Abstract
Applied nanotechnology has experienced tremendous advance over the last decade. In this study, the efficient synthesis of highly stable palladium-nanoparticles (PdNPs) biohybrids based on the application of an enzyme, which induces in situ the generation of spherical nanoparticles on the protein network, has been described. A heterogeneous material was synthesized formed with PdNPs with average sizes between 1.5 to 5 nm. These Pd nanocatalysts were successfully applied in different chemical processes: C-C bonding reactions (Suzuki and Heck reactions) and cascade processes combining enzymatic and metallic activities (hydrolysis-reduction, esterification-racemization).
1. Introduction
Palladium-nanoparticles (PdNPs) biohybrids have shown an important role in a widespread variety of chemical reactions [1,2,3,4]. The intrinsic properties of nanoparticles—with an extremely large surface-to-volume ratio—provided advantages as catalysts compared to bulk materials.
However, typical strategies for the transition metal nanoparticle syntheses require severe conditions, such as high T, pressure, flammable solvents, etc. [5]. Therefore, recently more simple, efficient, and sustainable synthetic strategies have been developed [6,7,8,9,10,11].
In this way, the use of biological agents as plants, microorganisms, or fungi have allowed synthesizing Pd nanoparticles by a green way. However, important parameters such as the localization or morphology of the nanoparticles are subject to the microorganism species, since mixtures of enzymes and proteins can be involved in the process, producing nanoparticles of dissimilar sizes [12].
Therefore, the most suitable scheme would be the use of proteins or enzymes directly [13,14,15,16]. Protein scaffolds could offer splendid metal coordination environments that encourage biomineralization procedures, allowing the control of dimension, particle growing, and avoiding nanoparticles aggregation [17,18,19].
Furthermore, using an enzyme, it could be possible to conjugate the activity of the metal nanoparticles with the enzymatic activity in a one-pot catalyst, being an additional advantage compared with other methods.
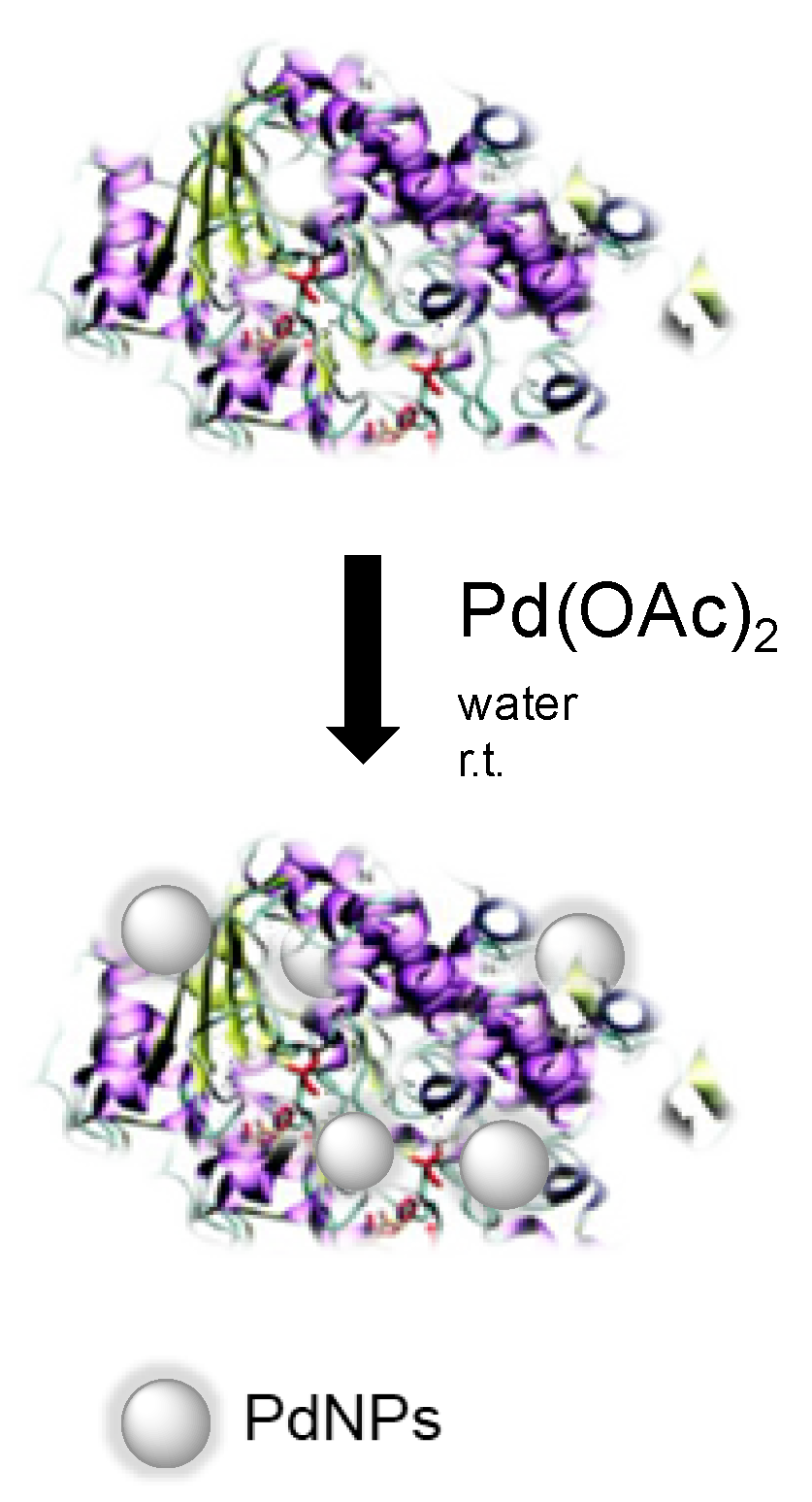
Here, we describe the synthesis of high stable heterogeneous Pd nanocatalysts (enzyme/PdNPs biohybrids), where NPs were created in situ from an aqueous solution containing an enzyme and a noble metal salt (Figure 1) [20]. Different enzymes, such as scaffolds, were tested evaluating their role in the metal reduction, stabilization of nanoparticles, and remaining biocatalytic activity.
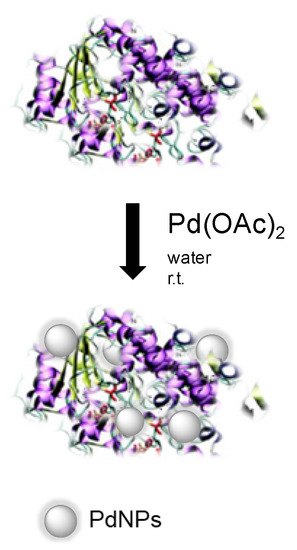
Figure 1.
Palladium-nanoparticles biohybrid synthesis.
The enzyme operated at once as a reducing, stabilizing, and supporting mediator as well as a bio-catalyst. These novel biohybrids were effectively used as catalysts in several chemical reactions, C-C bonding reaction, reductions, cascade processes in an aqueous or organic solvent as the reaction media, demonstrating their versatility as heterogeneous catalysts.
2. Materials and Methods
2.1. Materials
Candida antartica B lipase Lipozyme® CALB (CAL-B), Thermomyces lanuginose (TLL) Lipozyme® TL 100 L, and Rhizomucor miehei lipase Palatase® 20,000 L (Palatase) solutions were purchased from Novozymes (Bagsværd, Denmark). 4-Methoxybenzenediazonium tetrafluoroborate (MBDFB), p-nitrophenylpropionate (pNPP), p-nitrophenol (pNP), p-aminophenol (pAP), bromobenzene, iodobenzene, chlorobenzene, phenylboronic acid, benzene, 4-metoxianisol, tetrabutylammonium fluoride (TBAF), tetrabutylammonium chloride (TBACl), tetrabutylammonium bromide (TBABr), ethyl acrylate, and palladium acetate were purchased from Sigma-Aldrich (now Merk, Darmstadt, Germany). N-Acetyl-L-tryptophan methyl ester (TrpOMe) was bought from Alfa Aesar (Shanghai, China).
2.2. Characterization Techniques Used
The TGA was performed with the EXSTAR 6300 (Seiko, Tokyo, Japan) equipment in a range of temperatures: RT/1000 °C. FTIR spectra were recorded on the FT-IR 20SXC (Nicolet) spectrophotometer.
The X-ray diffraction (XRD) pattern was acquired using a Texture Analysis Diffractometer D8 Advance (Bruker) with Cu Kα radiation. The transmission electron microscopy (TEM) analysis was achieved on a JEOL 2100F microscope (Oxford Instruments, Oxford, UK). Inductively coupled plasma atomic emission spectrometry (ICP-OES) was performed on a Perkin Elmer OPTIMA 2100 DV equipment to determine the amount of Pd on the biohybrid. To recuperate the biohybrids, a Biocen 22 R (Orto-Alresa, Spain) refrigerated centrifuge was employed. The HPLC spectrum P100 (Thermo Separation products) was used. The analyses were performed at 25 °C using an L-7300 column oven and a UV6000LP detector.
2.3. Synthesis of PdNPs Biohybrids
Pd(OAc)2 (50 mg) were dissolved in 10 mL of solvent (DMF, MeOH, or acetonitrile (ACN)). This solution was added to 40 mL of a distilled water solution containing 16 mg of Cal-B protein (1.6 mL of commercial Lipozyme® CALB solution (10 mg protein·mL−1), 0.7 mL of Lipozyme® TL 100L (24 mg·mL−1), or 2.7 mL of Palatase® 20,000 L (6 mg·mL−1).
The final solution was kept under gentle magnetic stirring for 24 h at room temperature. After that, the resulting suspension was separated by centrifugation (10,000 rpm; 4 °C; 15 min). The recovered pellet was washed once with 10 mL of distilled water containing 20% (v/v) of the corresponding cosolvent and twice with distilled water (2 × 10 mL). Then, the suspension was directly lyophilized to obtain the catalyst in powder form for later use. The solid was characterized by TEM and XRD. The content of Pd in the solid was 25%, determined by ICP-OES.
2.4. Suzuki Reaction
The aryl halide (0.5 mmol) (0.051 mL of chlorobenzene, 0.053 mL of bromobenzene, or 0.059 mL iodobenzene) was added to a 1.5 mL screw-sealed vessel containing phenylboronic acid 5 (0.067 g, 0.55 mmol), NaOH (0.03 g, 0.75 mmol) and, where indicated, the phase transfer catalyst (TBAF: 0.043 g, 0.165 mmol; TBACl: 0.046 g, 0.165 mmol; TBABr: 0.054 g, 0.165 mmol) in distilled water (0.9 mL). The mixture was kept at 50 °C under strong magnetic stirring for 5 min. After that, to initialize the reaction, the PdNPs biohybrids was added. The final suspension was left under vigorous magnetic stirring at 50 °C for the indicated times. The outgoing reaction was examined by the HPLC analysis of the reaction’s samples withdrawn at different times. The analysis conditions were performed with a Kromasil-C4 (150 × 4.6 mm and 5 μmø), at a flow of 1.5 mL/min; λ: 254 nm; and water:ACN (1:1) as the mobile phase: RT of the biphenyl product was 9 min. The yields were acquired extrapolating the values through a calibration curve of biphenyl (R2 = 0.9973).
2.5. Heck Reaction
Iodobenzene (0.274 mmol, 306 µL) and ethyl acrylate (0.55 mmol, 59 µL,) were dissolved in DMF or DMF/distilled water (final volume of 1 mL). One mg of the hybrid (0.25 mg, 2.35 µmol of Pd, 0.0085 mol% of PdNPs) was added. The mixture was pre-warmed at 70 °C below vigorous magnetic stirring for 5 min. Next, to initialize the reaction, triethylamine (57 µL, 0.412 mmol) was added. The final suspension was left under vigorous magnetic stirring at 70 °C for the indicated times. The outgoing reaction was monitored by the HPLC analysis of the reaction’s samples withdrawn at different times. The analysis conditions performed were the same as in Section 2.4. The final product showed a RT of 6 min. The configuration was determined by HPLC using the (E) and (Z)-ethyl cinnamate standards.
2.6. C-H Activation
An amount of 0.192 mmol (50 mg) of TrpOMe or benzene and 0.192 mmol (42 mg) of MBDFB were added to a glass flask containing 5 mL of the solvent. The solution was left under magnetic stirring until homogenization. Then, 2 mg of the PdNPs hybrid were added. The mixture was kept at room temperature for the indicated time. The outgoing reaction was monitored by the HPLC analysis of the reaction’s samples withdrawn at different times. Samples (100 µL) were centrifuged and then 50 µL were diluted in 2 mL of bi-distilled water before the injection. The analysis conditions were achieved with a Kromasil-C8 (150 × 4.6 mm and 5 µmø), at a flow of 1.0 mL/min; λ: 270 nm; and 50% (v/v) ACN in MilliQ water as the mobile phase.
2.7. Enzymatic Hydrolysis of 4-Nitrophenyl Propionate (pNPP)
The enzymatic activity in the biohybrid was evaluated spectrophotometrically measuring the increment in absorbance at 348 nm produced by the release of p-nitrophenol (pNP) (є = 5150 M−1·cm−1) in the hydrolysis of 0.4 mM pNPP in 25 mM sodium phosphate at pH 7 and 25 °C. To start the reaction, 50–200 µL of the lipase solution or suspension were added to 2.5 mL of the substrate solution. The recovered enzymatic activity (%) was obtained comparing the obtained values to the catalytic activity expressed by a 0.054 mg CAL-B/mL water solution.
2.8. Transformation of 4-Nitrophenol (pNP) to 4-Aminophenol (pAP)
P-nitrophenol (pNP) was dissolved in 2 mL of distilled water at a 1 mM concentration. Then, the solid NaBH4 (3.2 mg) was added to the solution. After this addition, the light-yellow solution changes to a strong yellow color, generating the formation of 4-nitrophenolate ions (substrate UV-peak undergoes to an immediate shift from 317 to 400 nm). After 30 s, 3 mg of the different Pd hybrids were added under gentle stirring at room temperature in an orbital shaker. The reaction progress was monitored by taking out an aliquot of the solution (0.1 mL) at different times, diluting it with distilled water (2 mL), and measuring the absorption spectrum between 500 and 300 nm in a quartz cuvette.
2.9. Domino Synthesis of 4-Aminophenol (pAP) from 4-Nitrophenyl Butyrate (pNPP)
A solution of pNPP in ACN (0.1 M; 0.25 mL) was poured on a sodium phosphate buffer pH 7 solution (0.025 M; 2.25 mL) and the mixture was under magnetic stirring at 25 °C until homogenization. Next, the PdNPs-2 biohybrid was added. The reaction was kept on gentle stirring until complete conversion of pNPP to the product pNP (about 1 h). Then, to initialize the Pd-catalyzed reduction of pNP to pAP, the solid NaBH4 (0.001 mol; 0.0378 g) was added straight to the reaction mixture under gentle magnetic stirring at 25 °C. The reaction progress was checked by taking out an aliquot of the solution (20 µL), diluting it with water (2 mL), and measuring the absorption spectrum between 600 and 200 nm in a quartz cuvette.
2.10. Dynamic Kinetic Resolution (DKR) of (±)-1-Phenylethylamine
Ethyl acetate (6 µL, 0.06 mmol) was added to (±)-1-phenylethylamine (1.3 µL, 0.01 mmol) in toluene (1 mL). The mixture was kept at 70 °C under vigorous magnetic stirring for 5 min. After that, to initialize the reaction, 5 mg of PdNPs-2 was added. The final suspension was left under vigorous magnetic stirring at 70 °C for the indicated times. The Pd-catalyzed racemization process was studied using s-phenylethylamine, as the substrate in the conditions described above. The reactions were monitored by the RP-HPLC analysis of the reaction’s samples withdrawn at different times. The analysis conditions were column: Kromasil Ultrabase C18, 250 × 4.6 mm, 5 µmø; f: 1 mL/min; λ: 210 and 254 nm; mobile phase: 30% (v/v) ACN in MilliQ water 0.1% (v/v) trifluoroacetic acid; and temperature: 25 °C. The RT of phenylethylamine was 3.24 min, whereas the RT of phenylacetamide product was 7.0 min.
The enantiomeric excess (ee) value was determined by the chiral HPLC analysis of the reaction’s samples withdrawn at a final time. The analysis conditions were: Phenomenex Lux Cellulose-1 (250 × 4.6 mm, 3 µmø) column, flow of 0.5 mL/min; λ: 210 and 254 nm; mobile phase: 10% (v/v) IPA in n-Hexane; and temperature: 25 °C. The RT of s-enantiomer was 24 min, whereas the Rt of r-enantiomer was 26.5 min. Before their injection, the different samples were diluted with the mobile phase and centrifuged for 10 min at 10 k rpm. Each sample was injected in triplicate experiments.
3. Results
3.1. Synthesis of Pd Nanoparticles Biohybrids Using C. antarctica B (CAL-B) Lipase
The PdNPs biohybrids synthesis was performed in an aqueous medium by using a commercial liquid Candida antarctica B lipase (CAL-B). This enzyme was previously dissolved in distilled water (0.5 mg lipase/mL), and palladium acetate, previously dissolved in DMF (1 mg/mL), was added to this aqueous solution at room temperature and under gentle stirring. The total amount of DMF in the final solution was 20% (v/v). After 30 min, the initial clear solution turned to a slight cloudy suspension which was completely cloudy after 24 h of incubation. A solid was easily obtained after centrifugation which was washed with the same reaction solvent first, then distilled water, and finally frozen in liquid nitrogen and lyophilized, yielding the PdNPs-1 hybrid. No aggregation was observed in the enzyme solution without the Pd salt.
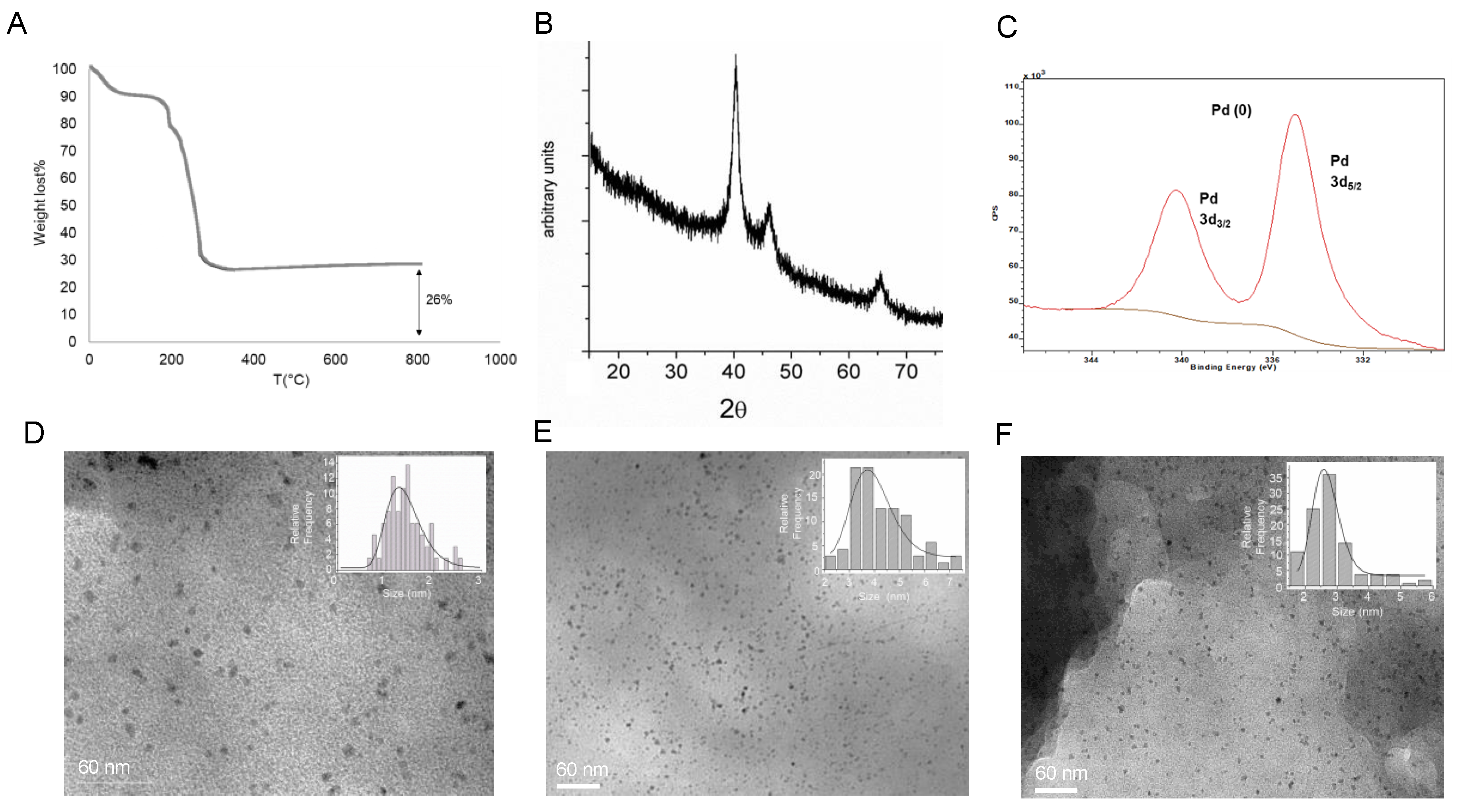
An ICP-OES analysis of the supernatants after complete formation (24 h) revealed that around 7 µmol of the Pd2+ amount was entrapped on the protein, whereas the protein determination by the Bradford method shows that 100% of the enzyme was in the heterogeneous hybrid. The thermo gravimetric analysis (TGA) of the lyophilized powder of PdNPs-1 (Figure 2A) showed that 26% (w/w) of the amount of Pd composed the solid material, confirming the value obtained previously by ICP-AES.
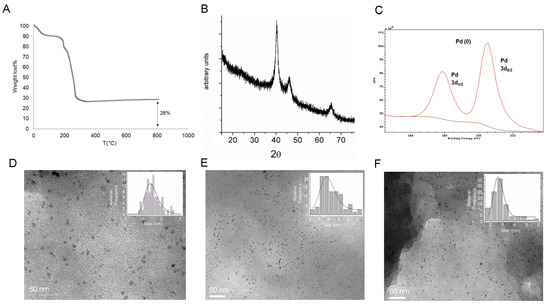
Figure 2.
Characterization of the palladium-nanoparticles (PdNPs) biohybrids synthesized using CAL-B. (A) TGA of the PdNPs-1 biohybrid after 24 h of incubation. (B) XRD analysis. (C) XPS. (D) TEM image of PdNPs-1. (E) TEM image of PdNPs-2, (F) TEM image of PdNPs-3.
The X-ray analysis (XRD and XPS) demonstrated that the Pd(0) was the only metal species in the hybrid (Figure 2B,C). The TEM analysis confirmed the formation of principally small nanoparticles (PdNPs), with a diameter magnitude between 1–2 nm embedded in the protein net (Figure 2D).
To estimate the effect of the solvent in the Pd hybrid synthesis, the protocol was repeated dissolving the Pd salt in methanol or acetonitrile. These hybrids, PdNPs-2 (Figure 2E) and PdNPs-3 (Figure 2F), respectively conserved the bimodal distribution in NPs, although slight larger sizes (2–3, 3–4.5 nm, respectively) were found.
These biohybrids were highly stable, without any changes in the particle size and morphology within 3 months in an aqueous solution or in a solid form at RT (data not shown). This offers an additional sign that the enzyme net acts as a stabilizing agent, as well as a physical support and reducing agent.
3.2. Synthesis of Pd Nanoparticles Biohybrids Using Other Enzymes
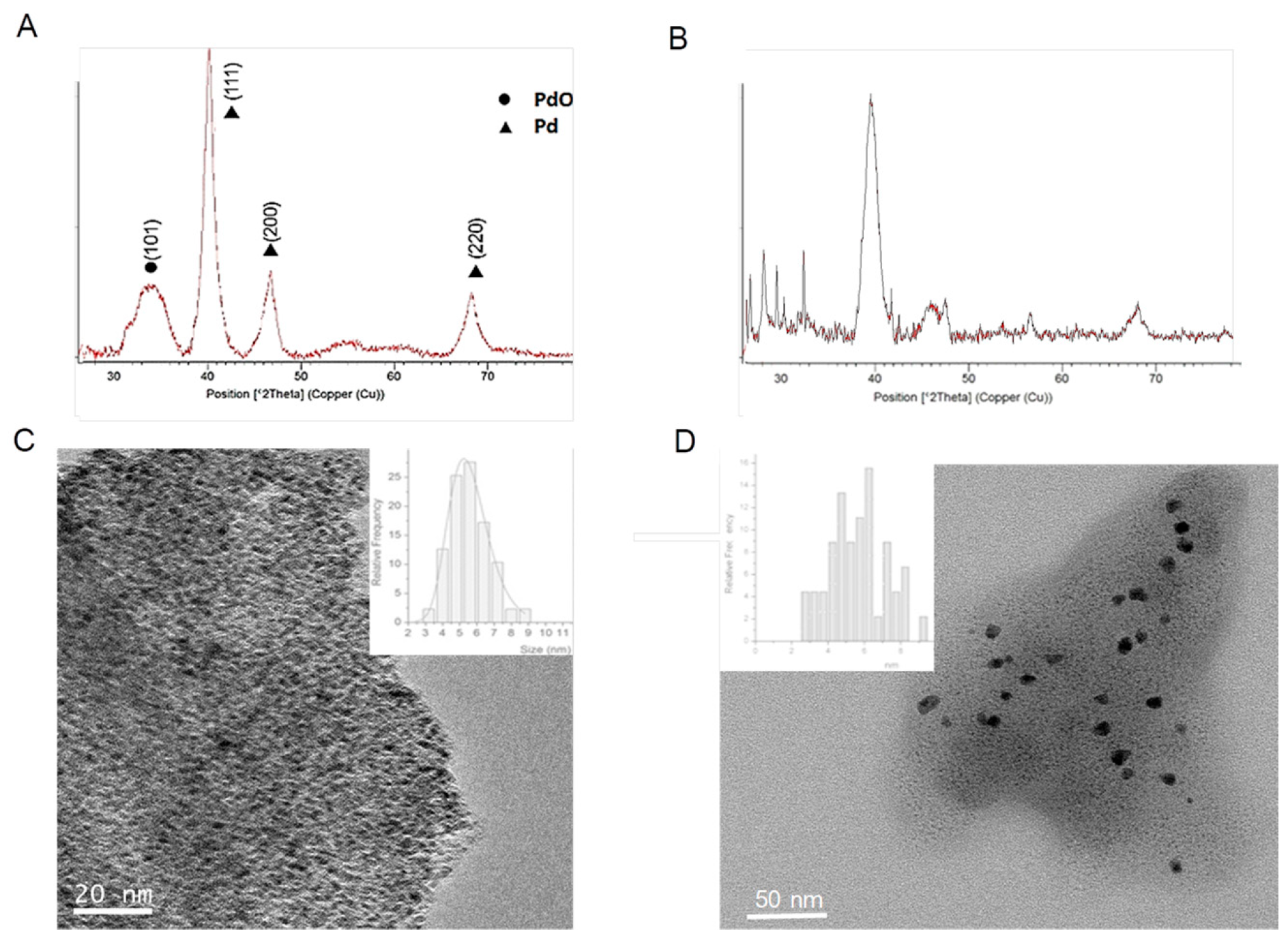
In order to ratify the effect of the enzyme, the synthesis of these PdNPs biohybrids was performed using two different lipases (Figure 3).
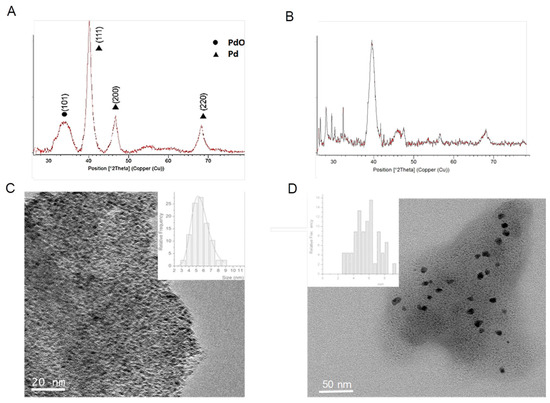
Figure 3.
Characterization of hybrids with TLL and RML lipases. (A) XRD of PdNPs-4. (B) XRD of PdNPs-5. (C) TEM image of PdNPs-4 (inset particle size distribution). (D) TEM image of PdNPs-5 (inset particle size distribution).
CAL-B has a particular characteristic, existing exclusively as a monomer in aqueous media, compared to other lipases, which normally exist mainly as a dimeric form [21].
In this phenomenon, the other lipases with a similar size [21] such as Rhizomucor miehei (Palatase) or Themomyces lanuginosus (TLL) have totally different behaviors.
Therefore, the effect of Pd nanoparticles formation using TLL was first evaluated. The protocol used in the synthesis was based on using methanol as a co-solvent for the synthesis, maintaining the same amount of protein per Pd. As previously with CAL-B, a solid was obtained, so called PdNPs-4. The ICP-OES analysis showed that the amount of Pd in this case was slightly higher (35%). The XRD pattern surprisingly demonstrated that Pd(0) was not the only metal species, containing also PdO in this case (Figure 3A). The TEM analysis revealed that the PdNPs synthesized were 2–4 times larger than using CAL-B (Figure 3B).
When the synthesis was made using Palatase, similar results were found, a hybrid (PdNPs-5) with larger nanoparticle sizes (3–8 nm), in this case only of the metallic Pd.
Therefore, the use of the dimeric protein rather than the monomeric one affected the diameter size of the final Pd nanoparticles synthesized, but also of the Pd species.
3.3. Application of PdNPs Biohybrids in C-C Bonding Formation
3.3.1. Suzuki Cross-Coupling Reaction
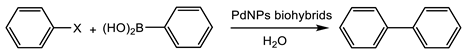
The first application of these PdNPs biohybrids was in a well-known reaction catalyzed by palladium, the Suzuki cross coupling.
The PdNPs-1 hybrid was tested as a catalyst in the Suzuki reaction against different aryl halides with phenylboronic acid at different conditions (Table 1). This catalyst was selected for the reaction, since it showed the smallest Pd nanoparticles.

Table 1.
Suzuki coupling of aryl halides with aryl boronic acid catalyzed by PdNPs biohybrids a.
Initially, chlorobenzene was used as an aryl halide. Additionally, in most cases, with or without the presence of phase transfer catalysts (PTC), a different base, or increasing the equivalent, the biaryl synthesis was barely observed, with the best results obtained using K2CO3 (20% yield at 24 h) (Table 1). A clear enhancement was observed when the halide used was iodine. In this case, a 55% yield of biphenyl was obtained, the value was not improved even using different PTCs or changing the base. Although it has been described that an extremely lower concentration of Pd in the commercial sodium carbonate could induce the biaryl formation in particular conditions [22], no conversion was observed when the reaction was performed without the catalyst or using a free enzyme (Table 1).
When the reaction was performed using bromobenzene, PdNPs-1 catalyzed the reaction producing 50% of biphenyl. However, in this case, the presence of the transfer catalyst improved the catalyst performance, obtaining full conversion in all cases. However, using TBACl, the quantitative yield of biphenyl was obtained in 2.5 h (Table 1). This PdNPs-1 hybrid was quite stable and was used during five reaction cycles without a significant activity loss after recovering (Table 1).
Moreover, in order to demonstrate the applicability for other biohybrids prepared with a different enzyme, the PdNPs-4 was used as a catalyst in the reaction with bromobenzene as aryl halide in an aqueous solution containing 20% acetonitrile and 45 °C. This catalyst produced 45% of biphenyl at these conditions after 24 h (Table 1).
These results represent an excellent example of the efficient catalysts in this C-C bonding reaction, as an alternative at different successful strategies, as recently described in [23,24,25,26].
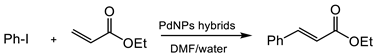
3.3.2. Heck Reaction
A second C-C bonding reaction tested for the applicability of these biohybrids in organic synthesis was the Heck reaction (Table 2). In this case, the hybrids were first tested in a well described condition in the Pd-catalysis, using DMF as a solvent at 120 °C. Pd-biohybrids exhibited an excellent catalytic activity and full conversion of iodobenzene and ethylacrylate in ethyl cinnamate was achieved after 24 h at these conditions (Table 2, entries 1–3). Decreasing the reaction T to 70 °C in DMF resulted in no conversion in any case.

Table 2.
Heck coupling of aryl iodide with ethyl acrylate catalyzed by PdNPs biohybrids a.
However, the presence of a protein embedded in the Pd nanoparticles could confirm a more sustainable property to the hybrids, permitting their application in aqueous media. Therefore, we performed for the first time the Pd-catalyzed Heck reaction by adding water as a co-solvent.
The addition of 25% of water in DMF as the reaction media surprisingly permitted the PdNPs-3 to completely perform the C-C bonding reaction in 18 h at 70 °C (Table 2, entry 5). The addition of more amounts of water resulted in worse results, reducing the catalytic capacity of the PdNPs (20% yield in 24 h). These results were also observed with the other hybrids, PdNPs-1 and PdNPs-2, yielding the product quantitatively after 18 h (Table 2, entries 7–8). No conversion was observed at these conditions when the reaction was performed without the catalyst or when using a free enzyme (data not shown).
These results demonstrate the potential applicability of these biohybrids in this C-C bonding reaction, for example, in the production of more complex molecules, key intermediates in the drug synthesis was similar for other Pd nanocatalysts, as previously described in [27,28,29].
3.3.3. C-H Activation
Considering the excellent results obtained in the previous C-C bonding formation, the direct C-H reaction for arylation was attempted (Table 3). Initially, the experiments were performed by directly using benzene and aryl halide at different conditions using PdNPs-2 as a catalyst. Different conditions were used by changing aryl halide (I or Br), base, solvent, or T from 50 to 120 °C. However, in any case, the synthetic biaryl product was observed (Table 3, entries 1–6). In order to promote the reaction, a more active aryl donor was used, 4-methoxybenzenediazonium tetrafluoroborate (MBDFB). In this case, PdNPs-2 and PdNPs-4 were tested as a heterogeneous catalyst.

Table 3.
C-H arylation reaction catalyzed by PdNPs biohybrids.
However, the reaction between the ionic liquid and 4-MeO-benzene was not produced even at different solvents, being only the metoxybenzene as a unique undesired product.
One interesting application consists of a site-specific modification of amino acids. Recently, some groups demonstrated the metal-catalyzed C-H arylation of the protected tryptophan using this ionic liquid [30,31].
Therefore, the PdNPs-2 hybrid was evaluated as a catalyst in the C-H activation of N-acetyl-L-tryptophan methyl ester (TrpOMe) (Table 3). Initially, the reaction was tested in ethylacetate as a solvent, working well with a full conversion of the C-H product at the C-2 position of the indol moiety in a Trp residue. The reaction was also tested in other water-soluble solvents, and surprisingly the reaction was extremely fast in pure methanol, with a full conversion in 2 h. The efficiency of the catalyst in other solvents such as THF, dioxane, or ethanol was lower (Table 3, entries 14–17). Considering the previous results in the use of water in chemical reactions with this hybrid, the process was tested in MeOH:water 1:1. At these conditions, the Pd hybrid catalyst was as efficient as in the pure solvent (Table 3, entry 17).
Therefore, this example demonstrates again the versatility and sustainability of these PdNPs biohybrids in chemistry and represent an advantage strategy in terms of sustainability of the process comparison with the previous work [30,31,32].
3.4. Application of PdNPs Biohybrids in Cascade Reactions
After an evaluation of the high versatility of Pd chemistry of the nanohybrid, one of the advantages of this system is the conserved intrinsic enzymatic activity on the material. Therefore, these PdNPs biohybrids could be excellent for cascade processes.
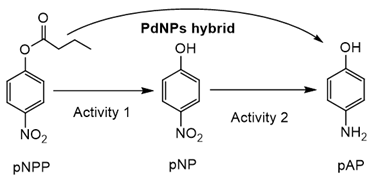
In order to demonstrate that, first a domino reaction in the transformation of p-nitrophenyl ester in p-aminophenol was attempted as a model reaction. The enzymatic hydrolytic activity and Pd reductive activity was evaluated (Table 4).

Table 4.
Dual (hydrolytic/reduction) activity of the PdNPs biohybrids for domino catalysis.
PdNPs-2 showed the highest enzymatic activity (around 47% activity in the hydrolysis of p-nitrophenyl propionate (pNPP compared to the soluble CAL-B) among the Pd nanohybrids. For the catalytic reduction of p-nitrophenol, the hybrid showed that the turnover frequency number (TOF) value (>140 min−1) was very interesting as other Pd catalysts developed for high yield production [33], whereas, for example, using the hybrid with TLL (PdNPs-4), the process was slower, demonstrating the effect of the nanoparticle size on the metal catalytic efficiency. The direct transformation of 10 mM of pNPP to pAP was completely achieved using PdNPs-2 first, the activity in around 1 h with 0.08 mg of the catalyst and just in 1 min by the simple addition of solid NaBH4 excess in the reaction mixture.
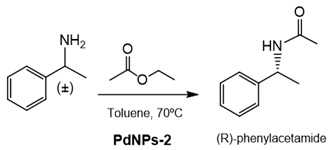
Finally, the capacity of these PdNPs biohybrids was attempted considering the production of enantiomerically pure products by the asymmetric catalysis.
In this case, the tandem catalysis by these hybrids (considering both the enzyme and Pd activity at the same time) was evaluated in the dynamic kinetic resolution (DKR) of rac-phenylethylamine. The soluble CAL-B was first used for studying the enzymatic transesterification, showing a very high enantioselectivity toward the R enantiomer (ee > 99%). PdNPs-2 was previously tested in the Pd-racemization of the enantiopure S-phenylethylamine. The enantiopure arylamine was totally converted to the rac mixture. Consequently, the tandem enzyme-Pd catalysis in the acetylation of rac-phenylethylamine, using ethylacetate as an acylation agent, was performed using PdNPs-2 in toluene at 70 °C (Table 5). The DRK worked perfectly, and an almost quantitative enantiopure R-phenylacetamide was obtained after 4 h at these conditions. The addition of bases in the process negatively affected the enantioselectivity of the product (Table 5).

Table 5.
Dynamic kinetic resolution ((±)-1-phenylethylamine catalyzed by PdNPs biohybrids a.
3.5. Recycling and Stability of the Heterogeneous Catalyst
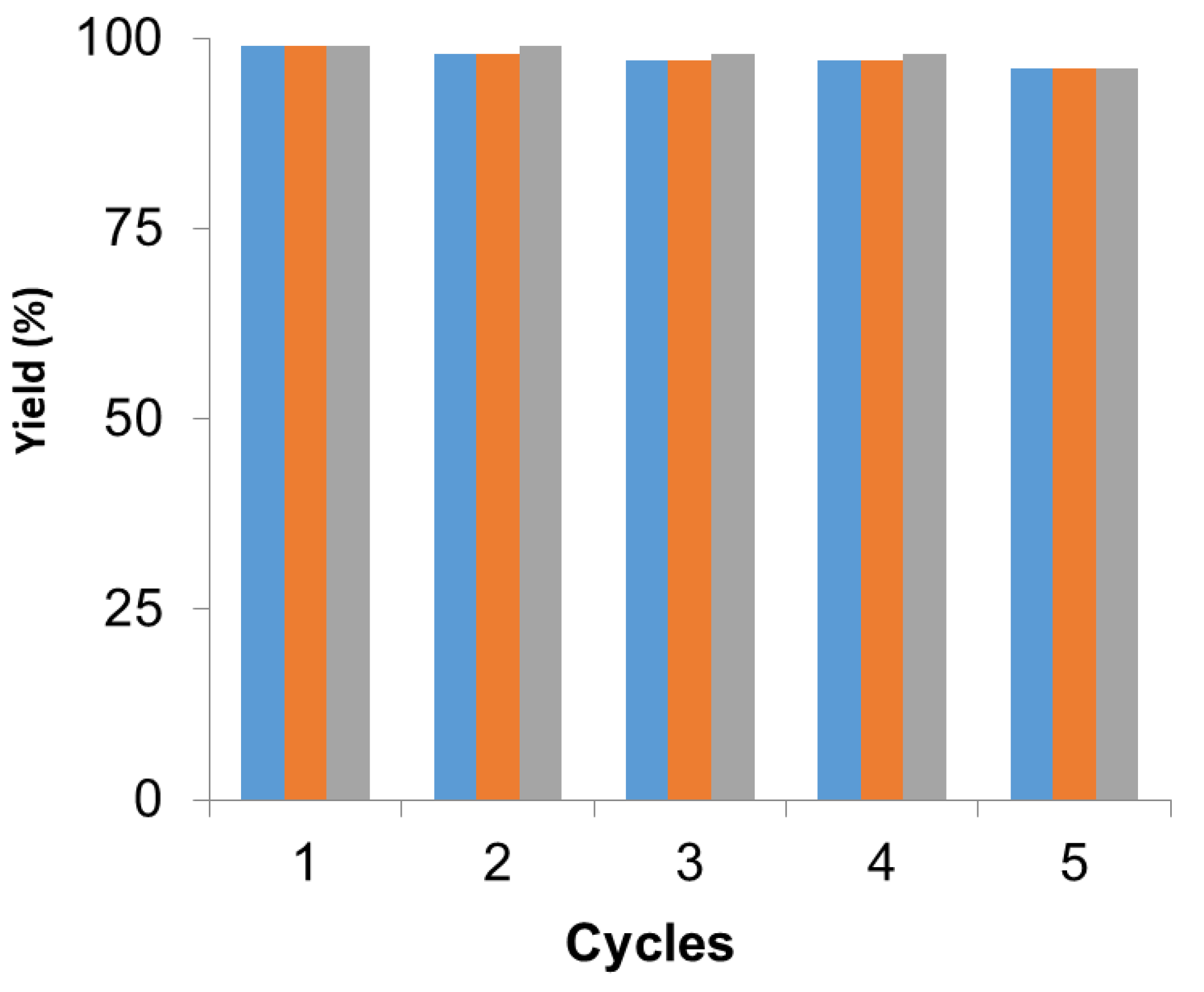
Furthermore, the Pd hybrid (PdNPs-2) was recycled in different reactions, as previously tested. This is quite interesting since the reactions were performed at different conditions; aqueous media and 50 °C (Suzuki), 70–120 °C (DMF) or toluene and 70 °C (DKR), demonstrating the versatility of this heterogeneous catalyst.
In all cases, the catalyst was reused up to five times maintaining more than 95% of the metallic and, in this case, the enzymatic catalytic efficiency (Figure 4). After each cycle, the catalyst was washed with the same solvent used in the reaction and then the fresh reaction media was added for the next cycle. After the fifth cycle, the catalyst was analyzed by XRD and ICP demonstrating that the Pd species and the amount of metal did not change.
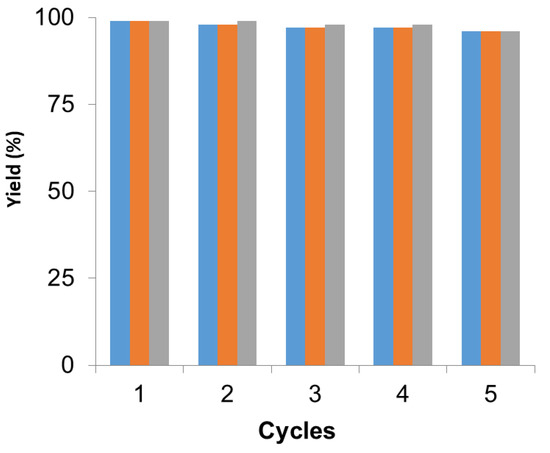
Figure 4.
Recyclability of PdNPs-2 in the different reactions. Suzuki (blue), Heck (orange), dynamic kinetic resolution (DKR) (grey). Reaction conditions in each reaction were performed as previously described.
4. Conclusions
Here, we showed the straightforward preparation of highly stable palladium-nanoparticles (PdNPs) biohybrids at very mild conditions (aqueous media, room temperature, no reductive agents), based on the application of an enzyme, which induces the in situ formation of these small spherical nanoparticles on the protein network. We have demonstrated the effect of the enzyme scaffold in the nanoparticle size and the Pd species.
The excellent applicability in chemistry of these PdNPs biohybrids has been demonstrated with excellent versatility to work in an aqueous, aqueous/organic solvent, organic solvent, moderate to high temperature, in addition to the excellent results in the reduction and C-C bonding reactions. Moreover, the cascade processes by dual activities of the hybrids have been demonstrated.
Therefore, these results open the possibility to easily combine together the desired enzyme and metallic catalytic activities for sustainable chemical processes.
Author Contributions
M.F., N.L.-G., M.M., C.P.-R., and M.d.P.M. performed the experiments; J.M.P. designed and supervised the study and experiments; J.M.P. and N.L.-G. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Government, the Spanish National Research Council (CSIC). We also thank the Ministry of Education, Youth and Sports of the Community of Madrid, and the European Social Fund for a contract to C.P.-R. (PEJD-2017PRE/SAL-3762).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Ramiro Martinez from Novozymes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, S.; Shin, K.; Wong, M.S.; Henkelman, G. Design of a Pd–Au Nitrite Reduction Catalyst by Identifying and Optimizing Active Ensembles. ACS Catal. 2019, 9, 7957–7966. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [PubMed]
- Mun, Y.; Lee, S.; Cho, A.; Kim, S.; Han, J.W.; Lee, J.S. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2019, 246, 82–88. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Liu, Q.; Du, P.; Liude, W.; He, Z. Biosynthesis of palladium nanoparticles using Shewanella loihica PV-4 for excellent catalytic reduction of chromium(vi). Environ. Sci. Nano 2018, 5, 730–739. [Google Scholar] [CrossRef]
- Veisi, H.; Ghorbani, M.; Hemmati, S. Sonochemical in situ immobilization of Pd nanoparticles on green tea extract coated Fe3O4 nanoparticles: An efficient and magnetically recyclable nanocatalyst for synthesis of biphenyl compounds un-der ultrasound irradiations. Mater. Sci. Eng. C 2019, 98, 584–593. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghorbani-Choghamarani, A. l-Methionine–Pd complex supported on hercynite as a highly efficient and reusable nanocatalyst for C–C cross-coupling reactions. New J. Chem. 2020, 44, 2919–2929. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, Y.; Xu, M.; Cui, H.; Tan, L.; Feng, N.; Liu, X.; Qiu, G.-Z.; Dong, H.; Xie, J. Microbial synthesis of Pd–Pt alloy nanoparticles using Shewanella oneidensis MR-1 with enhanced catalytic activity for nitrophenol and azo dyes reduction. Nanotechnology 2018, 30, 065607. [Google Scholar] [CrossRef]
- Garole, V.J.; Choudhary, B.C.; Tetgure, S.R.; Borse, A.U.; Garole, D.J. Palladium nanocatalyst: Green synthesis, characterization, and catalytic application. Int. J. Environ. Sci. Technol. 2019, 16, 7885–7892. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, D.; Sun, Y.; Wang, S.; Cai, J. Facile Fabrication of Highly Dispersed Pd@Ag Core-Shell Nanoparticles Embedded in Spirulina platensis by Electroless Deposition and Their Catalytic Properties. Adv. Funct. Mater. 2018, 28, 1707231. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Baruwati, B.; Sikdar, S.; Bhattacharyya, D. Nanostructured Membranes for Enzyme Catalysis and Green Synthesis of Nanoparticles. ChemSusChem 2011, 4, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Benavente, R.; Lopez-Tejedor, D.; Palomo, J.M. Synthesis of a superparamagnetic ultrathin FeCO3 nanorods–enzyme bionanohybrid as a novel heterogeneous catalyst. Chem. Commun. 2018, 54, 6256–6259. [Google Scholar] [CrossRef] [PubMed]
- Losada-Garcia, N.; Rodriguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme–copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Han, F. Protein coated gold nanoparticles as template for the directed synthesis of highly fluorescent gold nanoclusters. Nanotechnology 2018, 29, 165702. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, L.; Han, Y.; Chen, W.; Liu, H.; Zhang, M.; Deng, L.; Liu, Y. Dual Roles of Protein as a Template and a Sulfur Provider: A General Approach to Metal Sulfides for Efficient Photothermal Therapy of Cancer. Small 2018, 14. [Google Scholar] [CrossRef]
- Li, Y.; Song, K.; Cao, Y.; Peng, C.; Yang, G. Keratin-Templated Synthesis of Metallic Oxide Nanoparticles as MRI Contrast Agents and Drug Carriers. ACS Appl. Mater. Interfaces 2018, 10, 26039–26045. [Google Scholar] [CrossRef]
- Filice, M.; Marciello, M.; Morales, M.D.P.; Palomo, J.M. Synthesis of heterogeneous enzyme–metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General trend of li-pase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arvela, R.K.; Leadbeater, N.E.; Sangi, M.S.; Williams, V.A.; Granados, P.; Singer, R.D. A reassessment of the transi-tion-metal free Suzuki-type coupling methodology. J. Org. Chem. 2005, 70, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Mohazzab, B.F.; Jaleh, B.; Issaabadi, Z.; Nasrollahzadeh, M.; Varma, R.S. Stainless steel mesh-GO/Pd NPs: Catalytic applications of Suzuki–Miyaura and Stille coupling reactions in eco-friendly media. Green Chem. 2019, 21, 3319–3327. [Google Scholar] [CrossRef]
- Soltani, S.S.; Taheri-Ledari, R.; Farnia, S.M.F.; Maleki, A.; Foroumadi, A. Synthesis and characterization of a supported Pd complex on volcanic pumice laminates textured by cellulose for facilitating Suzuki–Miyaura cross-coupling reac-tions. RSC Adv. 2020, 10, 23359–23371. [Google Scholar] [CrossRef]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Parveen, N.; Sekar, G. Palladium Nanoparticles-Catalyzed Synthesis of Indanone Derivatives via Intramolecular Reductive Heck Reaction. Adv. Synth. Catal. 2019, 361, 4581–4595. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khorsandi, Z.; Farrokhpour, H. Regioselective Heck reaction catalyzed by Pd nanoparticles immobili-zed on DNA-modified MWCNTs. RSC Adv. 2016, 6, 59124–59130. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Leazer, J.; Varma, R.S. Magnetically separable Fe3O4@DOPA–Pd: A heterogeneous catalyst for aqueous Heck reaction. Clean Technol. Environ. Policy 2015, 17, 2073–2077. [Google Scholar] [CrossRef]
- Reay, A.J.; Hammarback, L.A.; Bray, J.T.; Sheridan, T.; Turnbull, D.; Whitwood, A.C.; Fairlamb, I.J. Mild and regioselective Pd(OAc)2-catalyzed C–H arylation of tryptophans by [ArN2] X, promoted by tosic acid. ACS Catal. 2017, 7, 5174–5179. [Google Scholar] [CrossRef]
- Lorion, M.M.; Kaplaneris, N.; Son, J.; Kuniyil, R.; Ackermann, L. Late-Stage Peptide Diversification through Cobalt-Catalyzed C− H Activation: Sequential Multicatalysis for Stapled Peptides. Angew. Chem. 2019, 131, 1698–1702. [Google Scholar] [CrossRef]
- Potukuchi, H.K.; Bach, T. Selective C-2 alkylation of tryptophan by a Pd (II)/norbornene-promoted C–H activation reaction. J. Org. Chem. 2013, 78, 12263–12267. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.-P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).