Water Management Strategies for Proton Exchange Membrane Fuel Cells: A Comprehensive Review
Abstract
1. Introduction
- (i)
- Summarizing the primary methodologies for regulating water transport and distribution within PEMFCs.
- (ii)
- Identifying the challenges and limitations of both traditional and novel water management techniques.
- (iii)
- Identifying prospective future strategies for enhancing PEMFC performance under various environmental and load conditions.
2. PEMFC Structure and Operation
2.1. Important Elements of PEMFC
2.1.1. Anode and Cathode Electrodes
2.1.2. Gas Diffusion Layers
2.1.3. Bipolar Plates
2.1.4. Catalyst Layers
2.1.5. Principle of Operation
2.2. PEMFC Manufacturing and the Negative Effects of Excessive Water Content
3. Water Transportation
3.1. Flow Channel Issues with Water
3.2. Water Transport Mechanisms
3.3. Effects of Dehydration and Harsh Operating Conditions on PEMFCs
3.4. Water Movement in the Catalyst Layer
3.5. PEMFC Simulation of Water Transport in Catalyst Layers
3.6. PEMFC Principle of Judging Water Failure Through Pressure Drop
4. Modeling Approaches for PEMFC Water Management
4.1. Zero-Dimensional Models
4.2. One-Dimensional and Multi-Dimensional Models
- The capillary pressure equation explains the necessary pressure differential to force liquid water through the hydrophobic GDL:
- 2.
- Darcy’s law-based drainage criterion determines when liquid water drains from the porous medium.
- 3.
- Evaporation source term models the rate of water phase change from liquid to vapor.
- 4.
- The condensation source term represents the condensation of vapor into droplets [83].
- 5.
- Water absorption rate simulates the re-humidification of the GDL.
4.3. Two-Phase Flow Models
4.4. Advanced Modeling Techniques
4.5. Water Content Adjustment in PEMFC by Changing the Pressure Drop
4.6. Improvement of Flow Channel Shape and Surface Tension Problems
4.7. Water Management Materials
4.8. Water Performance and Water Buildup in PEMFC by Changing the Pressure Drop
4.9. Nuclear Magnetic Resonance Imaging
4.10. Methods for X-Ray Irradiation
4.11. Changing the Material Structure
4.12. Improvement of Flow Channel Shape in PEMFCs
- Pressure drop, represented by Equation (11).
- 2.
- For multi-U inlet designs, the dimensional uniformity index, which measures reactant distribution across channel arrays, improved from 0.70 to 0.16 [153].
- 3.
- Interdigitated or curved channels enhance the under-rib convection effect, facilitating the evacuation of water and oxygen.
4.13. Proton Transport Mechanisms (Vehicle and Grotthus)
5. Future Challenges in Water Management
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbir, F. PEM Fuel Cells: Theory and Practice; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in Fuel Cell Technologies in the Transport Sector. Int. J. Hydrogen Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhang, J. (Ed.) PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Baschuk, J.J.; Li, X. A comprehensive, consistent, and practical PEM fuel cell model. J. Power Sources 2001, 86, 181–196. [Google Scholar]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life Cycle Assessment of Hydrogen Production via Electrolysis—A Review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Van Herle, J. Water Management of the Proton Exchange Membrane Fuel Cells: Optimizing the Effect of Microstructural Properties on the Gas Diffusion Layer Liquid Removal. Energy 2022, 256, 124712. [Google Scholar] [CrossRef]
- Baz, F.B.; Elzohary, R.M.; Osman, S.; Marzouk, S.A.; Ahmed, M. A Review of Water Management Methods in Proton Exchange Membrane Fuel Cells. Energy Convers. Manag. 2024, 302, 118150. [Google Scholar] [CrossRef]
- Xu, X.; Li, K.; Liao, Z.; Cao, J.; Wang, R. A Closed-Loop Water Management Methodology for PEM Fuel Cell System Based on Impedance Information Feedback. Energies 2022, 15, 7561. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Zhou, S.; Yang, J.; Kockelmann, W.; Han, Y.; Li, Q.; Chen, W.; Coppens, M.-O.; Shearing, P.R.; et al. Water management and mass transport of a fractal metal foam flow-field based polymer electrolyte fuel cell using operando neutron imaging. Appl. Energy 2024, 364, 123204. [Google Scholar] [CrossRef]
- Hwang, R.Y.; Moon, Y.; Kim, H.M.; Kim, E.; Park, S.; Han, O.H. Measuring the Water Content of Polymer Electrolyte Membranes Using Double Points of Proton Magic Angle Spinning Nuclear Magnetic Resonance Data. Polymer 2024, 309, 127431. [Google Scholar] [CrossRef]
- Sicilia, M.; Cervone, D.; Polverino, P.; Pianese, C. Advancements on Lumped Modelling of Membrane Water Content for Real-Time Prognostics and Control of PEMFC. Energies 2024, 17, 4841. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Smith, G. Modelling the Proton-Conductive Membrane in Practical Polymer Electrolyte Membrane Fuel Cell (PEMFC) Simulation: A Review. Membranes 2020, 10, 310. [Google Scholar] [CrossRef]
- Xu, L.; Trogadas, P.; Zhou, S.; Jiang, S.; Wu, Y.; Rasha, L.; Kockelmann, W.; Di Yang, J.; Neville, T.; Jervis, R.; et al. A Scalable and Robust Water Management Strategy for PEMFCs: Operando Electrothermal Mapping and Neutron Imaging Study. Adv. Sci. 2024, 11, e2404350. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khalid, M.; Kamal, K.; Ratlamwala, T.A.H.; Hussain, G.; Alkahtani, M. Modeling and Simulation of a Proton Exchange Membrane Fuel Cell Alongside a Waste Heat Recovery System Based on the Organic Rankine Cycle in MATLAB/SIMULINK Environment. Sustainability 2021, 13, 1218. [Google Scholar] [CrossRef]
- Liu, G.; Qin, Y.; Wang, J.; Liu, C.; Yin, Y.; Zhao, J.; Yin, Y.; Zhang, J.; Otoo, O.N. Thermodynamic Modeling and Analysis of a Novel PEMFC-ORC Combined Power System. Energy Convers. Manag. 2020, 217, 112998. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, C.Y. Numerical Modeling of Liquid Water Motion in a Polymer Electrolyte Fuel Cell. Int. J. Hydrogen Energy 2014, 39, 942–950. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Al-Makky, A.; Baroutaji, A.; James, J.C.; Thompson, J.G.; Olabi, A.G. Development of Bi-Polar Plate Design of PEM Fuel Cell Using CFD Techniques. Int. J. Hydrogen Energy 2017, 42, 25663–25685. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Hakenjos, A.; Muenter, H.; Wittstadt, U.; Hebling, C. A PEM fuel cell for combined measurement of current and temperature distribution, and flow field flooding. J. Power Sources 2004, 131, 213–216. [Google Scholar] [CrossRef]
- Yang, D.; Garg, H.; Andersson, M. Numerical Simulation of Two-Phase Flow in Gas Diffusion Layer and Gas Channel of Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2023, 48, 15677–15694. [Google Scholar] [CrossRef]
- Shimpalee, S.; Beuscher, U.; Van Zee, J.W. Analysis of GDL Flooding Effects on PEMFC Performance. Electrochim. Acta 2007, 52, 6748–6754. [Google Scholar] [CrossRef]
- Flipo, G.; Josset, C.; Giacoppo, G.; Squadrito, G.; Auvity, B.; Bellettre, J. Eruptive Water Transport in PEMFC: A Single-Drop Capillary Model. Int. J. Hydrogen Energy 2015, 40, 14667–14675. [Google Scholar] [CrossRef]
- Wasatch, B.; Tapas, K.; Thi, X.H.N.; Nam, H.K.; Kin-tak, L.; Joong, H.L. Polymer Membranes for High Temperature Proton Exchange Membrane Fuel Cell: Recent Advances and Challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Three-Dimensional Multiphase Simulation of PEMFC at High Current Density Utilizing Eulerian-Eulerian Model and Two-Fluid Model. Energy Convers. Manag. 2018, 176, 409–421. [Google Scholar] [CrossRef]
- Zhai, S.; Zhou, S.; Sun, P.; Chen, F.; Niu, J. Modeling study of anode water flooding and gas purge for PEMFCs. J. Fuel Cell Sci. Technol. 2012, 9, 031007. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y. Water Management in Proton Exchange Membrane Fuel Cells: An Analysis of Water Transport in Membranes. Electrochim. Acta 2007, 52, 8144–8154. [Google Scholar]
- Yousfi, N.S.; Hissel, D.; Mocoteguy, P.; Candusso, D. Diagnosis of polymer electrolyte fuel cells failure modes (flooding & drying out) by neural networks modeling. Int. J. Hydrogen Energy 2011, 36, 3067–3075. [Google Scholar] [CrossRef]
- Lee, D.; Bae, J. Visualization of flooding in a single cell and stacks by using a newly-designed transparent PEMFC. Int. J. Hydrogen Energy 2012, 37, 422–435. [Google Scholar] [CrossRef]
- Bazylak, A. Liquid Water Visualization in PEM Fuel Cells: A Review. Int. J. Hydrogen Energy 2009, 34, 3845–3857. [Google Scholar] [CrossRef]
- Caizhi, Z.; Lan, Z.; Weijiang, Z.; Youyi, W.; Siew, H.C. Investigation of Water Transport and Its Effect on Performance of High Temperature PEM Fuel Cells. Electrochim. Acta 2014, 149, 271–277. [Google Scholar] [CrossRef]
- Zamel, N.; Li, X. Effective Transport Properties for Polymer Electrolyte Membrane Fuel Cells—With a Focus on the Gas Diffusion Layer. Prog. Energy Combust. Sci. 2013, 39, 111–146. [Google Scholar] [CrossRef]
- Ghanbarian, A.; Kermani, M.J.; Scholta, J.; Abdollahzadeh, M. Polymer Electrolyte Membrane Fuel Cell Flow Field Design Criteria—Application to Parallel Serpentine Flow Patterns. Energy Convers. Manag. 2018, 166, 281–296. [Google Scholar] [CrossRef]
- Li, X.; Sabir, I. Review of Bipolar Plates in PEM Fuel Cells: Flow-Field Designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Bednarek, T.; Tsotridis, G. Issues Associated with Modelling of Proton Exchange Membrane Fuel Cell by Computational Fluid Dynamics. J. Power Sources 2017, 343, 550–563. [Google Scholar] [CrossRef]
- Kim, M.; Jung, N.; Eom, K.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Kim, H.J.; Hong, B.K.; Cho, E. Effects of Anode Flooding on the Performance Degradation of Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2014, 266, 332–340. [Google Scholar] [CrossRef]
- Qin, C.; Wang, J.; Yang, D.; Li, B.; Zhang, C. Proton Exchange Membrane Fuel Cell Reversal: A Review. Catalysts 2016, 6, 197. [Google Scholar] [CrossRef]
- Jang, J.-H.; Yan, W.-M.; Li, H.-Y.; Chou, Y.-C. Humidity of Reactant Fuel on the Cell Performance of PEM Fuel Cell with Baffle-Blocked Flow Field. J. Power Sources 2006, 159, 468–477. [Google Scholar] [CrossRef]
- Wen, D.-H.; Yin, L.-Z.; Piao, Z.-Y.; Lu, C.-D.; Li, G.; Leng, Q.-H. Performance Investigation of Proton Exchange Membrane Fuel Cell with Intersectant Flow Field. Int. J. Heat Mass Transf. 2018, 121, 775–787. [Google Scholar] [CrossRef]
- Lim, C.; Wang, C.Y. Effects of Water Flooding on Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2004, 49, 4149–4156. [Google Scholar] [CrossRef]
- Litster, S.; McLean, G. PEM Fuel Cell Electrodes. J. Power Sources 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Zawodzinski, T.A., Jr.; Derouin, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T.E.; Gottesfeld, S. Water Uptake by and Transport Through Nafion 117 Membranes. J. Electrochem. Soc. 1993, 140, 1041–1047. [Google Scholar] [CrossRef]
- Prater, K. The renaissance of the solid polymer fuel cell: Future perspectives. J. Power Sources 1990, 29, 239–250. [Google Scholar] [CrossRef]
- Xing, L.; Qiang, C.; Chun, X.; Cheng, L.; Scott, K.; Yan, Y. Numerical Study of the Effect of Relative Humidity and Stoichiometric Flow Ratio on PEM (Proton Exchange Membrane) Fuel Cell Performance with Various Channel Lengths: An Anode Partial Flooding Modelling. Energy 2016, 106, 631–645. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Ye, F.; Ma, C.F. Water Flooding and Pressure Drop Characteristics in Flow Channels of Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2007, 52, 3607–3614. [Google Scholar] [CrossRef]
- Carton, J.; Lawlor, V.; Olabi, A.; Hochenauer, C.; Zauner, G. Water Droplet Accumulation and Motion in PEM (Proton Exchange Membrane) Fuel Cell Minichannels. Sustain. Energy Environ. Prot. 2010, 39, 63–73. [Google Scholar] [CrossRef]
- Wang, B.; Lin, R.; Liu, D.; Xu, J.; Feng, B. Investigation of the Effect of Humidity at Both Electrode on the Performance of PEMFC Using Orthogonal Test Method. Int. J. Hydrogen Energy 2019, 44, 13737–13743. [Google Scholar] [CrossRef]
- Rakhshanpouri, S.; Rowshanzamir, S. Water Transport through a PEM (Proton Exchange Membrane) Fuel Cell in a Seven-Layer Model. Energy 2013, 50, 220–231. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of PEMFC Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Lin, R.; Liu, D.; Xia, S.; Ma, T.; Dutruel, B. Stack Shut-Down Strategy Optimisation of Proton Exchange Membrane Fuel Cell with the Segment Stack Technology. Int. J. Hydrogen Energy 2020, 45, 1030–1044. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High Temperature (HT) Polymer Electrolyte Membrane Fuel Cells (PEMFC)—A Review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Natarajan, D.; Van Nguyen, T. Three-Dimensional Effects of Liquid Water Flooding in the Cathode of a PEM Fuel Cell. J. Power Sources 2003, 115, 66–80. [Google Scholar] [CrossRef]
- Wilson, M.S.; Valerio, J.A.; Gottesfeld, S. Low Platinum Loading Electrodes for Polymer Electrolyte Fuel Cells Fabricated Using Thermoplastic Ionomers. Electrochim. Acta 1995, 40, 355–363. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Ahosseini, A.; Wang, X.; Yarlagadda, V.; Kwong, A.; Weber, A.Z.; Deevanhxay, P.; Tsushima, S.; Hirai, S. Hydrophobic Gas-Diffusion Media for Polymer-Electrolyte Fuel Cells by Direct Fluorination. J. Electrochem. Soc. 2015, 162, F1451–F1460. [Google Scholar] [CrossRef]
- Gostick, J.T.; Fowler, M.W.; Ioannidis, M.A.; Pritzker, M.D.; Volfkovich, Y.M.; Sakars, A. Capillary Pressure and Hydrophilic Porosity in Gas Diffusion Layers for Polymer Electrolyte Fuel Cells. J. Power Sources 2006, 156, 375–387. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Effects of Various Operating and Initial Conditions on Cold Start Performance of Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 8171–8184. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Lu, Z. Thermal Management Issues in a PEMFC Stack—A Brief Review of Current Status. Appl. Therm. Eng. 2009, 29, 1276–1280. [Google Scholar] [CrossRef]
- Gostick, J.T.; Fowler, M.W.; Ioannidis, M.A.; Pritzker, M.D.; Volfkovich, Y.M.; Sakars, A. Impact of GDL Structure and Wettability on Water Management in Polymer Electrolyte Fuel Cells. J. Mater. Chem. 2007, 17, 3187–3196. [Google Scholar] [CrossRef]
- Huang, Y.; Williams, S.; Heo, T.W.; Marshall, A.; Wood, B.; Kennedy, J.; Metson, J.; Woo, M.W.; Liu, J. Understanding the Impact of the Gas Diffusion Layer Structure on Catalyst Utilization in the PEM Water Electrolyzer. Next Energy 2025, 8, 100319. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Khandelwal, M.; Michael, M.M.; Anita, B. Pore Network Modeling of Phase Change in PEM Fuel Cell Fibrous Cathode. J. Appl. Electrochem. 2017, 47, 1323–1338. [Google Scholar] [CrossRef]
- Dullien, F.A.L. Porous Media: Fluid Transport and Pore Structure, 2nd ed.; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Ioannidis, M.A.; Chatzis, I. Network Modelling of Pore Structure and Transport Properties of Porous Media. Chem. Eng. Sci. 1993, 48, 951–972. [Google Scholar] [CrossRef]
- Athanasaki, G.; Jayakumar, A.; Kannan, A.M. Gas diffusion layers for PEM fuel cells: Materials, properties and manufacturing—A review. Int. J. Hydrogen Energy 2023, 48, 2294–2313. [Google Scholar] [CrossRef]
- Ohser, J.; Mucklich, F. Statistical Analysis of Microstructures in Materials Science; Wiley: Chichester, UK, 2000. [Google Scholar]
- Park, J.W.; Jiao, K.; Li, X. Numerical Investigations on Liquid Water Removal from the Porous Gas Diffusion Layer by Reactant Flow. Appl. Energy 2010, 87, 2180–2186. [Google Scholar] [CrossRef]
- Schulz, V.P.; Becker, J.; Wiegmann, A.; Mukherjee, P.P.; Wang, C.Y. Modeling of Two-Phase Behavior in the Gas Diffusion Medium of PEFCs via Full Morphology Approach. J. Electrochem. Soc. 2007, 154, B419–B426. [Google Scholar] [CrossRef]
- Park, J.; Li, X. Multi-phase micro-scale flow simulation in the electrodes of a PEM fuel cell by lattice Boltzmann method. J. Power Sources 2008, 178, 248–257. [Google Scholar] [CrossRef]
- Gostick, J.T.; Ioannidis, M.A.; Fowler, M.W.; Pritzker, M.D. Pore Network Modeling of Fibrous Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2007, 173, 277–290. [Google Scholar] [CrossRef]
- Mo, J.; Dehoff, R.R.; Peter, W.H.; Toops, T.J.; Green, J.B., Jr.; Zhang, F.-Y. Additive Manufacturing of Liquid/Gas Diffusion Layers for Low-Cost and High-Efficiency Hydrogen Production. Int. J. Hydrogen Energy 2016, 41, 3128–3135. [Google Scholar] [CrossRef]
- Renksizbulut, M. Lecture Notes for Convective Heat Transfer; University of Waterloo: Waterloo, KW, Canada, 2007. [Google Scholar]
- Lin, R.; Ren, Y.S.; Lin, X.W.; Jiang, Z.H.; Yang, Z.; Chang, Y.T. Investigation of the internal behavior in segmented PEMFCs of different flow fields during cold start process. Energy 2017, 123, 367–377. [Google Scholar] [CrossRef]
- Sun, S.; Shao, Z.; Yu, H.; Li, G.; Yi, B. Investigations on degradation of the long-term proton exchange membrane water electrolysis stack. J. Power Sources 2014, 267, 515–520. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Mo, J.; Yang, G.; Yu, S.; Talley, D.A.; Han, B.; Zhang, F.-Y. In-situ Investigation of Bubble Dynamics and Two-Phase Flow in Proton Exchange Membrane Electrolyzer Cells. Int. J. Hydrogen Energy 2018, 43, 11223–11233. [Google Scholar] [CrossRef]
- Djilali, N.; Lu, D. Influence of Heat Transfer on Gas and Water Transport in Fuel Cells. Int. J. Therm. Sci. 2002, 41, 29–40. [Google Scholar] [CrossRef]
- Zinser, A.; Papakonstantinou, G.; Sundmacher, K. Analysis of Mass Transport Processes in the Anodic Porous Transport Layer in PEM Water Electrolysers. Int. J. Hydrogen Energy 2019, 44, 28077–28087. [Google Scholar] [CrossRef]
- Kaiser, R.; Ahn, C.-Y.; Kim, Y.-H.; Park, J.-C. Performance and Mass Transfer Evaluation of PEM Fuel Cells with Straight and Wavy Parallel Flow Channels of Various Wavelengths Using CFD Simulation. Int. J. Hydrogen Energy 2023, 51, 1326–1344. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Wang, Z.; Shi, Z.; Wu, S.; Song, D.; Zhang, J.; Fatih, K.; Zhang, J.; Wang, H.; et al. A Review of Water Flooding Issues in the Proton Exchange Membrane Fuel Cell. J. Power Sources 2008, 178, 103–117. [Google Scholar] [CrossRef]
- Bernardi, D.M.; Verbrugge, M.W. A mathematical model of the solid-polymer-electrolyte fuel cell. J. Electrochem. Soc. 1992, 139, 2477–2491. [Google Scholar] [CrossRef]
- Stropnik, R.; Mlakar, N.; Lotrič, A.; Sekavčnik, M.; Mori, M. The Influence of Degradation Effects in Proton Exchange Membrane Fuel Cells on Life Cycle Assessment Modelling and Environmental Impact Indicators. Int. J. Hydrogen Energy 2022, 47, 24223–24241. [Google Scholar] [CrossRef]
- Afshari, E.; Jazayeri, S.A. Analyses of Heat and Water Transport Interactions in a Proton Exchange Membrane Fuel Cell. J. Power Sources 2009, 194, 423–432. [Google Scholar] [CrossRef]
- Das, P.K.; Li, X.; Liu, Z.-S. A Three-Dimensional Agglomerate Model for the Cathode Catalyst Layer of PEM Fuel Cells. J. Power Sources 2008, 179, 186–199. [Google Scholar] [CrossRef]
- Singh, D.; Lu, D.M.; Djilali, N. A Two-Dimensional Analysis of Mass Transport in Proton Exchange Membrane Fuel Cells. Int. J. Eng. Sci. 1999, 37, 431–452. [Google Scholar] [CrossRef]
- Najmi, A.-U.-H.; Anyanwu, I.S.; Xie, X.; Liu, Z.; Jiao, K. Experimental Investigation and Optimization of Proton Exchange Membrane Fuel Cell Using Different Flow Fields. Energy 2021, 217, 119313. [Google Scholar] [CrossRef]
- Malek, K.; Eikerling, M.; Wang, Q.; Navessin, T.; Liu, Z. Self-Organization in Catalyst Layers of Polymer Electrolyte Fuel Cells. J. Phys. Chem. C 2007, 111, 13627–13634. [Google Scholar] [CrossRef]
- Nam, J.H.; Kaviany, M. Effective Diffusivity and Water-Saturation Distribution in Single- and Two-Layer PEMFC Diffusion Medium. Int. J. Heat Mass Transf. 2003, 46, 4595–4611. [Google Scholar] [CrossRef]
- Kazim, A. Introduction of PEM fuel-cell vehicles in the transportation sector of the United Arab Emirates. Appl. Energy 2003, 74, 125–133. [Google Scholar] [CrossRef]
- Mammar, K.; Saadaoui, F.; Laribi, S. Design of a PEM Fuel Cell Model for Flooding and Drying Diagnosis Using Fuzzy Logic Clustering. Renew. Energy Focus 2019, 30, 123–130. [Google Scholar] [CrossRef]
- Manso, A.P.; Marzo, F.F.; Barranco, J.; Garikano, X.; Mujika, M.G. Influence of Geometric Parameters of the Flow Fields on the Performance of a PEM Fuel Cell: A Review. Int. J. Hydrogen Energy 2012, 37, 15256–15287. [Google Scholar] [CrossRef]
- Mosdale, R.; Srinivasan, S. Analysis of Performance and of Water and Thermal Management in Proton Exchange Membrane Fuel Cells. Electrochim. Acta 1995, 40, 413–421. [Google Scholar] [CrossRef]
- Culubret, S.; Rubio, M.A.; Sanchez, D.G.; Urquia, A. Dynamic Modeling of the Effect of Water Management on Polymer Electrolyte Fuel Cells Performance. Int. J. Hydrogen Energy 2020, 45, 5710–5722. [Google Scholar] [CrossRef]
- Owejan, J.P.; Gagliardo, J.J.; Sergi, J.M.; Kandlikar, S.G.; Trabold, T.A. Water Management Studies in PEM Fuel Cells, Part I: Fuel Cell Design and In Situ Water Distributions. Int. J. Hydrogen Energy 2009, 34, 3436–3444. [Google Scholar] [CrossRef]
- Mosdale, R.; Gebel, G.; Pineri, M. Water Profile Determination in a Running Proton Exchange Membrane Fuel Cell Using Small-Angle Neutron Scattering. J. Membr. Sci. 1996, 118, 269–277. [Google Scholar] [CrossRef]
- Natarajan, D.; Van Nguyen, T. A Two-Dimensional, Two-Phase, Multicomponent, Transient Model for the Cathode of a Proton Exchange Membrane Fuel Cell Using Conventional Gas Distributors. J. Electrochem. Soc. 2001, 148, A1324. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Yavarinasab, A.; Siavashi, M.; Matian, M.; Van Herle, J. Progress in the Proton Exchange Membrane Fuel Cells (PEMFCs) Water/Thermal Management: From Theory to the Current Challenges and Real-Time Fault Diagnosis Methods. Energy Rev. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Lemons, R.A. Fuel cells for transportation. J. Power Sources 1990, 29, 251–264. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, Technological Status, and Fundamentals of PEM Fuel Cells—A Review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Gass, R.; Li, Z.; Outbib, R.; Jemei, S.; Hissel, D. An Advanced 1D Physics-Based Model for PEM Hydrogen Fuel Cells with Enhanced Overvoltage Prediction. Int. J. Hydrogen Energy 2025, 97, 1108–1125. [Google Scholar] [CrossRef]
- Garche, J.; Jorissen, L. “PEMFC fuel cell systems” Fuel Cell Technology and Applications, Electric utility fuel cell systems. In Handbook of Fuel Cells: Fundamentals, Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Pasaogullari, U.; Wang, C.-Y. Two-Phase Modeling and Flooding Prediction of Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2005, 152, A380. [Google Scholar] [CrossRef]
- Sinha, P.K.; Wang, C.-Y. Pore-Network Modeling of Liquid Water Transport in Gas Diffusion Layer of a Polymer Electrolyte Fuel Cell. Electrochim. Acta 2007, 52, 7936–7945. [Google Scholar] [CrossRef]
- Kaya, M.F.; Demir, N.; Rees, N.V.; El-Kharouf, A. Improving PEM Water Electrolyser’s Performance by Magnetic Field Application. Appl. Energy 2020, 264, 114721. [Google Scholar] [CrossRef]
- Hassan, A.H.; Wang, X.; Liao, Z.; Xu, C. Numerical Investigation on the Effects of Design Parameters and Operating Conditions on the Electrochemical Performance of Proton Exchange Membrane Water Electrolysis. J. Therm. Sci. 2023, 32, 1989–2007. [Google Scholar] [CrossRef]
- Wang, X.R.; Ma, Y.; Gao, J.; Li, T.; Jiang, G.Z.; Sun, Z.Y. Review on Water Management Methods for Proton Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 12206–12229. [Google Scholar] [CrossRef]
- Li, G.; Xu, M.; Qin, Y.; Zhang, Y.; Wang, Y.; Yu, X.; Li, J. Numerical Simulation of Gradient Catalyst Layer Design in Proton Exchange Membrane Water Electrolyzer with Enhanced Performance. Fuel 2024, 368, 131444. [Google Scholar] [CrossRef]
- Zuo, J.; Steiner, N.Y.; Li, Z.; Hissel, D. Degradation Root Cause Analysis of PEM Fuel Cells Using Distribution of Relaxation Times. Appl. Energy 2025, 378, 124762. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Siavashi, M.; Moghimi, M. Design Optimization and Thermal Management of the PEMFC Using Artificial Neural Networks. Energy 2019, 182, 443–459. [Google Scholar] [CrossRef]
- Sorrentino, M.; Pianese, C.; Marano, V. A real-time model-based estimator for PEM fuel cell degradation diagnosis via EIS. J. Power Sources 2012, 218, 316–324. [Google Scholar]
- Luna, J.; Jemei, S.; Yousfi-Steiner, N.; Husar, A.; Serra, M.; Hissel, D. Nonlinear Predictive Control for Durability Enhancement and Efficiency Improvement in a Fuel Cell Power System. J. Power Sources 2016, 328, 250–261. [Google Scholar] [CrossRef]
- Chu, T.; Xie, M.; Yu, Y.; Wang, B.; Yang, D.; Li, B.; Ming, P.; Zhang, C. Experimental Study of the Influence of Dynamic Load Cycle and Operating Parameters on the Durability of PEMFC. Energy 2022, 239, 122356. [Google Scholar] [CrossRef]
- Arama, F.Z.; Laribi, S.; Mammar, K.; Aoun, N.; Ghaitaoui, T. Efficient Water-Related Failure Detection in PEM Fuel Cells: Combining a PEMFCs Fractional Order Impedance Model with FFT-PWM Techniques and Artificial Neural Network Classification. Heliyon 2024, 10, e29084. [Google Scholar] [CrossRef]
- Ait Ziane, M.; Join, C.; Benne, M.; Damour, C.; Steiner, N.Y.; Pera, M.C. A New Concept of Water Management Diagnosis for a PEM Fuel Cell System. Energy Convers. Manag. 2023, 285, 116986. [Google Scholar] [CrossRef]
- Sun, W.; Peppley, B.A.; Karan, K. An Improved Two-Dimensional Agglomerate Cathode Model to Study the Influence of Catalyst Layer Structural Parameters. Electrochim. Acta 2005, 50, 3359–3374. [Google Scholar] [CrossRef]
- Gong, D.; Xu, S.; Gao, Y. Investigation of Water and Heat Transfer Mechanism in PEMFCs Based on a Two-Phase Non-Isothermal Model. Energies 2023, 16, 697. [Google Scholar] [CrossRef]
- Wang, X.-D.; Yan, W.-M.; Won, W.-C.; Lee, D.-J. Effects of Operating Parameters on Transport Phenomena and Cell Performance of PEM Fuel Cells with Conventional and Contracted Flow Field Designs. Int. J. Hydrogen Energy 2012, 37, 15808–15819. [Google Scholar] [CrossRef]
- Chauhan, V.; Mortazavi, M.; Benner, J.Z.; Santamaria, A.D. Two-phase flow characterization in PEM fuel cells using machine learning. Energy Rep. 2020, 6, 2713–2719. [Google Scholar] [CrossRef]
- Li, S.; Peng, C.; Shen, Q.; Cheng, Y.; Wang, C.; Yang, G. Numerical Study on Thermal Stress of High Temperature Proton Exchange Membrane Fuel Cells during Start-Up Process. Membranes 2023, 13, 215. [Google Scholar] [CrossRef]
- Hussaini, I.S.; Wang, C.-Y. Visualization and Quantification of Cathode Channel Flooding in PEM Fuel Cells. J. Power Sources 2009, 187, 444–451. [Google Scholar] [CrossRef]
- Kerres, J.A. Development of ionomer membranes for fuel cells. J. Membr. Sci. 2001, 185, 3–27. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative Polymer Systems for Proton Exchange Membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Vengatesan, S.; Kim, H.-J.; Cho, E.A.; Jeong, S.U.; Ha, H.Y.; Oh, I.-H.; Hong, S.-A.; Lim, T.-H. Operation of a Proton-Exchange Membrane Fuel Cell under Non-Humidified Conditions Using Thin Cast Nafion Membranes with Different Gas-Diffusion Media. J. Power Sources 2006, 156, 294–299. [Google Scholar] [CrossRef]
- Kang, Z.; Chen, Y.; Wang, H.; Alia, S.M.; Pivovar, B.S.; Bender, G. Discovering and Demonstrating a Novel High-Performing 2D-Patterned Electrode for Proton-Exchange Membrane Water Electrolysis Devices. ACS Appl. Mater. Interfaces 2022, 14, 535–545. [Google Scholar] [CrossRef]
- Park, G.-G.; Sohn, Y.-J.; Yang, T.-H.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Effect of PTFE contents in the gas diffusion media on the performance of PEMFC. J. Power Sources 2004, 131, 182–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, P.; Yang, S.; Su, J.; Guo, L. Enhancing the Performance of Proton-Exchange Membrane Fuel Cell by Optimizing the Hydrophobicity and Porosity of Cathode Catalyst Layer. Sci. China Technol. Sci. 2025, 68, 1320101. [Google Scholar] [CrossRef]
- Heo, W.; Han, D.-H.; Oh, S.-J.; Yoon, J.U.; Woo, I.; Choi, S.-E.; Yoon, J.-M.; Bae, J.W. Enhancing Polymer Electrolyte Membrane Fuel Cell Performance and Efficiency: Plasma-Treated Effects of Expanded Polytetrafluoroethylene in Reinforced Composite Membranes. J. Power Sources 2025, 642, 237010. [Google Scholar] [CrossRef]
- Jha, N.; Jafri, R.I.; Rajalakshmi, N.; Ramaprabhu, S. Graphene-Multi Walled Carbon Nanotube Hybrid Electrocatalyst Support Material for Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2011, 36, 7284–7290. [Google Scholar] [CrossRef]
- Carcadea, E.; Varlam, M.; Marinoiu, A.; Raceanu, M.; Ismail, M.S.; Ingham, D.B. Influence of Catalyst Structure on PEM Fuel Cell Performance—A Numerical Investigation. Int. J. Hydrogen Energy 2019, 44, 12829–12841. [Google Scholar] [CrossRef]
- Aubertin, M.; Aachib, M.; Authier, K. Evaluation of Diffusive Gas Flux through Covers with a GCL. Geotext. Geomembr. 2000, 18, 215–233. [Google Scholar] [CrossRef]
- Calili-Cankir, M.F.; Can, E.M.; Ingham, D.B.; Hughes, K.J.; Ma, L.; Pourkashanian, M.; Lyth, S.M.; Ismail, M.S. Patterned Hydrophobic Gas Diffusion Layers for Enhanced Water Management in Polymer Electrolyte Fuel Cells. Chem. Eng. J. 2024, 484, 149711. [Google Scholar] [CrossRef]
- Adroher, X.C.; Wang, Y. Ex situ and Modeling Study of Two-Phase Flow in a Single Channel of Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2011, 196, 9544–9551. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, L.; Trogadas, P.; Rasha, L.; Du, W.; Shearing, P.R.; Coppens, M.-O.; Brett, D.J.; Jervis, R. Effects of an Easy-to-Implement Water Management Strategy on Performance and Degradation of Polymer Electrolyte Fuel Cells. J. Power Sources 2023, 575, 233184. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water Transport in Polymer Electrolyte Membrane Fuel Cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Ijaodola, O.S.; El-Hassan, Z.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Thompson, J.; Olabi, A.G. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- El-Hameed, M.A.; Saeed, M.; Kabbani, A.; El-Hay, E.A. Enhancing Performance of PEM Fuel Cell Powering SRM System Using Metaheuristic Optimization. Energies 2025, 18, 2004. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Shin, H.-S.; Cheon, S.-H.; Oh, B.S. A Study of Numerical Analysis for PEMFC Using a Multiphysics Program and Statistical Method. Int. J. Hydrogen Energy 2015, 40, 11577–11586. [Google Scholar] [CrossRef]
- Mortazavi, M.; Tajiri, K. Two-phase Flow Pressure Drop in PEM Fuel Cells: A Review. Renew. Sustain. Energy Rev. 2015, 45, 296–317. [Google Scholar] [CrossRef]
- Dunbar, Z.W.; Masel, R.I. Magnetic Resonance Imaging Investigation of Water Accumulation and Transport in Graphite Flow Fields in a Polymer Electrolyte Membrane Fuel Cell: Do Defects Control Transport? J. Power Sources 2008, 182, 76–82. [Google Scholar] [CrossRef]
- St-Pierre, J. PEMFC In Situ Liquid-Water-Content Monitoring Status. J. Electrochem. Soc. 2007, 154, B724–B731. [Google Scholar] [CrossRef]
- Tsushima, S.; Teranishi, K.; Hirai, S. Magnetic Resonance Imaging of the Water Distribution within a Polymer Electrolyte Membrane in Fuel Cells. Electrochem. Solid-State Lett. 2004, 7, A269–A272. [Google Scholar] [CrossRef]
- Tsushima, S.; Teranishi, K.; Nishida, K.; Hirai, S. Water content distribution in a polymer electrolyte membrane for advanced fuel cell system with liquid water supply. Magn. Reson. Imaging 2005, 23, 255–258. [Google Scholar] [CrossRef]
- Minard, K.; Viswanathan, V.; Majors, P.; Wang, L.; Rieke, P. Magnetic Resonance Imaging (MRI) of PEM Dehydration and Gas Manifold Flooding During Continuous Fuel Cell Operation. J. Power Sources 2006, 161, 856–863. [Google Scholar] [CrossRef]
- Bedet, J.; Maranzana, G.; Leclerc, S.; Lottin, O.; Moyne, C.; Stemmelen, D.; Mutzenhardt, P.; Canet, D. Magnetic Resonance Imaging of Water Distribution and Production in a 6 cm2 PEMFC under Operation. Int. J. Hydrogen Energy 2008, 33, 3146–3149. [Google Scholar] [CrossRef]
- Tötzke, C.; Nikolay, K.; Ingo, M.; Sebastian, C.B.; Alexander, H.; Markus, O.; Henning, M.; Tillmann, N.; Rebecca, B.; Michael, Z.; et al. Real-time in-operando visualization of liquid water transport in a PEM fuel cell using time-resolved neutron radiography. J. Power Sources 2017, 342, 136–144. [Google Scholar] [CrossRef]
- Zhan, Z.; Wang, C.; Fu, W.; Pan, M. Visualization of Water Transport in a Transparent PEMFC. Int. J. Hydrogen Energy 2012, 37, 1094–1105. [Google Scholar] [CrossRef]
- Lee, S.J.; Lim, N.-Y.; Kim, S.; Park, G.-G.; Kim, C.-S. X-ray Imaging of Water Distribution in a Polymer Electrolyte Fuel Cell. J. Power Sources 2008, 185, 867–870. [Google Scholar] [CrossRef]
- Manke, I.; Hartnig, C.; Grunerbel, M.; Lehnert, W.; Kardjilov, N.; Haibel, A. Investigation of Water Evolution and Transport in Fuel Cells with High Resolution Synchrotron X-Ray Radiography. Appl. Phys. Lett. 2007, 90, 174105. [Google Scholar] [CrossRef]
- Friess, B.R.; Hoorfar, M. Development of a Novel Radial Cathode Flow Field for PEMFC. Int. J. Hydrogen Energy 2012, 37, 7719–7729. [Google Scholar] [CrossRef]
- Han, S.H.; Choi, N.H.; Choi, Y.D. Study on the Flooding Phenomena and Performance Enhancement of PEM Fuel Cell Applying a Concus-Finn Condition. Renew. Energy 2012, 44, 88–98. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, Y.; Wang, Z.; Qu, L.; Shao, Z.; Yuan, Z.; Yi, B. Improvement of PEMFC Performance and Endurance by Employing Continuous Silica Film Incorporated Water Transport Plate. Electrochim. Acta 2016, 191, 116–123. [Google Scholar] [CrossRef]
- Jung, U.H.; Jeong, S.U.; Park, K.T.; Lee, H.M.; Chun, K.; Choi, D.W.; Kim, S. Improvement of water management in air-breathing and air-blowing PEMFC at low temperature using hydrophilic silica nano-particles. Int. J. Hydrogen Energy 2007, 32, 4459–4465. [Google Scholar] [CrossRef]
- Kumar, P.M.; Parthasarathy, V. A Passive Method of Water Management for an Air-Breathing Proton Exchange Membrane Fuel Cell. Energy 2013, 51, 457–461. [Google Scholar] [CrossRef]
- Chen, R.; Qin, Y.; Ma, S.; Du, Q. Numerical Simulation of Liquid Water Emerging and Transport in the Flow Channel of PEMFC Using the Volume of Fluid Method. Int. J. Hydrogen Energy 2020, 45, 29861–29873. [Google Scholar] [CrossRef]
- Xion, Z.; Liao, S.; Dang, D.; Tian, X.; Hou, S.; Liu, F.; Peng, H.; Fu, Z. Enhanced Water Management in the Cathode of an Air-Breathing PEMFC Using a Dual Catalyst Layer and Optimizing the Gas Diffusion and Microporous Layers. Int. J. Hydrogen Energy 2015, 40, 3961–3967. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Sun, S.; Shao, Z.; Yi, B. Improvement of PEMFC Water Management by Employing Water Transport Plate as Bipolar Plate. Int. J. Hydrogen Energy 2017, 42, 21922–21929. [Google Scholar] [CrossRef]
- Liu, Q.; Lan, F.; Chen, J.; Zeng, C.; Wang, J. A Review of Proton Exchange Membrane Fuel Cell Water Management: Membrane Electrode Assembly. J. Power Sources 2022, 517, 230723. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, X.; Liu, G.; Xu, H.; Guan, C.; Wang, H.; Li, H.; He, W.; Qin, Y. Review of Flow Field Designs for Polymer Electrolyte Membrane Fuel Cells. Energies 2023, 16, 4207. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent Advances on PEM Fuel Cells: From Key Materials to Membrane Electrode Assembly. Electrochem. Energy Rev. 2023, 6, 28. [Google Scholar] [CrossRef]
- Lü, X.; Qu, Y.; Wang, Y.; Qin, C.; Liu, G. A Comprehensive Review on Hybrid Power System for PEMFC-HEV: Issues and Strategies. Energy Convers. Manag. 2018, 171, 1273–1291. [Google Scholar] [CrossRef]
- Cao, J.; Su, C.; Ji, Y.; Yang, G.; Shao, Z. Recent Advances and Perspectives of Fluorite and Perovskite-Based Dual-Ion Conducting Solid Oxide Fuel Cells. J. Energy Chem. 2021, 57, 406–427. [Google Scholar] [CrossRef]
- Ogawa, T.; Kamiguchi, K.; Tamaki, T.; Imai, H.; Yamaguchi, T. Differentiating Grotthuss Proton Conduction Mechanisms by Nuclear Magnetic Resonance Spectroscopic Analysis of Frozen Samples. Anal. Chem. 2014, 86, 9362–9366. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.; Liu, C.; Chen, W. Molecular Study of Nonequilibrium Transport Mechanism for Proton and Water in Porous Proton Exchange Membranes. Int. J. Energy Res. 2023, 2023, 1138198. [Google Scholar] [CrossRef]
- Min, T.; Zhang, R.; Chen, L.; Zhou, Q. Reactive Transport Processes in Proton Exchange Membrane Fuel Cells. Encyclopedia 2023, 3, 746–758. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. Gas Diffusion Layer Modifications and Treatments for Improving the Performance of Proton Exchange Membrane Fuel Cells and Electrolysers: A Review. Int. J. Hydrogen Energy 2017, 42, 28515–28536. [Google Scholar] [CrossRef]
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing Water Transport Performance of Gas Diffusion Layers through Coupling Manipulation of Pore Structure and Hydrophobicity. J. Power Sources 2022, 525, 231121. [Google Scholar] [CrossRef]
- Li, L.; Ye, D.; Xiang, Y.; Guo, W. Effect of Deposition Temperature on Columnar Structure of α-C Nano-Coatings of PEMFC Metal Bipolar Plates. Int. J. Electrochem. Sci. 2023, 18, 100188. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, X.; Chang, L.; Dong, C. Response Characteristics of Platinum Coated Titanium Bipolar Plates for Proton Exchange Membrane Water Electrolysis under Fluctuating Conditions. Electrochem. Commun. 2024, 168, 107819. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wan, Z.; Chen, Y.; Tan, J.; Pan, M. Effect of Hydrophobic Additive on Oxygen Transport in Catalyst Layer of Proton Exchange Membrane Fuel Cells. J. Power Sources 2018, 379, 338–343. [Google Scholar] [CrossRef]
- Deng, S.; Hao, M.; Wang, R.; Zhang, J.; Zhang, X.; Li, Y. Improving Water Retention and Mass Transport for Low-Humidity Proton Exchange Membrane Fuel Cells via a Porous-Channel Interdigitated Flow Field. Int. J. Hydrogen Energy 2024, 95, 874–887. [Google Scholar] [CrossRef]
- Di Noto, V.; Bettiol, M.; Bassetto, F.; Boaretto, N.; Negro, E.; Lavina, S.; Bertasi, F. Hybrid Inorganic-Organic Nanocomposite Polymer Electrolytes Based on Nafion and Fluorinated TiO2 for PEMFCs. Int. J. Hydrogen Energy 2012, 37, 6169–6181. [Google Scholar] [CrossRef]
- Ahmed, M.; Shusheng, X. Comprehensive Review of Serpentine Flow Fields for Effective Thermal Management of Proton Exchange Membrane Fuel Cell. Heliyon 2024, 10, e39793. [Google Scholar] [CrossRef]
- Qi, L.; Liu, H.; Zhang, J.; Chen, Z. Effect of Channel Pass Number on Proton Exchange Membrane Fuel Cell (PEMFC) Performance and Flow Distribution Uniformity. Sustainability 2023, 15, 10389. [Google Scholar] [CrossRef]
- Asadi, M.R.; Ghasabehi, M.; Ghanbari, S.; Shams, M. The Optimization of an Innovative Interdigitated Flow Field Proton Exchange Membrane Fuel Cell by Using Artificial Intelligence. Energy 2024, 290, 130131. [Google Scholar] [CrossRef]
- Rocha, C.; Knöri, T.; Ribeirinha, P.; Gazdzicki, P. A Review on Flow Field Design for Proton Exchange Membrane Fuel Cells: Challenges to Increase the Active Area for MW Applications. Renew. Sustain. Energy Rev. 2024, 192, 114198. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Li, X.; Guo, X.; Wang, T.; Li, S.; Lei, H. Numerical Simulation and Visualization Study of a New Tapered-Slope Serpentine Flow Field in Proton Exchange Membrane Fuel Cell. Energy 2022, 246, 123406. [Google Scholar] [CrossRef]
- Palaniswamy, K.; Marappan, M.; Jothi, V.R. Influence of Porous Carbon Inserts on Scaling up Studies for Performance Enhancement on PEMFC. Int. J. Hydrogen Energy 2016, 41, 2867–2874. [Google Scholar] [CrossRef]
- Chen, C.-H.; Jung, S.-P.; Yen, S.-C. Flow Distribution in the Manifold of PEM Fuel Cell Stack. J. Power Sources 2007, 173, 249–263. [Google Scholar] [CrossRef]
- Aslam, R.M.; Ingham, D.B.; Ismail, M.S.; Hughes, K.J.; Ma, L.; Pourkashanian, M. Simultaneous Direct Visualisation of Liquid Water in the Cathode and Anode Serpentine Flow Channels of Proton Exchange Membrane (PEM) Fuel Cells. J. Energy Inst. 2018, 91, 1057–1070. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Transport in polymer-electrolyte membranes: I. J. Electrochem. Soc. 2004, 151, A311–A325. [Google Scholar] [CrossRef]
- Büchi, F.N.; Gupta, B.; Haas, O.; Scherer, G.G. Study of Radiation-Grafted FEP-G-Polystyrene Membranes as Polymer Electrolytes in Fuel Cells. Electrochim. Acta 1995, 40, 345–353. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Havaej, P. A Numerical Investigation of the Performance of Polymer Electrolyte Membrane Fuel Cell with the Converging-Diverging Flow Field Using Two-Phase Flow Modeling. Energy 2019, 182, 656–672. [Google Scholar] [CrossRef]
- Yuan, W.-W.; Ou, K.; Jung, S.; Kim, Y.-B. Analyzing and Modeling of Water Transport Phenomena in Open-Cathode Polymer Electrolyte Membrane Fuel Cell. Appl. Sci. 2021, 11, 5964. [Google Scholar] [CrossRef]
- Choi, H.; Choi, H.; Choi, H.J.; Kim, J.; Kim, O.-H.; Kim, Y.; Sung, S.Y.; Eom, D.; Park, S.; Ahn, C.-Y.; et al. Polymer Electrolyte Membrane Fuel Cell Durability Test Using Ship Operation Profile: A Comparative Study with Durability Test Protocols. J. Power Sources 2025, 632, 236396. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, J.; Liu, Z. Advancements and Insights in Thermal and Water Management of Proton Exchange Membrane Fuel Cells: Challenges and Prospects. Int. Commun. Heat Mass Transf. 2024, 153, 107376. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, C.; Zou, J.; Zaman, S.; Yu, Y.; Gong, H.; Wang, Y.; Chen, M.; Wang, M.; Lin, M.; et al. Recent Advances in Heat and Water Management of Forced-Convection Open-Cathode Proton Exchange Membrane Fuel Cells. Renew. Sustain. Energy Rev. 2022, 165, 112558. [Google Scholar] [CrossRef]
- Duan, Y.; Li, Y.; To, D.; Zhang, J.; Chen, J.; Ran, H.; Fan, M. Advanced Online Fuel Cell Stack Water Management Strategies for Fuel Cell Stacks in Vehicle Powertrain Control. Front. Energy Res. 2025, 13, 1457052. [Google Scholar] [CrossRef]
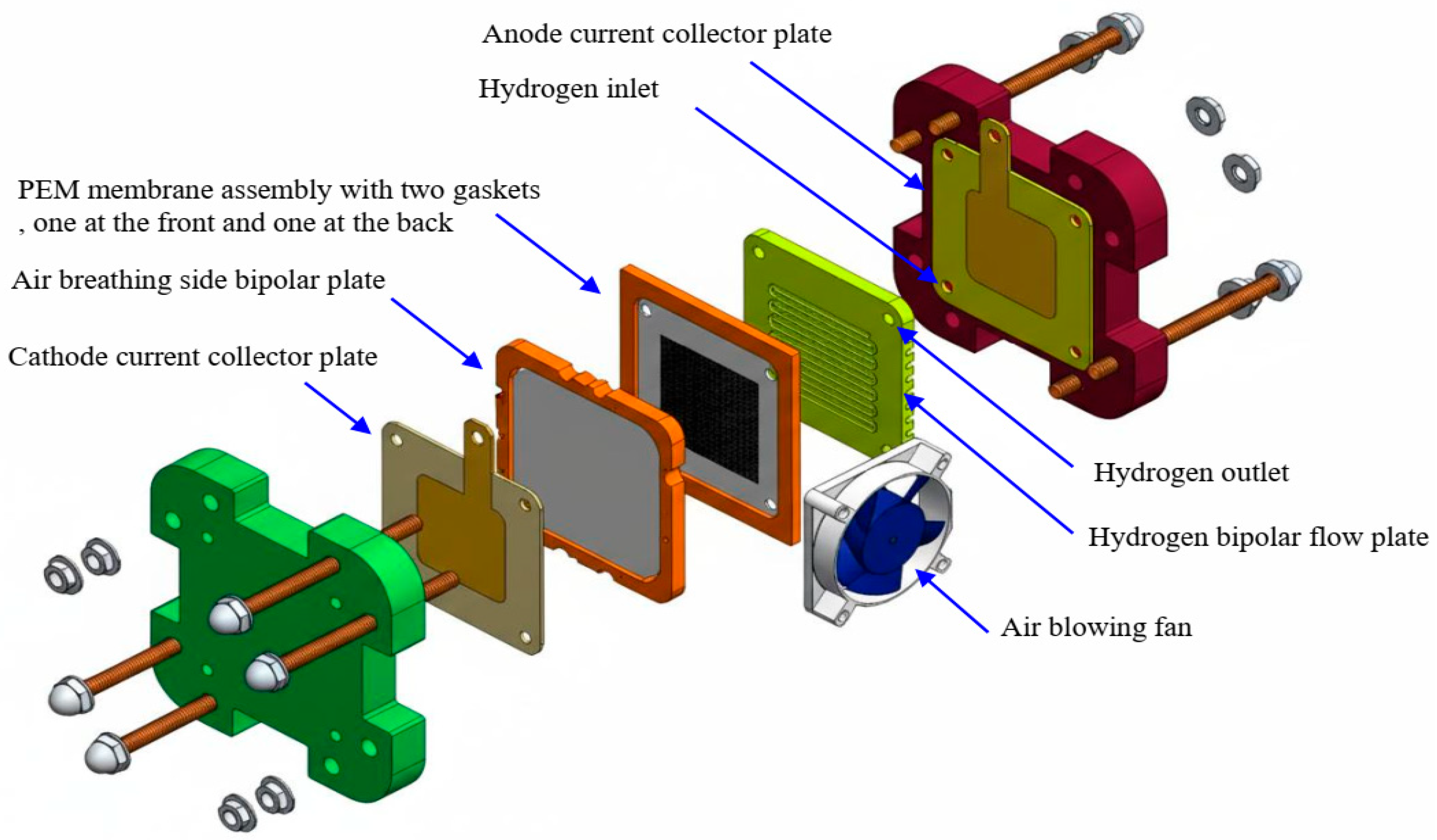
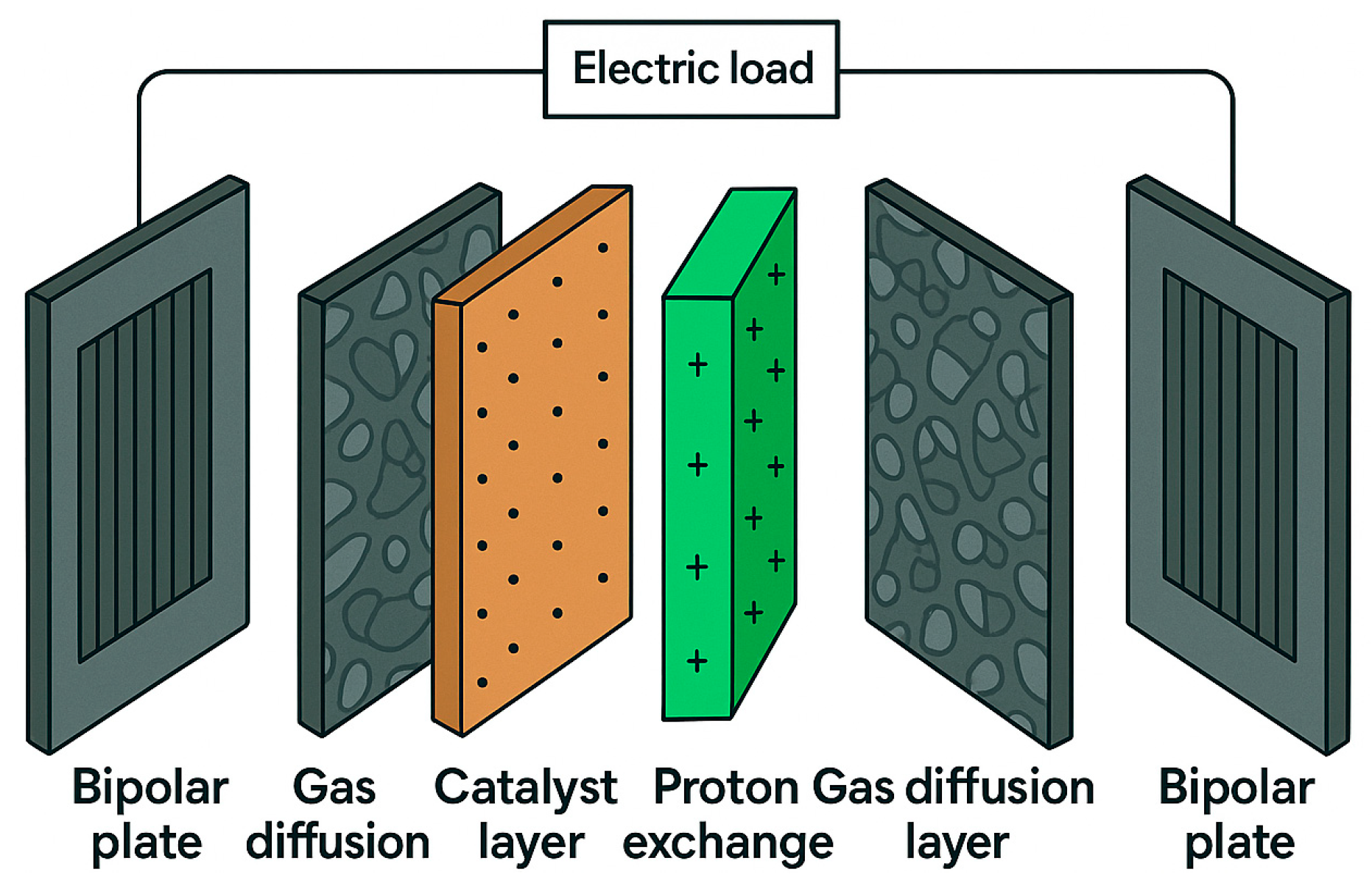
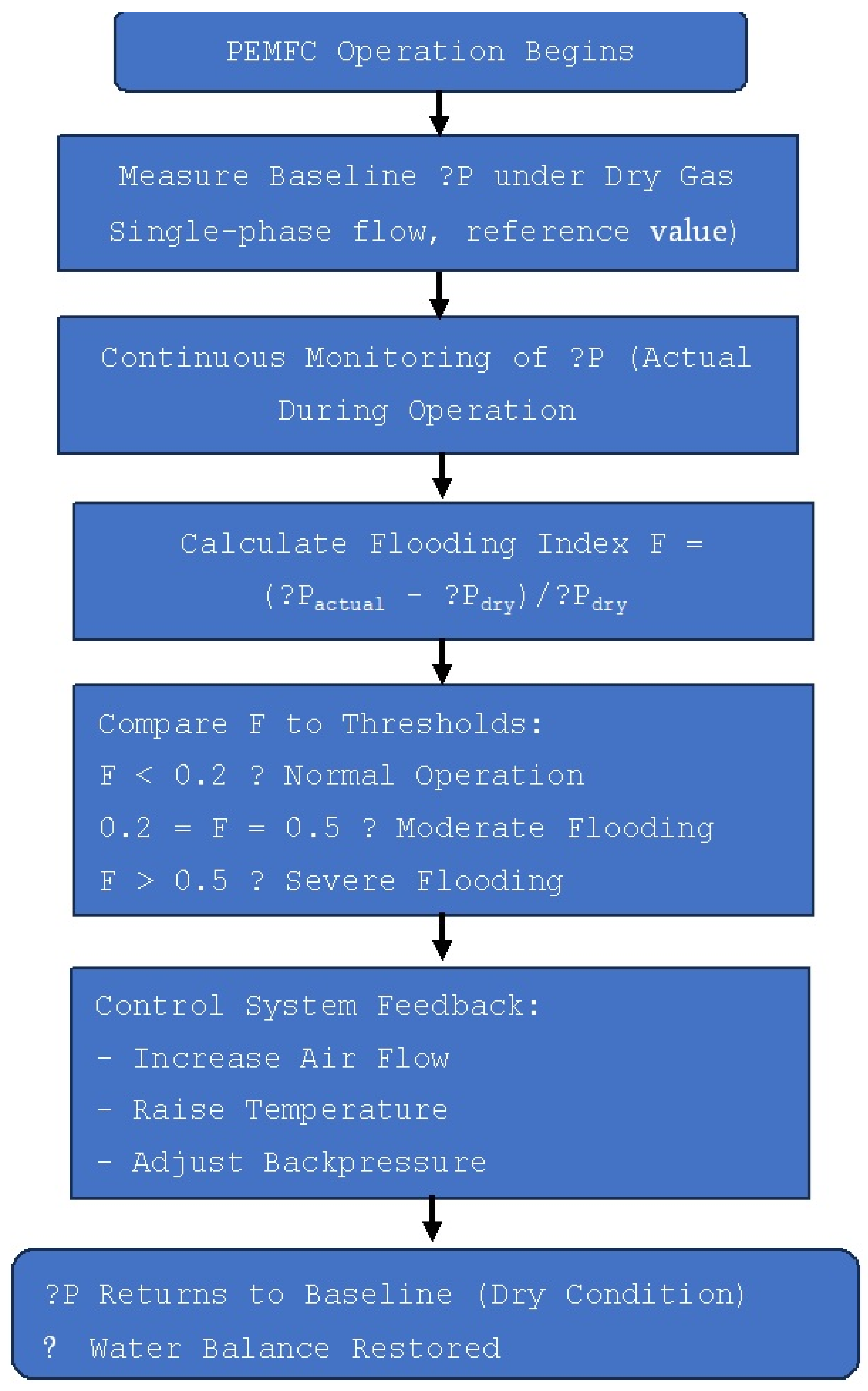
| Mechanism | Transport Principle | Typical Flow Field Designs | Advantages | Limitations | Operating Conditions |
|---|---|---|---|---|---|
| Under-Rib Convection | Pressure-driven flow forces liquid water from the gas diffusion layer beneath the rib towards the channel under differential pressure. | Serpentine flow fields with narrow ribs; interdigitated configurations | Strong water removal; prevents flooding under ribs; improves oxygen access | High pumping losses; risk of local dehydration; uneven current density | High current density; moderate–high stoichiometry |
| Shear-Driven Removal | Gas velocity exerts shear stress that detaches droplets from channel walls | Parallel or serpentine flow fields with moderate velocities | Promotes continuous droplet detachment; low structural complexity; stable operation | Less effective under low gas flow rates; sensitive to surface wettability | Mid-range flow rates; moderate humidity |
| Uniformity/Low Δp Design | Improves flow distribution uniformity across the active area with minimal pressure drop | Parallel, multi-pass, or bio-inspired flow fields | Low energy consumption; improved hydration stability; easier scaling | Less effective in severe flooding; limited forced convection effect | Low–medium current density; optimized water balance needed |
| Parameter | Condition | Nafion 117 (Thick, EW 1100) | Nafion 212 (Thin, EW 1100) | Impact on Water Balance |
|---|---|---|---|---|
| Electro-osmotic drag coefficient (nd) | 30 °C, 100% RH | 0.9–1.2 H2O/H+ | 1.1–1.4 H2O/H+ | Drives water from anode → cathode |
| Electro-osmotic drag coefficient (nd) | 80 °C, 50% RH | 1.6–2.0 H2O/H+ | 2.1–2.5 H2O/H+ | Increases with temperature |
| Back diffusion coefficient (Dw) | 30 °C, 50% RH | 1.5 × 10−10 m2/s | 2.0 × 10−10 m2/s | Opposes EOD; higher in thinner membranes |
| Membrane thickness | — | 178 µm | 50 µm | Thinner membranes → faster equilibration |
| Hydration level (λ) | Cathode | 14–22 | 12–20 | Depends on RH and current load |
| Dominant flux at low current | 0.1 A/cm2 | Back diffusion | Balanced | Cathode drying risk |
| Dominant flux at high current | ≥1 A/cm2 | EOD | Strong EOD | Cathode flooding risk |
| Stress Factor/Condition | Water Management Impact | Durability Mechanism | Observed Effect | Relevance to Load Cycling |
|---|---|---|---|---|
| Membrane dehydration (low RH, insufficient humidification) | Reduced membrane water content | Increase in ohmic resistance (ΔR_ohmic), polymer chain scission | Voltage decay, membrane thinning | Repeated dry/wet cycles lead to mechanical fatigue |
| Cyclic dry-out and rehydration | Osmotic stress in the membrane | Crack formation, pinhole initiation | Gas crossover, reduced OCV | Strongly linked to duty cycling in automotive PEMFCs |
| Cathode flooding (excess water in GDL/flow channels) | Water accumulation blocks O2 transport | Mass transport loss in ORR | Voltage loss at high current | Starts during dynamic load shifts + inadequate water removal |
| High current density (>1.2 A cm−2) | Increased water production | Local thermal stress and dehydration at the inlet | Hot spots, carbon support corrosion | Accelerated by transient high power demand |
| High temperature operation (>80 °C) | Faster dehydration and membrane shrinkage | Radical attack (•OH), chemical degradation | Loss of proton conductivity | Thermal cycling increases stress on the membrane |
| Potential cycling (start-stop/load cycling) | Water imbalance in the catalyst layer | Pt dissolution, agglomeration, carbon corrosion | ECSA loss, voltage decay | Major factor in automotive PEMFC durability |
| Low-pressure operation | Poor liquid water removal | Flooding-induced transport limitation | Efficiency reduction | Load cycling worsens transient water control |
| Model Type | Complexity | Spatial Detail | Computational Cost | Typical Applications | Accuracy Range | Validation Status |
|---|---|---|---|---|---|---|
| 0D | Low | None | Very low | System-level control, energy management | Low–Moderate | Experimental, literature |
| 1D | Moderate | Through-plane | Low–Moderate | MEA hydration, voltage-current prediction | Moderate | Partially validated |
| 2D/3D | High | Full geometry | High | Design studies, performance optimization | High | Validated with experiments |
| Two-phase | Very High | Multiphase | Very high | Water removal, flooding analysis | High | Validated, CFD comparison |
| Advanced | Varies | Embedded/learned | Low–Moderate | Real-time prediction, control systems | Moderate–High | Validated, data-driven |
| Modality | Spatial/Temporal Resolution | What It Measures | Operational Constraints | Key PEMFC Water Findings |
|---|---|---|---|---|
| Neutron Imaging | ~10–25 μm spatial; ~1–2 s temporal | Liquid water distribution | Requires access to reactor facility; limited portable use; beam time required. | Reveals initial water accumulation near the GDL–channel interface; shows channel vs. under-rib flooding. |
| X-ray Computed Tomography (XCT) | ~1–5 μm spatial; static imaging | Liquid water saturation, GDL structure | High radiation dose; complex during dynamic PEMFC operation | Shows water transport paths in GDL and MPL; capillary behavior |
| Magnetic Resonance Imaging (MRI) | 50–200 μm spatial; 100–500 ms temporal | Water distribution in porous layers | Magnetic field limits materials; complex setup | Reveals internal membrane hydration dynamics |
| Optical Visualization | ~1–10 μm spatial; video-rate temporal | Liquid droplets in channels | Requires transparent cell/window; only 2D surface data | Shows droplet growth and detachment regime; channel blockages |
| Infrared Thermography | ~50 μm spatial; ms temporal | Surface temperature → water effects indirectly | Limited to surface; emissivity correction needs | Identifies hot spots from dry-out or flooding |
| Water Management Strategy | Description | Cost Implication | Performance Impact | Technology Readiness Level (TRL) | Notes |
|---|---|---|---|---|---|
| Hydrophobic GDL (PTFE-treated carbon paper) | Enhances liquid water transport and prevents flooding | Low–Medium | Improves water removal and gas diffusion | TRL 9 (Commercial) | Widely used in automotive PEMFC |
| Microporous Layer (MPL) | Added between GDL and catalyst layer to improve water distribution | Medium | Better water balance, reduced membrane dehydration | TRL 8–9 | Common in commercial stacks |
| Hydrophilic/Hydrophobic Gradient GDLs | Gradient design enhances capillary-driven water transport | Medium–High | Significant flooding control, stable performance | TRL 6–7 | Used in advanced research systems |
| Membrane Humidification (external humidifier) | Humidifies inlet gases to prevent dry-out | Medium–High | Ensures membrane conductivity | TRL 9 | Older commercial systems still use this |
| Self-Humidifying Membranes (Nafion + hygroscopic fillers) | Water generated retained by membrane additives | Medium | Reduces system complexity | TRL 7–8 | Used in portable/low-temp PEMFC |
| Thermal Management (coolant plates) | Controls cell temperature to manage vapor–liquid balance | Medium–High | Improves stability under transient load | TRL 9 | Standard in high-power systems |
| Wicks/Capillary Structures | Passive water transport via porous wicking layers | Low | Simplifies water control | TRL 4–5 | Prototype systems |
| Electro-Osmotic Drag (EOD) Enhancement | Adjusting membrane chemistry for balanced water transport | Medium–High | Uniform hydration | TRL 3–4 | Active research |
| Two-Phase Flow Control via Back Pressure | Gas flow pressure control aids water removal | Low | Effective, but increases parasitic loss | TRL 8–9 | Common method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; El-Hameed, M.A.; Al-Hajri, E.; Kabbani, A. Water Management Strategies for Proton Exchange Membrane Fuel Cells: A Comprehensive Review. Electrochem 2025, 6, 38. https://doi.org/10.3390/electrochem6040038
Saeed M, El-Hameed MA, Al-Hajri E, Kabbani A. Water Management Strategies for Proton Exchange Membrane Fuel Cells: A Comprehensive Review. Electrochem. 2025; 6(4):38. https://doi.org/10.3390/electrochem6040038
Chicago/Turabian StyleSaeed, Mahfouz, Mohamed A. El-Hameed, Essa Al-Hajri, and Adnan Kabbani. 2025. "Water Management Strategies for Proton Exchange Membrane Fuel Cells: A Comprehensive Review" Electrochem 6, no. 4: 38. https://doi.org/10.3390/electrochem6040038
APA StyleSaeed, M., El-Hameed, M. A., Al-Hajri, E., & Kabbani, A. (2025). Water Management Strategies for Proton Exchange Membrane Fuel Cells: A Comprehensive Review. Electrochem, 6(4), 38. https://doi.org/10.3390/electrochem6040038







