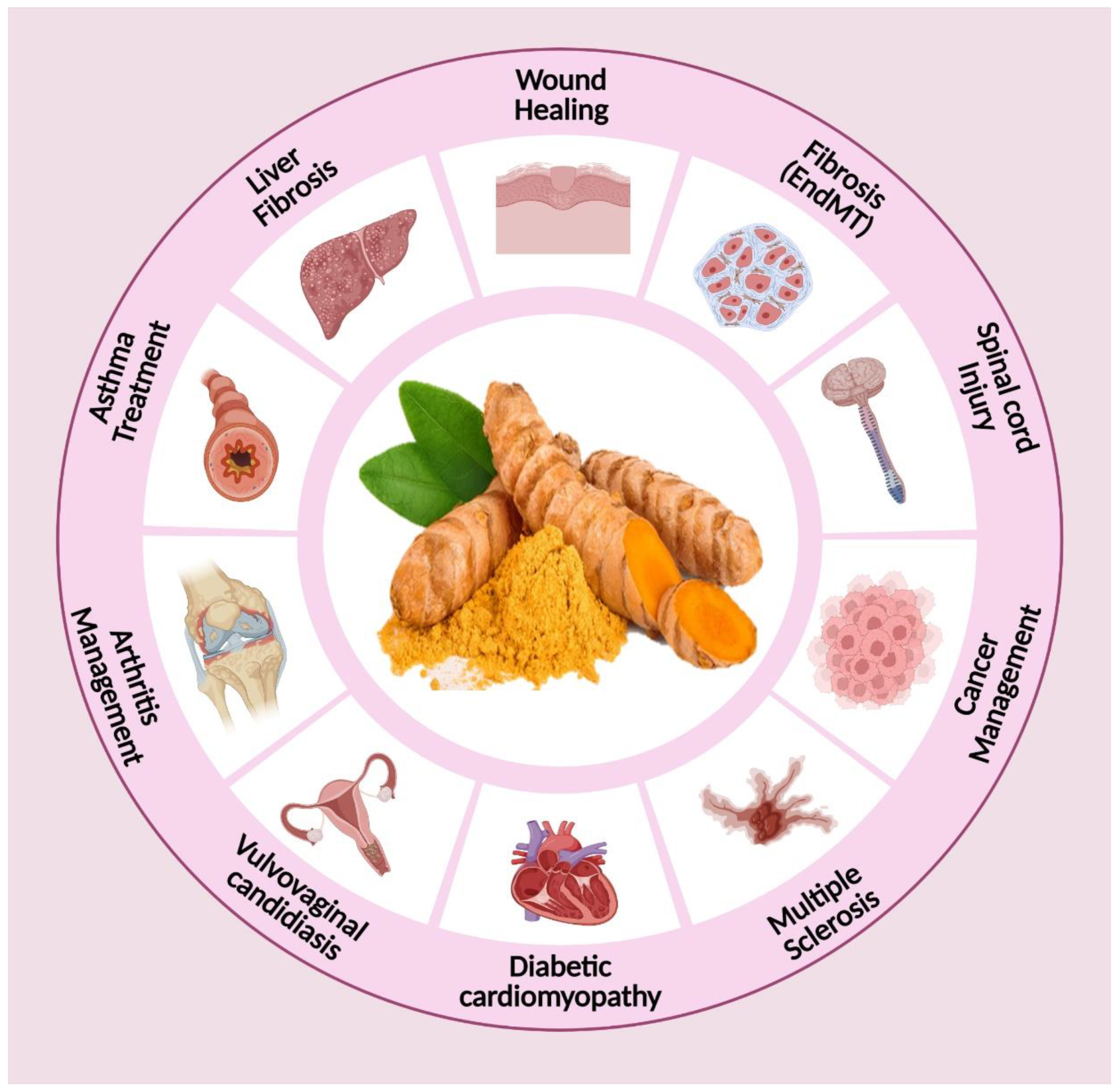
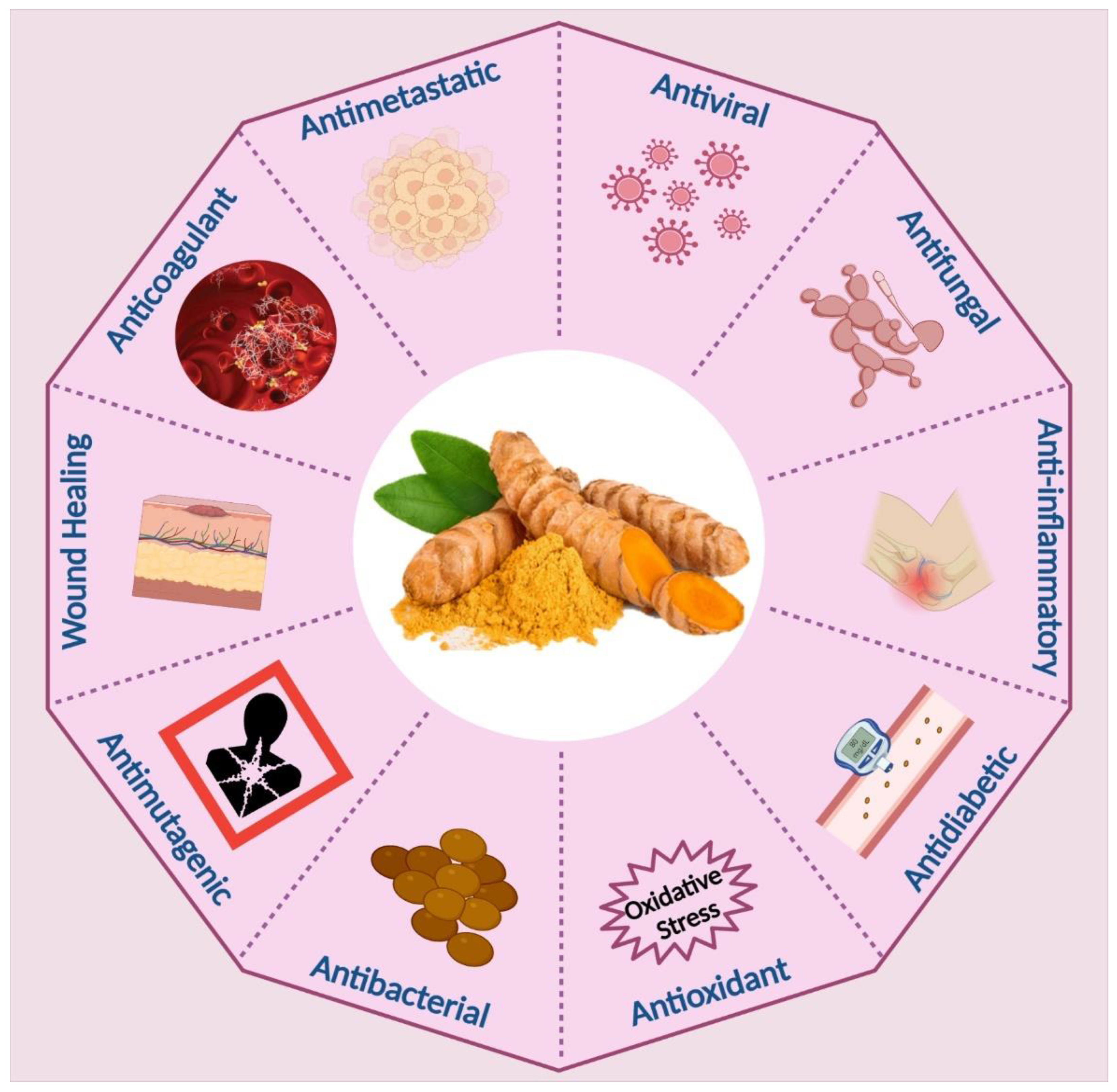
3. Biomedical Applications of Curcumin
4. Safety Profile of Curcumin
| S. No | Safety Profile of Curcumin | Ref |
|---|---|---|
| 1 | The Food and Drug Administration has acknowledged curcumin as a safe substance. | [77,78,79] |
| 2 | Daily consumption of 12,000 mg is considered safe in healthy persons since no adverse effects were seen in participants. | [13] |
| 3 | Healthy patients given up to 8000 mg per day did not have curcumin in their blood serum. Two persons given 10,000 or 12,000 mg had low levels. | [13] |
| 4 | Curcumin at 500 to 8000 mg per day for three months was safe for patients with internal organ pre-malignant lesions and cardiovascular risk. | [80] |
| 5 | Advanced pancreatic cancer patients taking 8000 mg per day of curcumin for two months and advanced breast cancer patients receiving radiation while taking up to 6000 mg per day of curcumin have also shown this safety. | [81,82,83] |
| 6 | In healthy participants and patients with ulcerative colitis, cholangitis, and advanced colorectal cancer, up to 8000 mg of curcumin daily caused moderate and controllable gastrointestinal complications. | [14,84,85,86] |
| 7 | A tiny proportion of sclerosing cholangitis patients receiving up to 1400 mg per day of curcumin experienced headache or nausea. | [87] |
| 8 | Individuals with advanced pancreatic cancer taking gemcitabine reported severe stomach discomfort after starting 8000 mg of curcumin daily. | [88] |
| 9 | Short-term IV liposomal curcumin administration to healthy volunteers was safe up to 120 mg/m. | [89] |
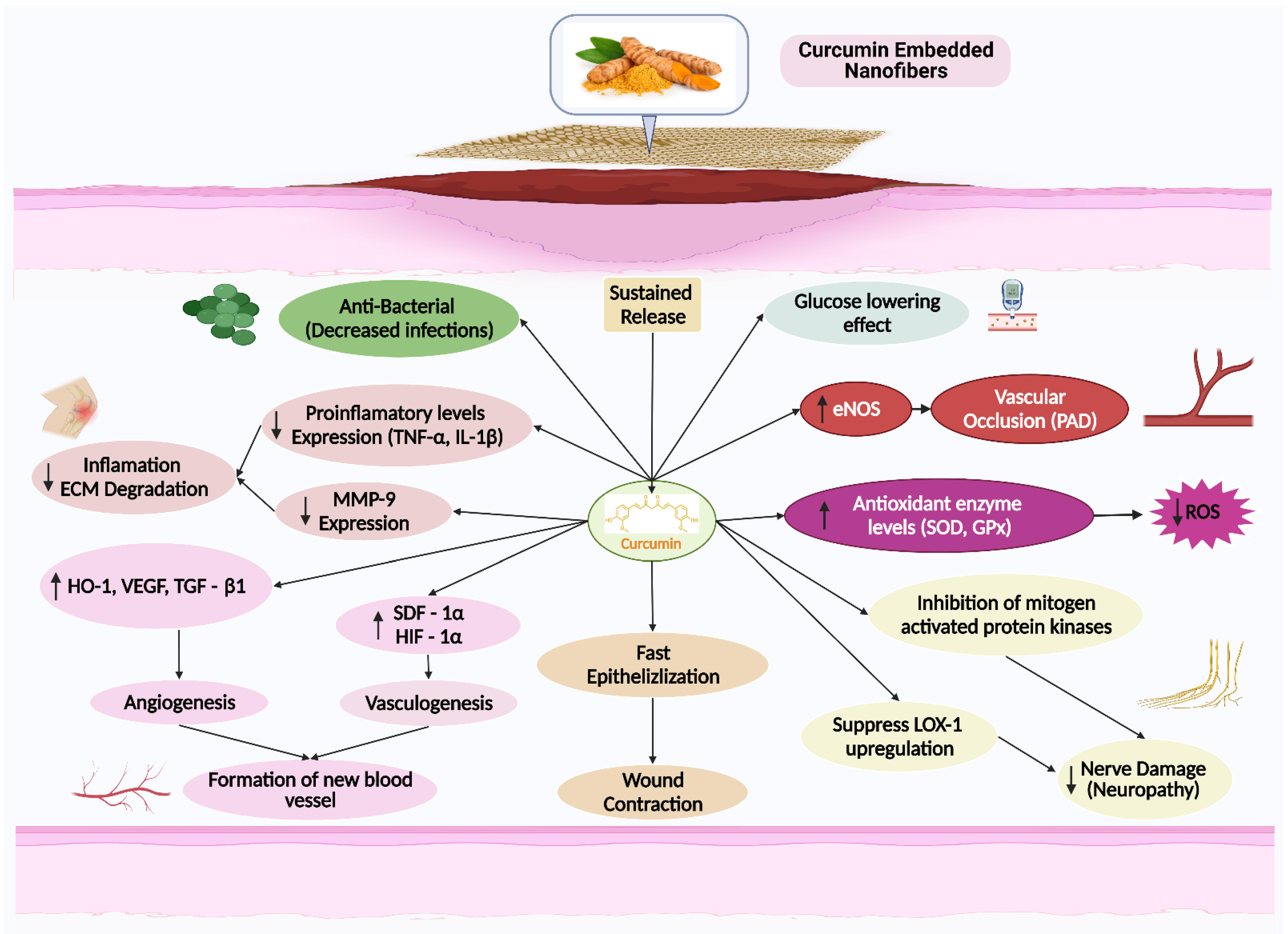
5. The Effects of Curcumin on Wound Healing
5.1. Inflammation
5.2. Antioxidant
5.3. Fibroblast Proliferation
5.4. Angiogenesis
5.5. Granulation Tissue Formation
5.6. Collagen Deposition
5.7. Apoptosis
5.8. Wound Contraction
5.9. Re-Epithelialization and Remodeling
6. Nanofibers
| S. No | Nanocarrier | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| 1 | Fibers |
|
| [155] |
| 2 | Polymeric nanoparticles |
|
| [156] |
| 3 | Liposomes |
|
| [157] |
| 4 | Films |
|
| [158] |
| 5 | Sponges |
|
| [159] |
| 6 | Hydrogels |
|
| [160] |
| 7 | Hydrocolloids |
|
| [161] |
7. Curcumin Embedded Electrospun Nanofibers for Wound Healing
| S. No | Curcumin & Additives | Solvents | Dosage | Electrospinning Setting | Diameter (nm) | Drug Release Profile | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| kV | cm | mL/h | |||||||
| 1 | PCL | CHCl3: Methanol | 3 & 17% w/w | 25 | 10 | 2 | 300–400 | 3%—20 µg at 3 d 17%—35 µg at 3 d | [27] December 2009 |
| 2 | p(HEMA) | Ethanol: H2O | 3 & 5 wt% | 25 | 17 | 0.5 | 20–110 | 63% at 120 h 72% at 240 h | [162] January 2015 |
| 3 | PLA | CHCl3: DMAc | 0.125, 1.250, 6.250 wt% | 11 | 12 | 1 | 300–1200 | – | [163] June 2013 |
| 4 | CA | IPA: EA | 5, 10, 15, 17.5, 20 wt% | 12 | 15 | 1.5 | 300 | 309.02 µg/cm2 at 24 h | [164] September 2017 |
| 5 | PCL/GT | Acetic acid | 1, 3, 8, 24% | 15 | 15 | 1 | 667 ± 33 | 42.6% at 10 d 65% at 20 d | [166] March 2016 |
| 6 | PCL | Acetic acid: Formic acid | 0.5 wt% | 11 | 10 | 0.4 | 499 | 80% at 2 h | [167] November 2019 |
| 7 | PLA | CHCl3: Acetone | 10 wt% | 20 | 15 | 0.5 | 430–750 | 9 µg/cm2 at 24 h | [168] April 2020 |
| 8 | PHBV | CHCl3: DMF | 0.1, 0.3 0.5% w/v | 17 | 20 | 0.01 | 207–519 | 45%, 63%, 78% at 200 min | [169] February 2018 |
| 9 | PCL | DCM: DMF | 0.5 wt% | 8.5 | 16 | 0.8 | 300–1200 | 5, 4.1 µg at 24 h | [170] November 2014 |
| 10 | PCL-PEG -PCL | Acetone: CHCl3 CH3OH | 5%, 10% w/w | 28–30 | 10 | 2 | 50–300 | 59, 68, 81.5%, respectively | [171] December 2019 |
| 11 | PLA | CHCl3 | 10, 15 wt/wt% | 13–15 | 12 | 0.5 | 516 | 32 µg/mL at 72 h | [172] March 2017 |
| 12 | PCL-PEG | CHCl3: Acetone | 8.7% | 18 | 15 | 2 | 400 ± 20 | 95.11% at 24 h | [173] April 2018 |
| 13 | PCL | HFIP | 5, 10 wt% | 20 | 21 | 0.5 | 427–651 | burst release at 24 h | [174] February 2020 |
| 14 | PCEC | DCM | 20 wt% | 18 | 12 | 6 | 535 | burst release within the first 24 h | [175] April 2014 |
| 15 | PLLA-PCL | HFIP | 2.0%, 4.0% 6.0% w/w | 10 | 15 | 1.2 | 293 ± 110 | sustain released over 72 h | [176] Nov. 2014 |
| 16 | CA-PVP | Acetone: H2O | 10% | 25 | 15 | 3 | 1560 ± 145 | 22% (1.2 μg/mL) at 120 min | [177] April 2017 |
| 17 | PU-CA | DMF: THF DMF: Acetone | 4.0 wt% | 17 | 15 | 0.4 | 222 ± 44 | – | [180] February 2020 |
| 18 | PLLA | DCM: DMF | 0.2, 0.5, 1.0% w/w | 24 | 15 | - | 380 ± 113 | 22, 34, 58% at 50 h | [181] October 2019 |
| 19 | CA | Acetone: DMAc | 5, 10, 15, 20 wt% | 17 | 15 | 1 | 340 ± 98 | 90 to 95% at 50 h | [183] December 2007 |
| 20 | PCL | HFIP | 2.5 mg/mL | 18 | 20 | 0.3 | 1548 µm | 23% at 6 h Until 106 h | [185] June 2020 |
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.C.N.; Lim, L.L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on Diabetes: Using Data to Transform Diabetes Care and Patient Lives. Lancet 2020, 396, 2019–2082. [Google Scholar] [CrossRef]
- Mishra, S.C.; Chhatbar, K.C.; Kashikar, A.; Mehndiratta, A. Diabetic Foot. BMJ 2017, 359, j5064. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Armstrong, D.G.; Lin, C.H.; Liu, P.H.; Hung, S.Y.; Lee, S.R.; Huang, C.H.; Huang, Y.Y. Nationwide Trends in the Epidemiology of Diabetic Foot Complications and Lower-Extremity Amputation over an 8-Year Period. BMJ Open Diabetes Res. Care 2019, 7, e000795. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.C.; Fleetwood, K.; Wild, S.H.; Colhoun, H.M.; Lindsay, R.S.; Petrie, J.R.; McCrimmon, R.J.; Gibb, F.; Philip, S.; Sattar, N.; et al. Foot Ulcer and Risk of LowerLimb Amputation or Death in People With Diabetes: A National Population-Based Retrospective Cohort Study. Diabetes Care 2022, 45, 83–91. [Google Scholar] [CrossRef]
- Bus, S.A. Preventing Foot Ulcers in Diabetes Using Plantar Pressure Feedback. Lancet Digit. Health 2019, 1, e250–e251. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Asadi, N.; Annabi, N.; Mostafavi, E.; Anzabi, M.; Khalilov, R.; Saghfi, S.; Mehrizadeh, M.; Akbarzadeh, A. Synthesis, Characterization and in Vitro Evaluation of Magnetic Nanoparticles Modified with PCL–PEG–PCL for Controlled Delivery of 5FU. Artif. Cells Nanomed. Biotechnol. 2018, 46, 938–945. [Google Scholar] [CrossRef]
- Peschel, D.; Koerting, R.; Nass, N. Curcumin Induces Changes in Expression of Genes Involved in Cholesterol Homeostasis. J. Nutr. Biochem. 2007, 18, 113–119. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma Longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a Low Bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin IV, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Hsu, C.H.; Cheng, A.L. Clinical Studies with Curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle Encapsulation Improves Oral Bioavailability of Curcumin by at Least 9-Fold When Compared to Curcumin Administered with Piperine as Absorption Enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin Loaded Chitosan Nanoparticles Impregnated into Collagen-Alginate Scaffolds for Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef]
- Ignatova, M.; Rashkov, I.; Manolova, N. Drug-Loaded Electrospun Materials in Wound-Dressing Applications and in Local Cancer Treatment. Expert Opin. Drug Deliv. 2013, 10, 469–483. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun Nanofibers Promote Wound Healing: Theories, Techniques, and Perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 1, 1–31. [Google Scholar] [CrossRef]
- Memic, A.; Abdullah, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- da Costa, P.R.A.; de Menezes, L.R.; Dias, M.L.; da Silva, E.O. Advances in the Use of Electrospinning as a Promising Technique for Obtaining Nanofibers to Guide Epithelial Wound Healing in Diabetics—Mini-Review. Polym. Adv. Technol. 2022, 33, 1031–1046. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent Advances in Electrospun Nanofibers for Wound Healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.G.; Schilling, A.F. Nano-Formulated Curcumin Accelerates Acute Wound Healing through Dkk-1-Mediated Fibroblast Mobilization and MCP-1-Mediated Anti-Inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-Loaded Poly(ε-Caprolactone) Nanofibres: Diabetic Wound Dressing with Anti-Oxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef]
- Guo, R.; Lan, Y.; Xue, W.; Cheng, B.; Zhang, Y.; Wang, C.; Ramakrishna, S. Collagen-Cellulose Nanocrystal Scaffolds Containing Curcumin-Loaded Microspheres on Infected Full-Thickness Burns Repair. J. Tissue Eng. Regen. Med. 2017, 11, 3544–3555. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In Situ Injectable Nano-Composite Hydrogel Composed of Curcumin, N,O-Carboxymethyl Chitosan and Oxidized Alginate for Wound Healing Application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancement of Curcumin Wound Healing Ability by Complexation with 2-Hydroxypropyl-γ-Cyclodextrin in Sacran Hydrogel Film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Wu, Q.J.; Wang, Y.J.; Zhang, D.D.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal Wound Healing Processes with Curcumin Incorporated Collagen Films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Qian, Z.; Dai, M.; Zheng, X.; Xu, X.; Kong, X.; Li, X.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.Q. Chitosan-Alginate Sponge: Preparation and Application in Curcumin Delivery for Dermal Wound Healing in Rat. J. Biomed. Biotechnol. 2009, 2009, 595126. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of Curcumin Loaded Nanostructured Lipid Carrier Based Thermosensitive in Situ Gel for Dermal Delivery. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 356–362. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Li, X.; Ye, X.; Qi, J.; Fan, R.; Gao, X.; Wu, Y.; Zhou, L.; Tong, A.; Guo, G. EGF and Curcumin Co-Encapsulated Nanoparticle/Hydrogel System as Potent Skin Regeneration Agent. Int. J. Nanomed. 2016, 11, 3993–4009. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-Encapsulated Nanoparticles as Innovative Antimicrobial and Wound Healing Agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef]
- Jagannath, J.H.; Radhika, M. Antimicrobial Emulsion (Coating) Based on Biopolymer Containing Neem (Melia Azardichta) and Turmeric (Curcuma Longa) Extract for Wound Covering. Biomed. Mater. Eng. 2006, 16, 329–336. [Google Scholar]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In Vivo Evaluation of Curcumin Nanoformulation Loaded Methoxy Poly (Ethylene Glycol)-Graft-Chitosan Composite Film for Wound Healing Application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of Curcumin Loaded Sodium Hyaluronate Immobilized Vesicles (Hyalurosomes) and Their Potential on Skin Inflammation and Wound Restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Novel Curcumin-Loaded Gel-Core Hyaluosomes with Promising Burn-Wound Healing Potential: Development, in-Vitro Appraisal and in-Vivo Studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of Quercetin and Curcumin Bionanovesicles for the Prevention and Rapid Regeneration of Full-Thickness Skin Defects on Mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Des Rieux, A.; Vandermeulen, G.; Préat, V. Combined Effect of PLGA and Curcumin on Wound Healing Activity. J. Control Release 2013, 171, 208–215. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Dréno, B. Bacteriological Resistance in Acne: A Call to Action. Eur. J. Dermatol. 2016, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Katsambas, A. Propionibacterium Acnes and Antimicrobial Resistance in Acne. Clin. Dermatol. 2017, 35, 163–167. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Topical and Oral Antibiotics for Acne Vulgaris. Semin. Cutan. Med. Surg. 2016, 35, 57–61. [Google Scholar] [CrossRef]
- Liu, C.H.; Huang, H.Y. Antimicrobial Activity of Curcumin-Loaded Myristic Acid Microemulsions against Staphylococcus Epidermidis. Chem. Pharm. Bull. 2012, 60, 1118–1124. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Krausz, A.E.; Souza, A.C.O.; Adler, B.L.; Landriscina, A.; Musaev, T.; Nosanchuk, J.D.; Friedman, A.J. Trichophyton Rubrum Is Inhibited by Free and Nanoparticle Encapsulated Curcumin by Induction of Nitrosative Stress after Photodynamic Activation. PLoS ONE 2015, 10, e0120179. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Mata, I.R.d.; Mata, S.R.d.; Menezes, R.C.R.; Faccioli, L.S.; Bandeira, K.K.; Bosco, S.M.D. Benefits of Turmeric Supplementation for Skin Health in Chronic Diseases: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3421–3435. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.A.; Patel, P.M.; Wilson, C.; Wang, H.; Ashack, K.A. Complementary and Alternative Medicine Treatments for Common Skin Diseases: A Systematic Review and Meta-Analysis. JAAD Int. 2021, 2, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of Curcumin and Curcumin Analogue Effects in Skin Diseases: A Narrative Review. J. Cell. Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial Role of Curcumin in Skin Diseases. Adv. Exp. Med. Biol. 2007, 595, 343–357. [Google Scholar] [CrossRef]
- Waghule, T.; Gorantla, S.; Rapalli, V.K.; Shah, P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Emerging Trends in Topical Delivery of Curcumin Through Lipid Nanocarriers: Effectiveness in Skin Disorders. AAPS PharmSciTech 2020, 21, 284. [Google Scholar] [CrossRef]
- Liang, G.; Li, X.; Chen, L.; Yang, S.; Wu, X.; Studer, E.; Gurley, E.; Hylemon, P.B.; Ye, F.; Li, Y.; et al. Synthesis and Anti-Inflammatory Activities of Mono-Carbonyl Analogues of Curcumin. Bioorganic Med. Chem. Lett. 2008, 18, 1525–1529. [Google Scholar] [CrossRef]
- Williams, M.D.; Nadler, J.L. Inflammatory Mechanisms of Diabetic Complications. Curr. Diab. Rep. 2007, 7, 242–248. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an Antioxidant and Anti-Inflammatory Agent, Induces Heme Oxygenase-1 and Protects Endothelial Cells against Oxidative Stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Anusuya, T. Investigation on Curcumin Nanocomposite for Wound Dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef]
- Gowthamarajan, K. Multiple Biological Actions of Curcumin in the Management of Diabetic Foot Ulcer Complications: A Systematic Review. Trop. Med. Surg. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Bhatia, M.; Bhalerao, M.; Cruz-Martins, N.; Kumar, D. Curcumin and Cancer Biology: Focusing Regulatory Effects in Different Signalling Pathways. Phyther. Res. 2021, 35, 4913–4929. [Google Scholar] [CrossRef]
- Shen, X.Y.; Li, Y.; Zhang, Z. Research Progress of Curcumin in the Treatment of Osteoarthritis. Zhonghua Wai Ke Za Zhi 2021, 59, 554–557. [Google Scholar] [CrossRef]
- Ghanaatian, N.; Lashgari, N.A.; Abdolghaffari, A.H.; Rajaee, S.M.; Panahi, Y.; Barreto, G.E.; Butler, A.E.; Sahebkar, A. Curcumin as a Therapeutic Candidate for Multiple Sclerosis: Molecular Mechanisms and Targets. J. Cell. Physiol. 2019, 234, 12237–12248. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Singh, D.K.; Dash, D.; Singh, R. Intranasal Curcumin Regulates Chronic Asthma in Mice by Modulating NF-ĸB Activation and MAPK Signaling. Phytomedicine 2018, 51, 29–38. [Google Scholar] [CrossRef]
- Andrade, J.T.; Fantini de Figueiredo, G.; Cruz, L.F.; Eliza de Morais, S.; Souza, C.D.F.; Pinto, F.C.H.; Ferreira, J.M.S.; Araújo, M.G.d.F. Efficacy of Curcumin in the Treatment of Experimental Vulvovaginal Candidiasis. Rev. Iberoam. Micol. 2019, 36, 192–199. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Lai, S.; Liu, J.; Qi, J. Curcumin Activates Nrf2/HO-1 Signaling to Relieve Diabetic Cardiomyopathy Injury by Reducing ROS in Vitro and in Vivo. FASEB J. 2022, 36, e22505. [Google Scholar] [CrossRef] [PubMed]
- Reena, K.; Singh, L. Curcumin: A Review of Its’ Efficacy in the Management of Psoriasis. Drug Deliv. Lett. 2022, 12, 163–183. [Google Scholar] [CrossRef]
- Chamani, S.; Moossavi, M.; Naghizadeh, A.; Abbasifard, M.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Immunomodulatory Effects of Curcumin in Systemic Autoimmune Diseases. Phyther. Res. 2022, 36, 1616–1632. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, H.J.; Tong, Y.; Chen, Y.Z.; Ye, C.; Qiu, Y.; Zhang, F.; Chen, A.D.; Qi, X.H.; Chen, Q.; et al. Curcumin Attenuates Migration of Vascular Smooth Muscle Cells via Inhibiting NFκB-Mediated NLRP3 Expression in Spontaneously Hypertensive Rats. J. Nutr. Biochem. 2019, 72, 108212. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Krawczyk, M.; Wozniak, L.A. Antidiabetic Activity of Curcumin. Nutr. Ther. Interv. Diabetes Metab. Syndr. 2018, 385–401. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic Potential of Curcumin in Diabetic Complications. Pharmacol. Res. 2018, 136, 181–193. [Google Scholar] [CrossRef]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The Effect of Curcumin on Cognition in Alzheimer’s Disease and Healthy Aging: A Systematic Review of Pre-Clinical and Clinical Studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Coelho, M.R.; Romi, M.D.; Ferreira, D.M.T.P.; Zaltman, C.; Soares-Mota, M. The Use of Curcumin as a Complementary Therapy in Ulcerative Colitis: A Systematic Review of Randomized Controlled Clinical Trials. Nutrients 2020, 12, 2296. [Google Scholar] [CrossRef]
- Rahimnia, A.R.; Panahi, Y.; Alishiri, G.; Sharafi, M.; Sahebkar, A. Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2014, 65, 521–525. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a Component of Golden Spice: From Bedside to Bench and Back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Chen, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; She, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Wu, M.S.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and Safety of Turmeric and Curcumin in Lowering Blood Lipid Levels in Patients with Cardiovascular Risk Factors: A Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for Radiation Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Thirty Breast Cancer Patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Euden, S.A.; Steward, W.P.; Gescher, A.J.; Ireson, C.R.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma Longa) and Its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Eaton, J.E.; Nelson, K.M.; Gossard, A.A.; Carey, E.J.; Tabibian, J.H.; Lindor, K.D.; LaRusso, N.F. Efficacy and Safety of Curcumin in Primary Sclerosing Cholangitis: An Open Label Pilot Study. Scand. J. Gastroenterol. 2019, 54, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and Gemcitabine in Patients with Advanced Pancreatic Cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A Phase 1 Dose-Escalation Study on the Safety, Tolerability and Activity of Liposomal Curcumin (LipocurcTM) in Patients with Locally Advanced or Metastatic Cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Storka, A.; Vcelar, B.; Klickovic, U.; Gouya, G.; Weisshaar, S.; Aschauer, S.; Bolger, G.; Helson, L.; Wolzt, M. Safety, Tolerability and Pharmacokinetics of Liposomal Curcumin in Healthy Humans. Int. J. Clin. Pharmacol. Ther. 2015, 53, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Lukefahr, A.L.; McEvoy, S.; Alfafara, C.; Funk, J.L. Drug-Induced Autoimmune Hepatitis Associated with Turmeric Detary Supplement Use. BMJ Case Rep. 2018, 2018, bcr2018224611. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The Metabolism and Excretion of Curcumin (1,7-Bis-(4-Hydroxy-3-Methoxyphenyl)-1,6-Heptadiene-3,5-Dione) in the Rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and Anti-Inflammatory Potential of Curcumin Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin Enhances Wound Healing in Streptozotocin Induced Diabetic Rats and Genetically Diabetic Mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Chandra, D.; Gupta, S.S. Anti-Inflammatory and Anti-Arthritic Activity of Volatile Oil of Curcuma Longa (Haldi). Indian J. Med. Res. 1972, 60, 138–142. [Google Scholar]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial Effects of Curcumin: An in Vitro Minimum Inhibitory Concentration Study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic Effects of Curcumin in Inflammatory and Immune-Mediated Diseases: A Nature-Made Jack-of-All-Trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef]
- Javadi, B.; Sahebkar, A. Natural Products with Anti-Inflammatory and Immunomodulatory Activities against Autoimmune Myocarditis. Pharmacol. Res. 2017, 124, 34–42. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin Modulates Nuclear Factor ΚB (Nf-ΚB)-Mediated Inflammation in Human Tenocytes in Vitro: Role of the Phosphatidylinositol 3-Kinase/Akt Pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef]
- Yen, Y.H.; Pu, C.M.; Liu, C.W.; Chen, Y.C.; Chen, Y.C.; Liang, C.J.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin Accelerates Cutaneous Wound Healing via Multiple Biological Actions: The Involvement of TNF-α, MMP-9, α-SMA, and Collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Mito, S.; Harima, M.; Thandavarayan, R.A.; Suzuki, K.; Nagata, M.; Takagi, R.; et al. Curcumin Prevents Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Rats: Possible Involvement of PKC-MAPK Signaling Pathway. Eur. J. Pharm. Sci. 2012, 47, 604–614. [Google Scholar] [CrossRef]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. Effect of Curcumin on the Advanced Glycation and Cross-Linking of Collagen in Diabetic Rats. Biochem. Pharmacol. 1998, 56, 1607–1614. [Google Scholar] [CrossRef]
- Srivastava, G.; Mehta, J.L. Currying the Heart: Curcumin and Cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 22–27. [Google Scholar] [CrossRef]
- Farhangkhoee, H.; Khan, Z.A.; Chen, S.; Chakrabarti, S. Differential Effects of Curcumin on Vasoactive Factors in the Diabetic Rat Heart. Nutr. Metab. 2006, 3, 27. [Google Scholar] [CrossRef]
- Rungseesantivanon, S.; Thenchaisri, N.; Ruangvejvorachai, P.; Patumraj, S. Curcumin Supplementation Could Improve Diabetes-Induced Endothelial Dysfunction Associated with Decreased Vascular Superoxide Production and PKC Inhibition. BMC Complement. Altern. Med. 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Patumraj; Rungseesantivanon; Thengchaisri; Ruangvejvorachai Curcumin Improves Prostanoid Ratio in Diabetic Mesenteric Arteries Associated with Cyclooxygenase-2 and NF-κB Suppression. Diabetes, Metab. Syndr. Obes. Targets Ther. 2010, 3, 421. [CrossRef]
- Hassan, N.; El-Bassossy, H.M.; Zakaria, M.N.M. Heme Oxygenase-1 Induction Protects against Hypertension Associated with Diabetes: Effect on Exaggerated Vascular Contractility. Naunyn. Schmiedebergs. Arch. Pharmacol. 2013, 386, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism. Front. Immunol. 2021, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent Developments in Curcumin and Curcumin Based Polymeric Materials for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring Recent Developments to Improve Antioxidant, Anti-Inflammatory and Antimicrobial Efficacy of Curcumin: A Review of New Trends and Future Perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rajanikant, G.K. Curcumin Stimulates the Antioxidant Mechanisms in Mouse Skin Exposed to Fractionated γ-Irradiation. Antioxidants 2015, 4, 25–41. [Google Scholar] [CrossRef]
- Hassan, F.U.; Rehman, M.S.U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef]
- Loughlin, D.T.; Artlett, C.M. Modification of Collagen by 3-Deoxyglucosone Alters Wound Healing through Differential Regulation of P38 MAP Kinase. PLoS ONE 2011, 6, e18676. [Google Scholar] [CrossRef]
- Martin, P. Wound Healing—Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The Molecular Biology of Chronic Wounds and Delayed Healing in Diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Mutsaers, H.A.M.; Pennings, S.W.C.; Szarek, W.A.; Russel, F.G.M.; Wagener, F.A.D.T.G. Curcumin-Induced Fibroblast Apoptosis and in Vitro Wound Contraction Are Regulated by Antioxidants and Heme Oxygenase: Implications for Scar Formation. J. Cell. Mol. Med. 2009, 13, 712–725. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, R.; Zhang, Y.; Xue, W.; Cheng, B.; Zhang, Y. Controlled Dual Delivery of Angiogenin and Curcumin by Electrospun Nanofibers for Skin Regeneration. Tissue Eng. Part A 2017, 23, 597–608. [Google Scholar] [CrossRef]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The Effects of Topical Treatment with Curcumin on Burn Wound Healing in Rats. J. Mol. Histol. 2013, 44, 83–90. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-Induced Angiogenesis Hastens Wound Healing in Diabetic Rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Gibran, N.S.; Boyce, S.; Greenhalgh, D.G. Cutaneous Wound Healing. J. Burn Care Res. 2007, 28, 577–579. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Longaker, M.T.; Whitby, D.J.; Adzick, N.S.; Crombleholme, T.M.; Langer, J.C.; Duncan, B.W.; Bradley, S.M.; Stern, R.; Ferguson, M.W.J.; Harrison, M.R. Studies in Fetal Wound Healing VI. Second and Early Third Trimester Fetal Wounds Demonstrate Rapid Collagen Deposition without Scar Formation. J. Pediatr. Surg. 1990, 25, 63–69. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin Improves Wound Healing by Modulating Collagen and Decreasing Reactive Oxygen Species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Leng, Q.Q.; Li, Y.; Pang, X.L.; Wang, B.Q.; Wu, Z.X.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S.Z. Curcumin Nanoparticles Incorporated in PVA/Collagen Composite Films Promote Wound Healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A Basic Physiologic Process in Wound Healing. Int. J. Low. Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.P.; Odland, G.F.; Clark, R.A.F. Temporal Relationships of F-Actin Bundle Formation, Collagen and Fibronectin Matrix Assembly, and Fibronectin Receptor Expression to Wound Contraction. J. Cell Biol. 1990, 110, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Orci, L. Transforming Growth Factor Beta Stimulates Collagen-Matrix Contraction by Fibroblasts: Implications for Wound Healing. Proc. Natl. Acad. Sci. USA 1988, 85, 4894–4897. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of Wound Healing by Curcumin in Animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef]
- Mani, H.; Sidhu, G.S.; Kumari, R.; Gaddipati, J.P.; Seth, P.; Maheshwari, R.K. Curcumin Differentially Regulates TGF-Β1, Its Receptors and Nitric Oxide Synthase during Impaired Wound Healing. BioFactors 2002, 16, 29–43. [Google Scholar] [CrossRef]
- Koivisto, L.; Heino, J.; Häkkinen, L.; Larjava, H. Integrins in Wound Healing. Adv. Wound Care 2014, 3, 762–783. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing Diabetes with Nanomedicine: Challenges and Opportunities. Nat. Rev. Drug Discov. 2014, 14, 45–57. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Ji, W.; Yao, H.; Lin, L.; Cui, H.; Santos, H.A.; Pan, G. Emerging Theranostic Nanomaterials in Diabetes and Its Complications. Adv. Sci. 2022, 9, 2102466. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as New-Generation Materials: From Spinning and Nano-Spinning Fabrication Techniques to Emerging Applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.J. Current Progresses of 3D Bioprinting Based Tissue Engineering. Quant. Biol. 2017, 5, 136–142. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing Electrospun Nanofiber Mats to Promote Wound Healing-a Review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef]
- Brennan, D.A.; Conte, A.A.; Kanski, G.; Turkula, S.; Hu, X.; Kleiner, M.T.; Beachley, V. Mechanical Considerations for Electrospun Nanofibers in Tendon and Ligament Repair. Adv. Healthc. Mater. 2018, 7, 1701277. [Google Scholar] [CrossRef]
- Ghafoor, B.; Aleem, A.; Najabat Ali, M.; Mir, M. Review of the Fabrication Techniques and Applications of Polymeric Electrospun Nanofibers for Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2018, 48, 82–87. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the Fabrication and Application of Electrospun Nanofiber Composites. Membranes 2020, 10, 1–35. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Sabra, S.; Ragab, D.M.; Agwa, M.M.; Rohani, S. Recent Advances in Electrospun Nanofibers for Some Biomedical Applications. Eur. J. Pharm. Sci. 2020, 144, 105224. [Google Scholar] [CrossRef]
- Nayl, A.; Abd-Elhamid, A.; Awwad, N.; Abdelgawad, M.; Wu, J.; Mo, X.; Gomha, S.; Aly, A.; Brase, S. Recent Progress and Potential Biomedical Applications of Electrospun Nanofibers in Regeneration of Tissues and Organs. Polymers 2022, 14, 1508. [Google Scholar] [CrossRef]
- Ekrami, E.; Khodabandeh Shahraky, M.; Mahmoudifard, M.; Mirtaleb, M.S.; Shariati, P. Biomedical Applications of Electrospun Nanofibers in Industrial World: A Review. Int. J. Polym. Mater. Polym. Biomater. 2022. [Google Scholar] [CrossRef]
- Chen, S.; Boda, S.K.; Batra, S.K.; Li, X.; Xie, J. Emerging Roles of Electrospun Nanofibers in Cancer Research. Adv. Healthc. Mater. 2018, 7, 1701024. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound Healing Performance of PCL/Chitosan Based Electrospun Nanofiber Electrosprayed with Curcumin Loaded Chitosan Nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Development and Characterization of Cefazolin Loaded Zinc Oxide Nanoparticles Composite Gelatin Nanofiber Mats for Postoperative Surgical Wounds. Mater. Sci. Eng. C 2016, 58, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Khoshnevisan, K.; Sajjadi-Jazi, S.M.; Baharifar, H.; Doostan, M.; Khoshnevisan, N.; Sharifi, F. Nanofiber-Based Systems Intended for Diabetes. J. Nanobiotechnol. 2021, 19, 317. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber Technology: Current Status and Emerging Developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Souto, E.B.; Yoshida, C.M.P.; Leonardi, G.R.; Cano, A.; Sanchez-Lopez, E.; Zielinska, A.; Viseras, C.; Severino, P.; da Silva, C.F.; Barbosa, R. de M. Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair. Pharmaceutics 2021, 13, 1199. [Google Scholar] [CrossRef]
- Henry, L.A.; Hart, M. Regeneration from Injury and Resource Allocation in Sponges and Corals—A Review. Int. Rev. Hydrobiol. 2005, 90, 125–158. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Dumville, J.C.; Deshpande, S.; O’Meara, S.; Speak, K. Hydrocolloid Dressings for Healing Diabetic Foot Ulcers. Cochrane Database Syst. Rev. 2013, 2013, CD009099. [Google Scholar] [CrossRef]
- Ramalingam, N.; Natarajan, T.S.; Rajiv, S. Preparation and Characterization of Electrospun Curcumin Loaded Poly(2-Hydroxyethyl Methacrylate) Nanofiber-A Biomaterial for Multidrug Resistant Organisms. J. Biomed. Mater. Res. Part A 2015, 103, 16–24. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.G.; Tran, L.D.; Park, J.S. Characteristics of Curcumin-Loaded Poly (Lactic Acid) Nanofibers for Wound Healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Ravikumar, R.; Ganesh, M.; Ubaidulla, U.; Young Choi, E.; Tae Jang, H. Preparation, Characterization, and in Vitro Diffusion Study of Nonwoven Electrospun Nanofiber of Curcumin-Loaded Cellulose Acetate Phthalate Polymer. Saudi Pharm. J. 2017, 25, 921–926. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun Curcumin Loaded Poly(ε-Caprolactone)/Gum Tragacanth Nanofibers for Biomedical Application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Amir Afshar, H.; Bogheiri, H. Biomimetic Nanocomposite Scaffolds Based on Surface Modified PCL-Nanofibers Containing Curcumin Embedded in Chitosan/Gelatin for Skin Regeneration. Compos. Part B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The Effect of Molecular Weight and Content of PEG on in Vitro Drug Release of Electrospun Curcumin Loaded PLA/PEG Nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-Loaded Electrospun PHBV Nanofibers as Potential Wound-Dressing Material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Bui, H.T.; Chung, O.H.; Dela Cruz, J.; Park, J.S. Fabrication and Characterization of Electrospun Curcumin-Loaded Polycaprolactone-Polyethylene Glycol Nanofibers for Enhanced Wound Healing. Macromol. Res. 2014, 22, 1288–1296. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Sharif Zak, M.; Majdi, H.; Mostafavi, E.; Barati, M.; Lotfimehr, H.; Ghaseminasab, K.; Pazoki-Toroudi, H.; Webster, T.J.; Akbarzadeh, A. The Effect of Chrysin–Curcumin-Loaded Nanofibres on the Wound-Healing Process in Male Rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1642–1652. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and Characterization of Curcumin Loaded PLA—Hyperbranched Polyglycerol Electrospun Blend for Wound Dressing Applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Ganesh, M.; Senthil, V.; Ramesh, Y.V.; Jakki, S.L.; Choi, E.Y. Tetrahydro Curcumin Loaded PCL-PEG Electrospun Transdermal Nanofiber Patch: Preparation, Characterization, and in Vitro Diffusion Evaluations. J. Drug Deliv. Sci. Technol. 2018, 44, 342–348. [Google Scholar] [CrossRef]
- Shababdoust, A.; Zandi, M.; Ehsani, M.; Shokrollahi, P.; Foudazi, R. Controlled Curcumin Release from Nanofibers Based on Amphiphilic-Block Segmented Polyurethanes. Int. J. Pharm. 2020, 575, 118947. [Google Scholar] [CrossRef]
- Fu, S.Z.; Meng, X.H.; Fan, J.; Yang, L.L.; Wen, Q.L.; Ye, S.J.; Lin, S.; Wang, B.Q.; Chen, L.L.; Wu, J.B.; et al. Acceleration of Dermal Wound Healing by Using Electrospun Curcumin-Loaded Poly(ε-Caprolactone)-Poly(Ethylene Glycol)-Poly(ε-Caprolactone) Fibrous Mats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 533–542. [Google Scholar] [CrossRef]
- Lian, Y.; Zhan, J.C.; Zhang, K.H.; Mo, X.M. Fabrication and Characterization of Curcumin-Loaded Silk Fibroin/P(LLA-CL) Nanofibrous Scaffold. Front. Mater. Sci. 2014, 8, 354–362. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun Curcumin-Loaded Cellulose Acetate/Polyvinylpyrrolidone Fibrous Materials with Complex Architecture and Antibacterial Activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-Dissolving Antioxidant Curcumin/Cyclodextrin Inclusion Complex Electrospun Nanofibrous Webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef]
- Saeed, S.M.; Mirzadeh, H.; Zandi, M.; Barzin, J. Designing and Fabrication of Curcumin Loaded PCL/PVA Multi-Layer Nanofibrous Electrospun Structures as Active Wound Dressing. Prog. Biomater. 2017, 6, 39–48. [Google Scholar] [CrossRef]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The Biomedical Potential of Cellulose Acetate/Polyurethane Nanofibrous Mats Containing Reduced Graphene Oxide/Silver Nanocomposites and Curcumin: Antimicrobial Performance and Cutaneous Wound Healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The Use of Electrospun Curcumin-Loaded Poly(L-Lactic Acid) Fiber Mats as Wound Dressing Materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled Release of Curcumin from Electrospun Fiber Mats with Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun Cellulose Acetate Fiber Mats Containing Curcumin and Release Characteristic of the Herbal Substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, S.; Zhao, Z.; Wu, T.; Wang, R.; Xu, S.; Liu, L.; Xie, R.; Zheng, Z.; Li, G.; et al. Silk Fibroin/Polyethylene Glycol Nanofibrous Membranes Loaded with Curcumin. Therm. Sci. 2017, 21, 1587–1594. [Google Scholar] [CrossRef][Green Version]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (ε-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef]
- Liao, H.T.; Lai, Y.T.; Kuo, C.Y.; Chen, J.P. A Bioactive Multi-Functional Heparin-Grafted Aligned Poly(Lactide-Co-Glycolide)/Curcumin Nanofiber Membrane to Accelerate Diabetic Wound Healing. Mater. Sci. Eng. C 2021, 120, 111689. [Google Scholar] [CrossRef]
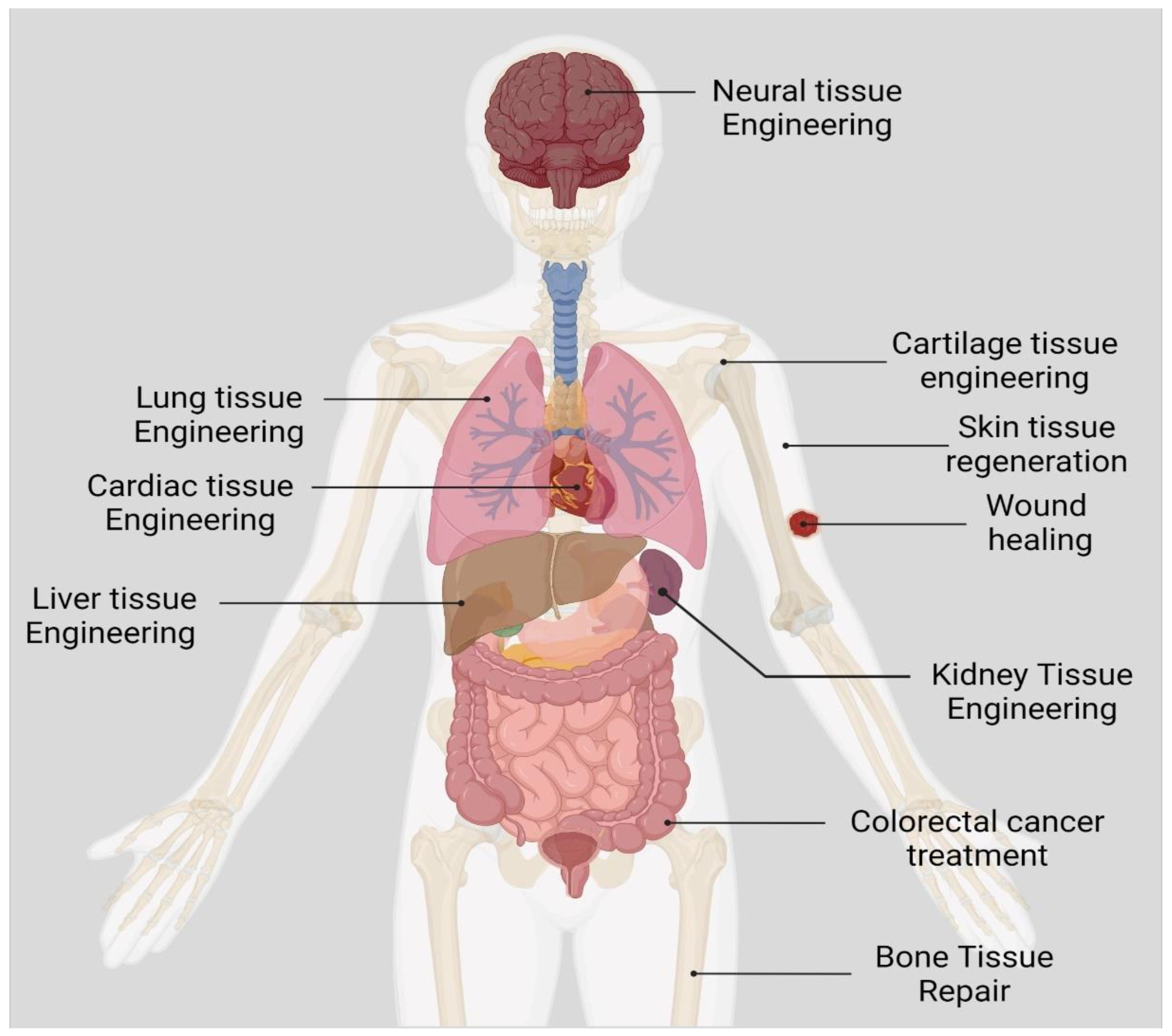
| S. No | Target Disease | Mechanism of Action | Ref |
|---|---|---|---|
| 1 | Liver Diseases | Curcumin down-regulates expression of TGF-β1 to enhance VE-cadherin, DDAH1 and Nrf2 levels, and diminish MMP-9 and ERK1/2 levels. Consequently, TGF-b-mediated EndMT is inhibited to suppress endothelial cell fibrosis | [63] |
| 2 | Skin cancer | Inhibits pAKT, pS6, p-4EBP1, pSTAT3 and pERK1/2 Improved skin penetration, deposition and antimelanoma activity of curcumin | [64] |
| 3 | Osteoarthritis | Decreases Visual Analog Score (VAS), CRP, CD4+ and CD8+ T cells, Th17 cells and B cells frequency | [65] |
| 4 | Multiple sclerosis | Enhancing expression of anti-inflammatory factors such as IL-4, IL-5 and TGF-β is a promising strategy in multiple sclerosis therapy | [66] |
| 5 | Asthma | The inhibitory effect on the expression and level of TGF-β is critical in asthma therapy. | [67] |
| 6 | Vulvovaginal candidiasis | By lowering the level of IL-1β (a pro-inflammatory factor) in comparison to TGF-β (an anti-inflammatory factor), Vulvovaginal candidiasis improves, paving the way for effective treatment of this infection. | [68] |
| 7 | Diabetic cardiomyopathy | Curcumin down-regulates the expression of TGF-β1 via inhibition of JAK/STAT signaling pathway, leading to reducing inflammation and improving diabetic cardiomyopathy. | [69] |
| 8 | Psoriasis | Inhibits phosphorylase kinase activity and decreases the epidermal CD8+ T-cell density resulting in reduced autoimmune-mediated cell damage and resolution of psoriasis | [70] |
| 9 | Scleroderma | Inhibits the TGF-β-mediated phosphorylation of smad2 by upregulation of TGF-β-induced factor (TGIF) which is a negative regulator of TGF-β signalling | [71] |
| 10 | Antihypertensive | Inhibits ACE thereby preventing overexpression of RAAS, curcumin scavenges superoxide anion (O2-) generated under the diabetic conditions, thereby preventing its reaction with potent vasodilator nitric oxide (NO) to form the much more powerful oxidant peroxynitrite (ONOO-); curcumin prevents cadmium-mediated inhibition of catechol-O-methyltransferase by its chelating effect which decreases adrenaline and noradrenaline level. | [72] |
| 11 | Antidiabetic | Decreases hepatic glucose level, increases glucose uptake by upregulating GLUT2, GLUT3 and GLUT4 gene expressions, enhancing secretion of insulin from pancreatic cells, decreases insulin resistance. | [73] |
| 12 | Diabetic foot ulcer | Inhibits the growth of bacteria that are associated with the onset of foot infections in patients with diabetes | [74] |
| 14 | Alzheimer | Improves memory due to its antioxidant effect which decreases degradation of neurons, beta-amyloid plaques and microglia formation | [75] |
| 15 | Ulcerative colitis | Decreases TNF-α, IL-6 | [76] |
| S. No | Polymers | Advantages |
|---|---|---|
| 1 | PCL |
|
| 2 | PLA |
|
| 3 | Cellulose Acetate |
|
| 4 | PEG |
|
| 5 | PHBV |
|
| 6 | Polyurethane |
|
| 7 | PVP |
|
| 8 | PVA |
|
| 9 | SF |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamilarasi, G.P.; Krishnan, M.; Sabarees, G.; Gouthaman, S.; Alagarsamy, V.; Solomon, V.R. Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing. Appl. Nano 2022, 3, 202-232. https://doi.org/10.3390/applnano3040015
Tamilarasi GP, Krishnan M, Sabarees G, Gouthaman S, Alagarsamy V, Solomon VR. Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing. Applied Nano. 2022; 3(4):202-232. https://doi.org/10.3390/applnano3040015
Chicago/Turabian StyleTamilarasi, Ganesan Padmini, Manikandan Krishnan, Govindaraj Sabarees, Siddan Gouthaman, Veerachamy Alagarsamy, and Viswas Raja Solomon. 2022. "Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing" Applied Nano 3, no. 4: 202-232. https://doi.org/10.3390/applnano3040015
APA StyleTamilarasi, G. P., Krishnan, M., Sabarees, G., Gouthaman, S., Alagarsamy, V., & Solomon, V. R. (2022). Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing. Applied Nano, 3(4), 202-232. https://doi.org/10.3390/applnano3040015