Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil and Formulation Preparation
2.2. Shoot Culture, Inoculation and Treatments
2.3. X. euvesicatoria Quantification
2.4. Reactive Oxygen Species’ Content
2.4.1. Hydrogen Peroxide (H2O2) Quantification
2.4.2. Superoxide Radical (O2·−) Quantification
2.5. Lipid Peroxidation Assessment by Quantification of MDA
2.6. Gene Expression Analysis
2.7. Hormone Extraction and Quantification
2.8. Statistical Analysis
3. Results
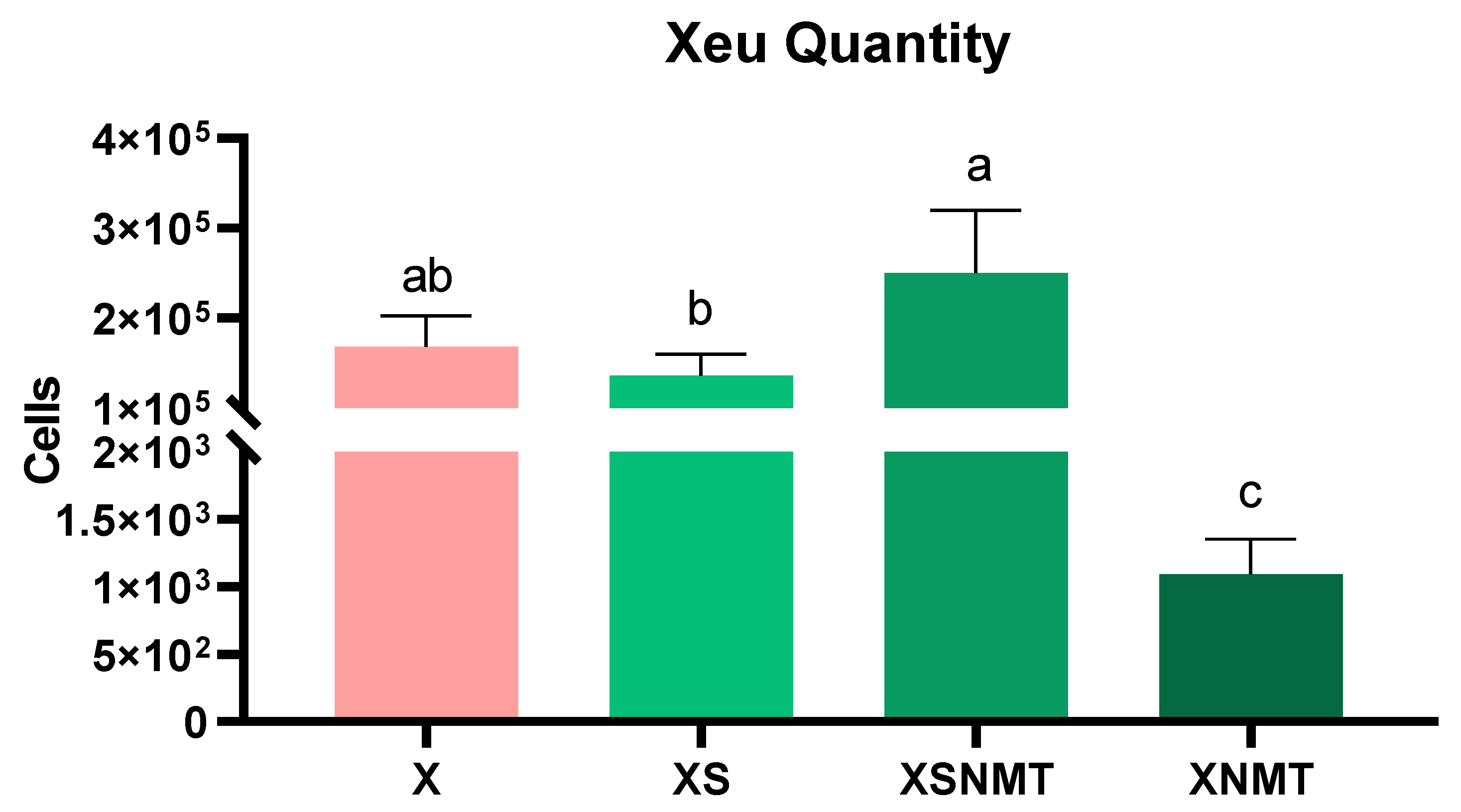
3.1. X. euvesicatoria Quantification
3.2. Reactive Oxygen Species’ Content
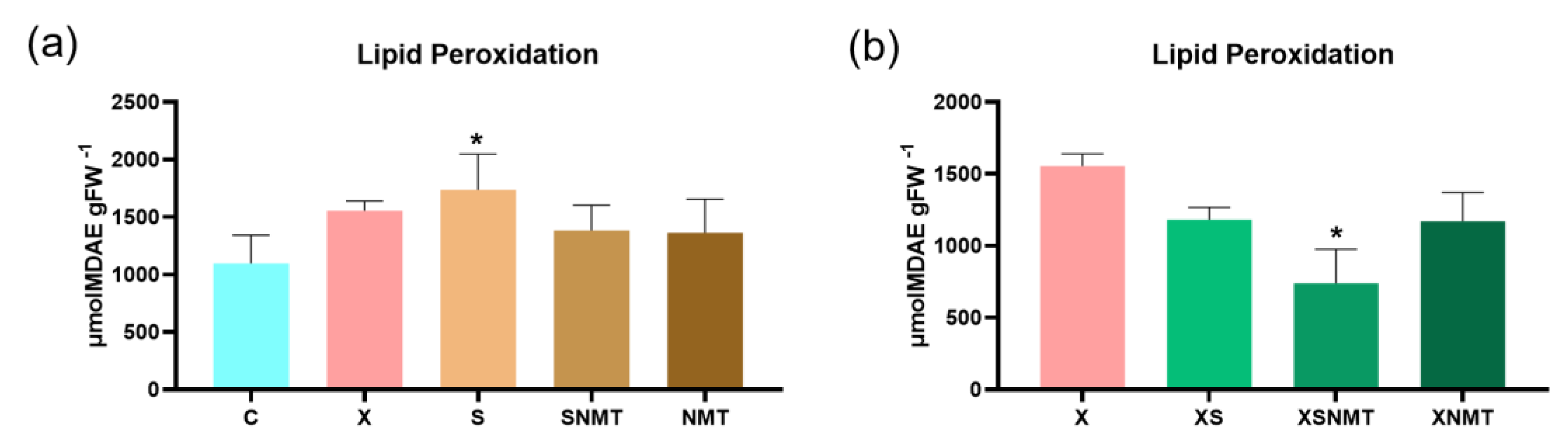
3.3. Lipid Peroxidation Assessment by Quantification of MDA
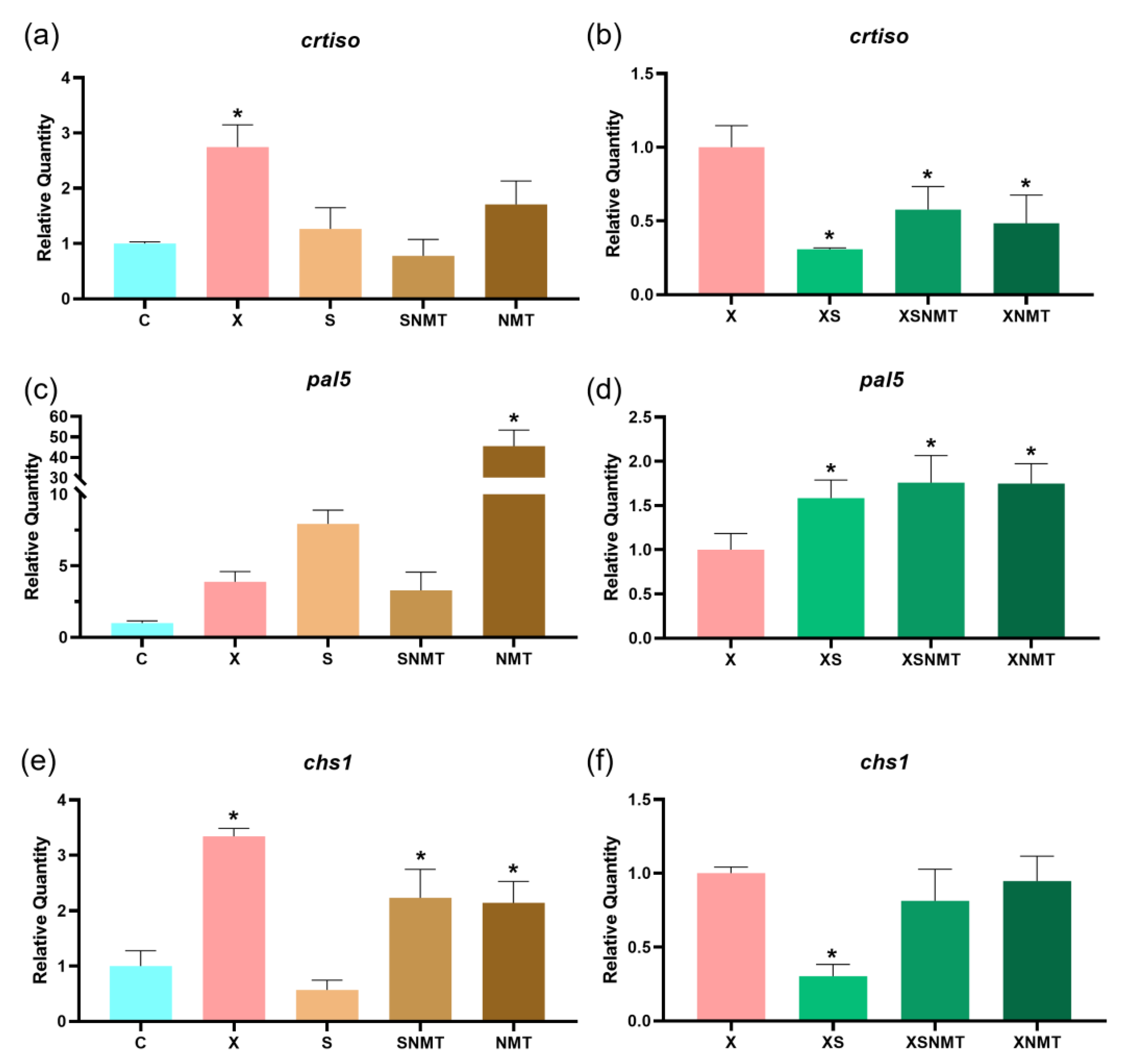
3.4. Gene Expression Analysis
3.4.1. Phenylpropanoid Pathway
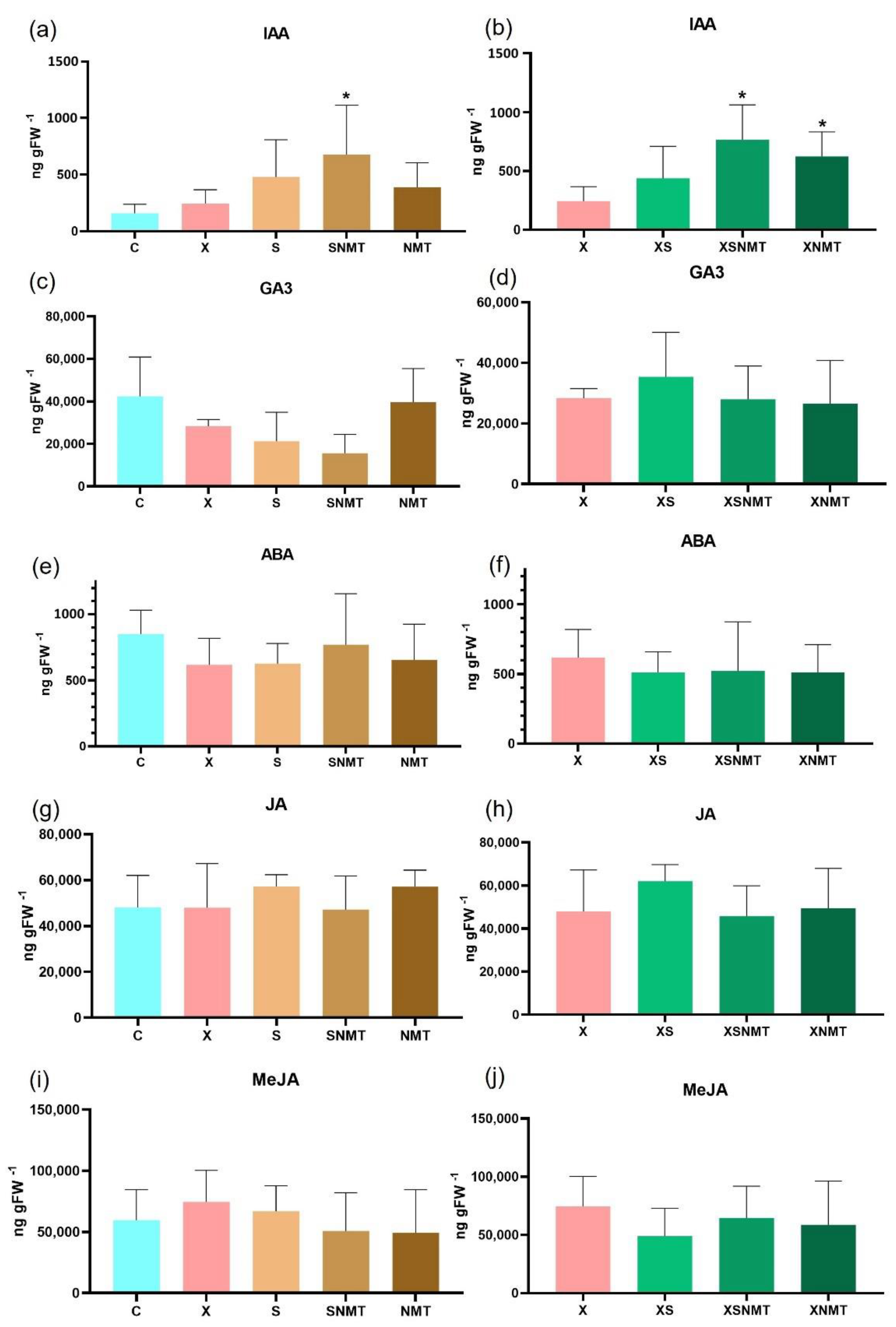
3.4.2. Hormone Pathways
3.5. Hormone Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y.Y.; Strayer-Scherer, A.L.; White, J.; Mukherjee, A.; De La Torre-Roche, R.; Ritchie, L.; Colee, J.; Vallad, G.E.; Freeman, J.H.; Jones, J.B. Nano-Magnesium oxide: A novel bactericide against copper-tolerant Xanthomonas perforans causing tomato bacterial spot. Phytopathology 2019, 109, 52–62. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Huang, Y.; Carvalho, R.; Choudhary, M.; Da Silva, S.; Colee, J.; Huerta, A.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Magnesium Oxide Nanomaterial, an Alternative for Commercial Copper Bactericides: Field-Scale Tomato Bacterial Spot Disease Management and Total and Bioavailable Metal Accumulation in Soil. Environ. Sci. Technol. 2021, 55, 13561–13570. [Google Scholar] [CrossRef]
- Qiao, K.; Liu, Q.; Huang, Y.; Xia, Y.; Zhang, S. Management of bacterial spot of tomato caused by copper-resistant Xanthomonas perforans using a small molecule compound carvacrol. Crop Prot. 2020, 132, 105114. [Google Scholar] [CrossRef]
- Smith, S.L.; Campos, M.G.; Ozcan, A.; Mendis, H.C.; Young, M.; Myers, M.E.; Atilola, M.; Doomra, M.; Thwin, Z.; Johnson, E.G.; et al. Multifunctional Surface, Subsurface, and Systemic Therapeutic (MS3T) Formulation for the Control of Citrus Canker. J. Agric. Food Chem. 2021, 69, 10807–10818. [Google Scholar] [CrossRef]
- El Miz, M.; Salhi, S.; Chraibi, I.; El Bachiri, A.; Fauconnier, M.L.; Tahani, A. Characterization and adsorption study of thymol on pillared bentonite. Open J. Phys. Chem. 2014, 4, 98–116. [Google Scholar] [CrossRef]
- De Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with essential oils as antimicrobial agents, packaging, repellents, and insecticides: An overview. Colloids Surf. B 2022, 209, 112186. [Google Scholar] [CrossRef]
- Yendluri, R. Nanoclays: A new avenue for drug delivery. EC Pharmacol. Toxicol. ECO 2019, 2, 20–22. [Google Scholar]
- Rawat, K.; Agarwal, S.; Tyagi, A.; Verma, A.; Bohidar, H.B. Aspect ratio dependent cytotoxicity and antimicrobial properties of nanoclay. Appl. Biochem. Biotechnol. 2014, 174, 936–944. [Google Scholar] [CrossRef]
- Giannakas, A. Na-Montmorillonite vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Loukidi, A.; Chatzimichailidou, S.; Salmas, C.E.; Giannakas, A.E.; Achilias, D.S. Effect of Na-and Organo-Modified Montmorillonite/Essential Oil Nanohybrids on the Kinetics of the In Situ Radical Polymerization of Styrene. Nanomaterials 2021, 11, 474. [Google Scholar] [CrossRef]
- Merino, D.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Preparation, characterization, and in vitro testing of nanoclay antimicrobial activities and elicitor capacity. J. Agric. Food Chem. 2018, 66, 3101–3109. [Google Scholar] [CrossRef]
- Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Torres, A.; Tavares, F.; Fernandes-Ferreira, M.; Sousa, R.M.; Santos, C. Satureja montana L. essential oil, montmorillonite and nanoformulation reduce Xanthomonas euvesicatoria infection, modulating redox and hormonal pathways of tomato plants. Sci. Hortic. 2022, 295, 110861. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research, 1st ed.; Malik, S., Ed.; Springer: Berlin, Germany, 2019; pp. 3–17. [Google Scholar]
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils, 1st ed.; Baser, K.S.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 41–83. [Google Scholar]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 49. [Google Scholar] [CrossRef]
- Ozogul, Y.; Kuley, E.; Ucar, Y.; Ozogul, F. Antimicrobial impacts of essential oils on food borne-pathogens. Recent Pat. Food Nutr. Agric. 2015, 7, 53–61. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Dadasoglu, F.; Aydin, T.; Cakmakci, R.; Ozer, H.; Kordali, S.; Mete, E.; Dikbas, N. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J. Sci. Food Agric. 2010, 90, 145–160. [Google Scholar] [CrossRef]
- Kotan, R.; Dadasoğlu, F.; Karagoz, K.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dikbas, N. Antibacterial activity of the essential oil and extracts of Satureja hortensis against plant pathogenic bacteria and their potential use as seed disinfectants. Sci. Hortic. 2013, 153, 34–41. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Goncalves, S.; Romano, A. Cyclodextrins enhance the antioxidant activity of essential oil. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Li, C.; Chen, X.; Lin, L. Antibacterial efficacy of Satureja montana L. essential oil encapsulated in methyl-β-cyclodextrin/soy soluble polysaccharide hydrogel and its assessment as meat preservative. LWT 2021, 152, 112427. [Google Scholar] [CrossRef]
- Cadena, M.B.; Preston, G.M.; Van der Hoorn, R.A.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodriguéz-González, J.A.; et al. Controlled release of essential oils using laminar nanoclay and porous halloysite/essential oil composites in a multilayer film reservoir. Microporous Mesoporous Mat. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Catara, V.; Cubero, J.; Pothier, J.F.; Bosis, E.; Bragard, C.; Đermić, E.; Holeva, M.C.; Jacques, M.A.; Petter, F.; Pruvost, O.; et al. Trends in molecular diagnosis and diversity studies for phytosanitary regulated Xanthomonas. Microorganisms 2021, 9, 862. [Google Scholar] [CrossRef]
- Horvath, D.M.; Stall, R.E.; Jones, J.B.; Pauly, M.H.; Vallad, G.E.; Dahlbeck, D.; Staskawicz, B.J.; Scott, J.W. Transgenic resistance confers effective field level control of bacterial spot disease in tomato. PLoS ONE 2012, 7, e42036. [Google Scholar] [CrossRef]
- Stall, R.E.; Jones, J.B.; Minsavage, G.V. Durability of resistance in tomato and pepper to xanthomonads causing bacterial spot. Annu. Rev. Phytopathol. 2009, 47, 265–284. [Google Scholar] [CrossRef]
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015, 16, 907–920. [Google Scholar] [CrossRef]
- Jones, J.B.; Zitter, T.A.; Momol, T.M.; Miller, S.A. Compendium of Tomato Diseases and Pests, American Phytopathological Society; APS Press: St. Paul, MN, USA, 2014. [Google Scholar]
- Egel, D.S.; Jones, J.B.; Minsavage, G.V.; Creswell, T.; Ruhl, G.; Maynard, E.; Marchino, C. Distribution and characterization of Xanthomonas strains causing bacterial spot of tomato in Indiana. Plant Health Prog. 2018, 19, 319–321. [Google Scholar] [CrossRef]
- Rojas, M.; Peña, M.; Peña-Vera, M.J.; Sulbaran, M.; Perez, E.; Velasquez, C.L. Characterization and determination of antimicrobial and metal resistant profiles of Xanthomonas strains isolated from natural environments. J. Anal. Pharm. Res. 2019, 8, 55–60. [Google Scholar] [CrossRef][Green Version]
- Moretti, C.; Amatulli, M.T.; Buonaurio, R. PCR-based assay for the detection of Xanthomonas euvesicatoria causing pepper and tomato bacterial spot. Lett. Appl. Microbiol. 2009, 49, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Cell Rep. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Gajewska, E.; Sklodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. BioMetals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Costa-Santos, M.; Mariz-Ponte, N.; Dias, M.C.; Moura, L.; Marques, G.; Santos, C. Effect of Bacillus spp. and Brevibacillus sp. on the Photosynthesis and Redox Status of Solanum lycopersicum. Horticulturae 2021, 7, 24. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef]
- Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Sousa, R.M.O.F.; Torres, A.; Tavares, F.; Ribeiro, A.; Cavaco-Paulo, A.; Fernandes-Ferreira, M.; Santos, C. Satureja montana Essential Oil, Zein Nanoparticles and Their Combination as a Biocontrol Strategy to Reduce Bacterial Spot Disease on Tomato Plants. Horticulturae 2021, 7, 584. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; De Oliveira, J.F.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Suzuki, T.; Takebayashi, Y.; Ishiguro, R.; Higashiyama, T.; Sakakibara, H.; Ishiguro, S. Jasmonic acid facilitates flower opening and floral organ development through the upregulated expression of SlMYB21 transcription factor in tomato. Biosci. Biotechnol. Biochem. 2018, 82, 292–303. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, L.; Zhang, L.; Lv, H.; He, Q.; Guo, L.; Zhang, X.; He, H.; Ren, S.; Zhang, N.; et al. Melatonin promotes carotenoid biosynthesis in an ethylene-dependent manner in tomato fruits. Plant Sci. 2020, 298, 110580. [Google Scholar] [CrossRef] [PubMed]
- Van Meulebroek, L.; Bussche, J.V.; Steppe, K.; Vanhaecke, L. Ultra-high performance liquid chromatography coupled to high resolution Orbitrap mass spectrometry for metabolomic profiling of the endogenous phytohormonal status of the tomato plant. J. Chromatogr. A 2012, 1260, 67–80. [Google Scholar] [CrossRef]
- ICH. ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2 (R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Lai, Y.R.; Lin, C.H.; Chang, C.P.; Ni, H.F.; Tsai, W.S.; Huang, C.J. Distribution of copper resistance gene variants of Xanthomonas citri subsp. citri and Xanthomonas euvesicatoria pv. perforans. Plant Prot. Sci. 2021, 57, 206–216. [Google Scholar] [CrossRef]
- Wu, Y.W.; Sun, S.Q.; Zhou, Q.; Tao, J.X.; Noda, I. Volatility-dependent 2D IR correlation analysis of traditional Chinese medicine ‘Red Flower Oil’ preparation from different manufacturers. J. Mol. Struct. 2008, 882, 107–115. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, S. Preparation of active antimicrobial methyl cellulose/carvacrol/montmorillonite nanocomposite films and investigation of carvacrol release. LWT 2011, 44, 465–472. [Google Scholar] [CrossRef]
- Roy, A.; Joshi, M.; Butola, B.S. Antimicrobial performance of polyethylene nanocomposite monofilaments reinforced with metal nanoparticles decorated montmorillonite. Colloids Surf. B. 2019, 178, 87–93. [Google Scholar] [CrossRef]
- He, Y.; Fei, X.; Li, H. Carboxymethyl cellulose-based nanocomposites reinforced with montmorillonite and ε-poly-l-lysine for antimicrobial active food packaging. J. Appl. Polym. Sci. 2020, 137, 48782. [Google Scholar] [CrossRef]
- Hong, S.I.; Rhim, J.W. Antimicrobial activity of organically modified nanoclays. J. Nanosci. Nanotechnol. 2008, 8, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.G.; Dos Santos, N.M.A.; Torin, R.F.S.; Rosa, D.S. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical composition and cytotoxic and antioxidant activities of Satureja montana L. essential oil and its antibacterial potential against Salmonella spp. strains. J. Chem. 2013, 2013, 275698. [Google Scholar] [CrossRef]
- Lucas, G.C.; Alves, E.; Pereira, R.B.; Perina, F.J.; Souza, R.M.D. Antibacterial activity of essential oils on Xanthomonas vesicatoria and control of bacterial spot in tomato. Pesqui. Agropecu. Bras. 2012, 47, 351–359. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Yang, J.; Yang, W. Comparative transcriptome analysis of resistant and susceptible tomato lines in response to infection by Xanthomonas perforans race T3. Front. Plant Sci. 2015, 6, 1173. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van Loon, L.C. Salicylic acid-independent plant defence pathways. Trends Plant Sci. 1999, 4, 52–58. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Long, Q.; Xie, Y.; He, Y.; Li, Q.; Zou, X.; Chen, S. Abscisic acid promotes jasmonic acid accumulation and plays a key role in citrus canker development. Front. Plant Sci. 2019, 10, 1634. [Google Scholar] [CrossRef]
- Ecker, J.R.; Davis, R.W. Plant defense genes are regulated by ethylene. Proc. Natl. Acad. Sci. USA 1987, 84, 5202–5206. [Google Scholar] [CrossRef]
- Norman-Setterblad, C.; Vidal, S.; Palva, E.T. Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol. Plant Microbe Interact. 2000, 13, 430–438. [Google Scholar] [CrossRef]
- Guan, R.; Su, J.; Meng, X.; Li, S.; Liu, Y.; Xu, J.; Zhang, S. Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae. Plant Physiol. 2015, 169, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Cho, Y.G.; Park, M.Y.; Lee, M.C.; Eun, M.Y.; Kang, B.G.; Kim, W.T. Hormonal cross-talk between auxin and ethylene differentially regulates the expression of two members of the 1-aminocyclopropane-1-carboxylate oxidase gene family in rice (Oryza sativa L.). Plant Cell Physiol. 2000, 41, 354–362. [Google Scholar] [CrossRef] [PubMed]
- European Community. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]


| Gene | Primer | Sequence | Annealing Temperature (°C) | Product Size (bp) | Reference |
|---|---|---|---|---|---|
| ubi | Forward | GGACGGACGTACTCTAGCTGAT | 60 | 134 | [42] |
| Reverse | AGCTTTCGACCTCAAGGGTA | ||||
| tub | Forward | AACCTCCATTCAGGAGATGTTT | 60 | 180 | [42] |
| Reverse | TCTGCTGTAGCATCCTGGTATT | ||||
| crtiso | Forward | GTTTGTAATCTTGGGTTTCCAGCA | 60 | 117 | [43] |
| Reverse | TTGCCTTGTGGGTTTCAAGC | ||||
| pal5 | Forward | TGGAGGAGAATTTGAAGAATGCTG | 60 | 136 | [43] |
| Reverse | TCCCTTTCCACCACTTGTAGC | ||||
| chs1 | Forward | ACCAACAAGGTTGCTTTGCC | 60 | 135 | [44] |
| Reverse | GAGATTCACTGGGTCCACGG | ||||
| aao | Forward | CCAGGCACAAACACAATCAA | 60 | 154 | [45] |
| Reverse | GTCGTAAATAATATCAGACTG | ||||
| opr3 | Forward | ATGGACTCTAATCCACTCAGCCTTG | 60 | 152 | [46] |
| Reverse | TCACTGCCAAGTCTGCCTGCTTCAG | ||||
| aco3 | Forward | GAAGGCTATGGAAGCTAATGTGG | 60 | 104 | [47] |
| Reverse | ATGTGTTTAATTAGCTACGTTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Gil, R.L.; Cunha, E.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Fernandes-Ferreira, M.; Sousa, R.M.O.F.; Santos, C. Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum. Appl. Nano 2022, 3, 126-142. https://doi.org/10.3390/applnano3030009
Oliveira-Pinto PR, Mariz-Ponte N, Gil RL, Cunha E, Amorim CG, Montenegro MCBSM, Fernandes-Ferreira M, Sousa RMOF, Santos C. Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum. Applied Nano. 2022; 3(3):126-142. https://doi.org/10.3390/applnano3030009
Chicago/Turabian StyleOliveira-Pinto, Paulo R., Nuno Mariz-Ponte, Renato L. Gil, Edite Cunha, Célia G. Amorim, Maria C. B. S. M. Montenegro, Manuel Fernandes-Ferreira, Rose M. O. F. Sousa, and Conceição Santos. 2022. "Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum" Applied Nano 3, no. 3: 126-142. https://doi.org/10.3390/applnano3030009
APA StyleOliveira-Pinto, P. R., Mariz-Ponte, N., Gil, R. L., Cunha, E., Amorim, C. G., Montenegro, M. C. B. S. M., Fernandes-Ferreira, M., Sousa, R. M. O. F., & Santos, C. (2022). Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum. Applied Nano, 3(3), 126-142. https://doi.org/10.3390/applnano3030009











