Metal–Organic Frameworks for Seawater Electrolysis and Hydrogen Production: A Review
Abstract
1. Introduction
2. Seawater for Green Hydrogen Production
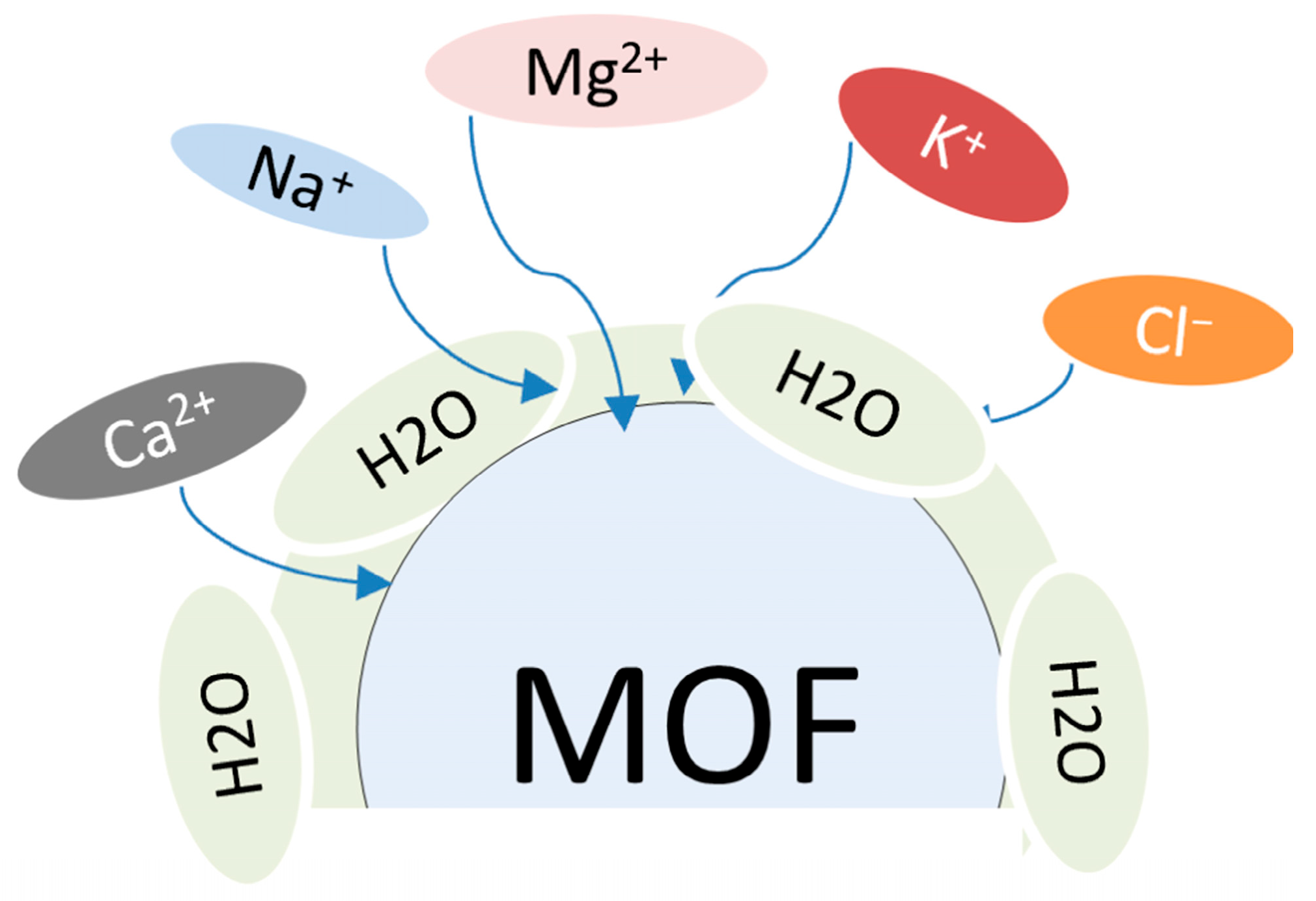
The Content of Salts in Seawater
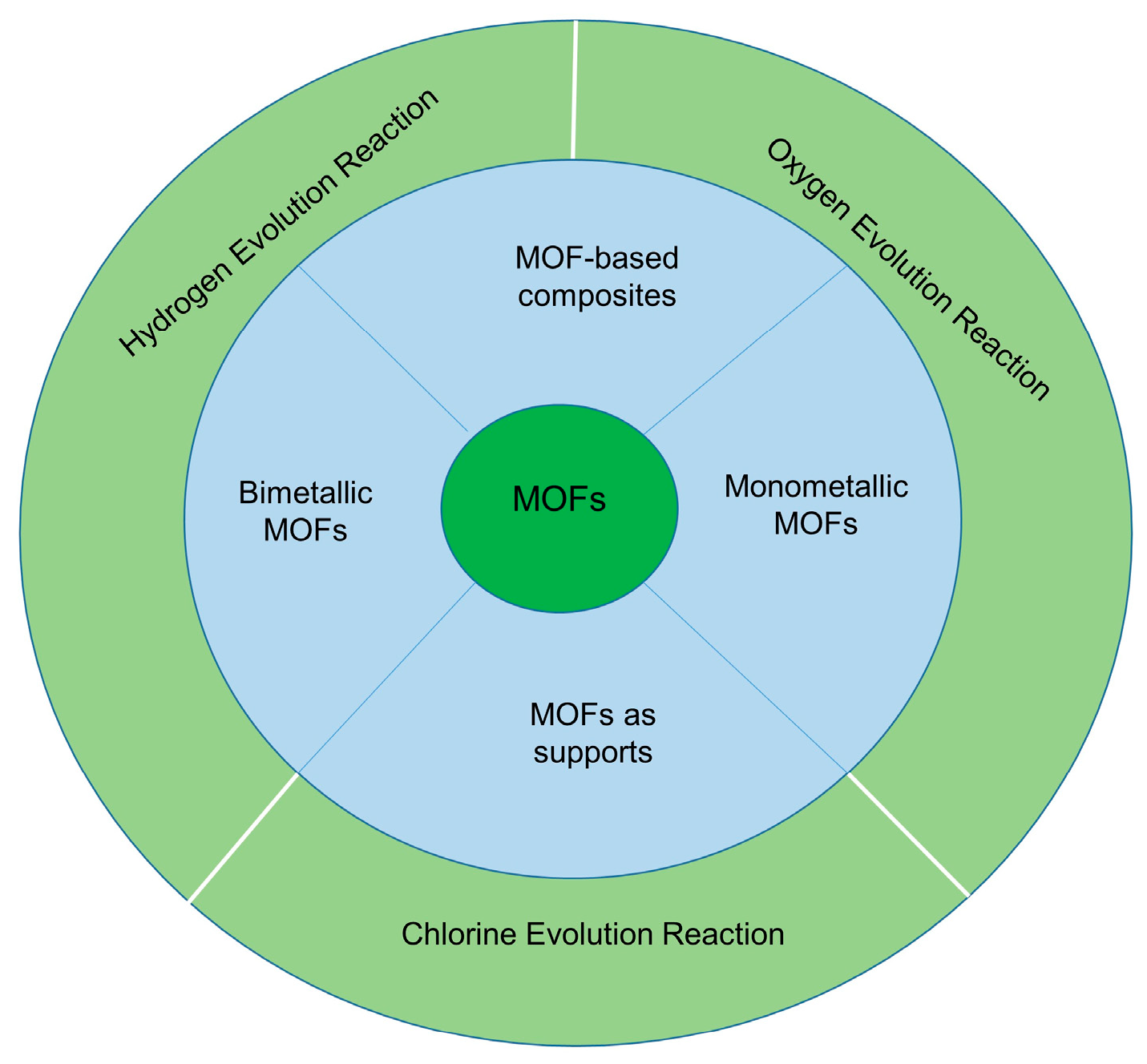
3. Metal–Organic Framework Electrocatalysts for Seawater Electrolysis
- MOFs possess high surface area and porosity, which provides additional active sites for catalytic reactions, making them very promising for advancing sustainable energy solutions.
- The tunable chemical structure of MOFs by modifying its surface with various metal nodes and organic linkers allows tailoring the structure depending on the further application.
- MOFs are very versatile, as they can be easily functionalized with various metal nanoparticles, improving catalytic sites and performance in hydrogen production.
- MOFs can be easily synthesized by green synthesis methods, facilitating scalable and environmentally friendly production [55].
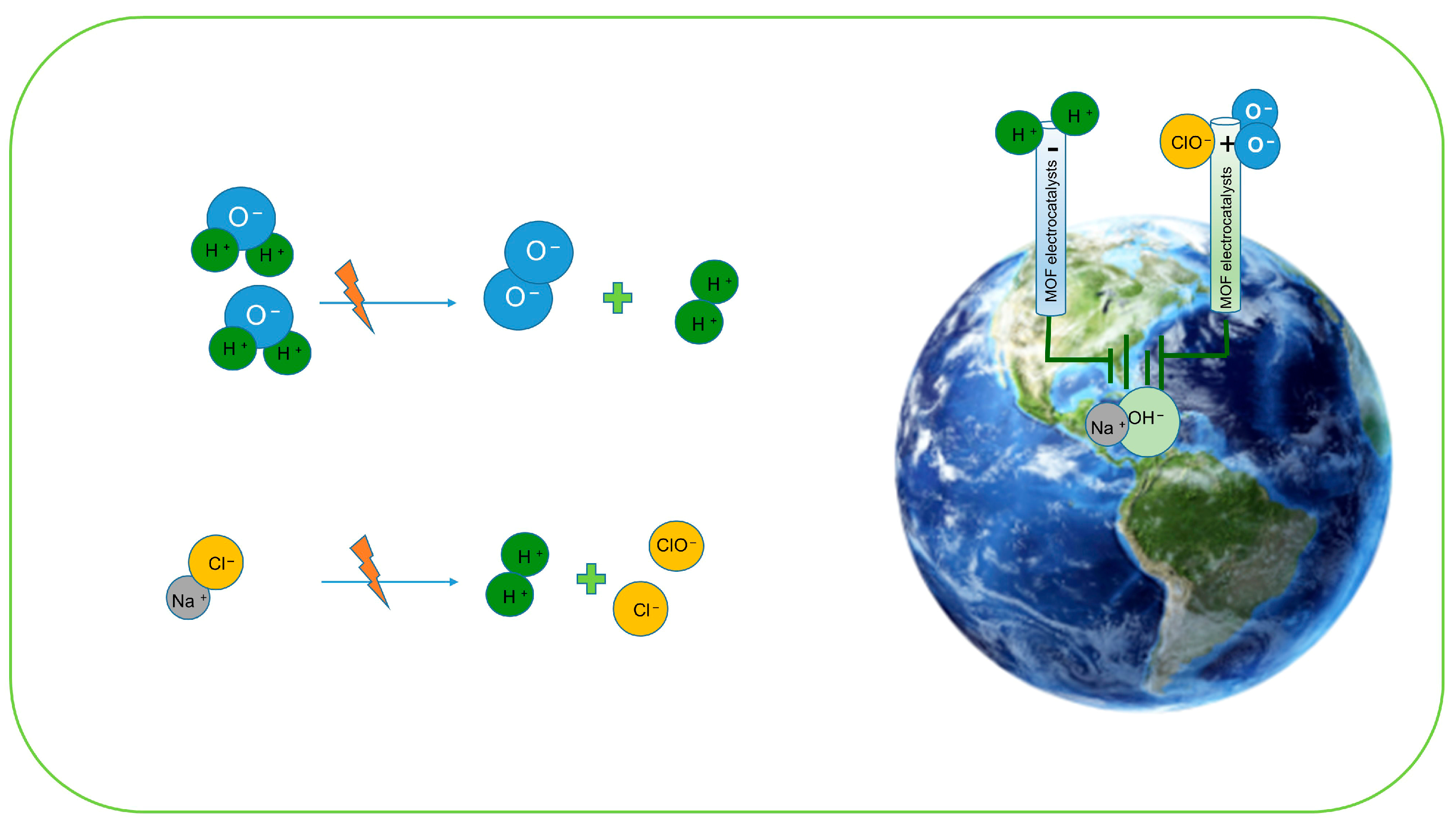
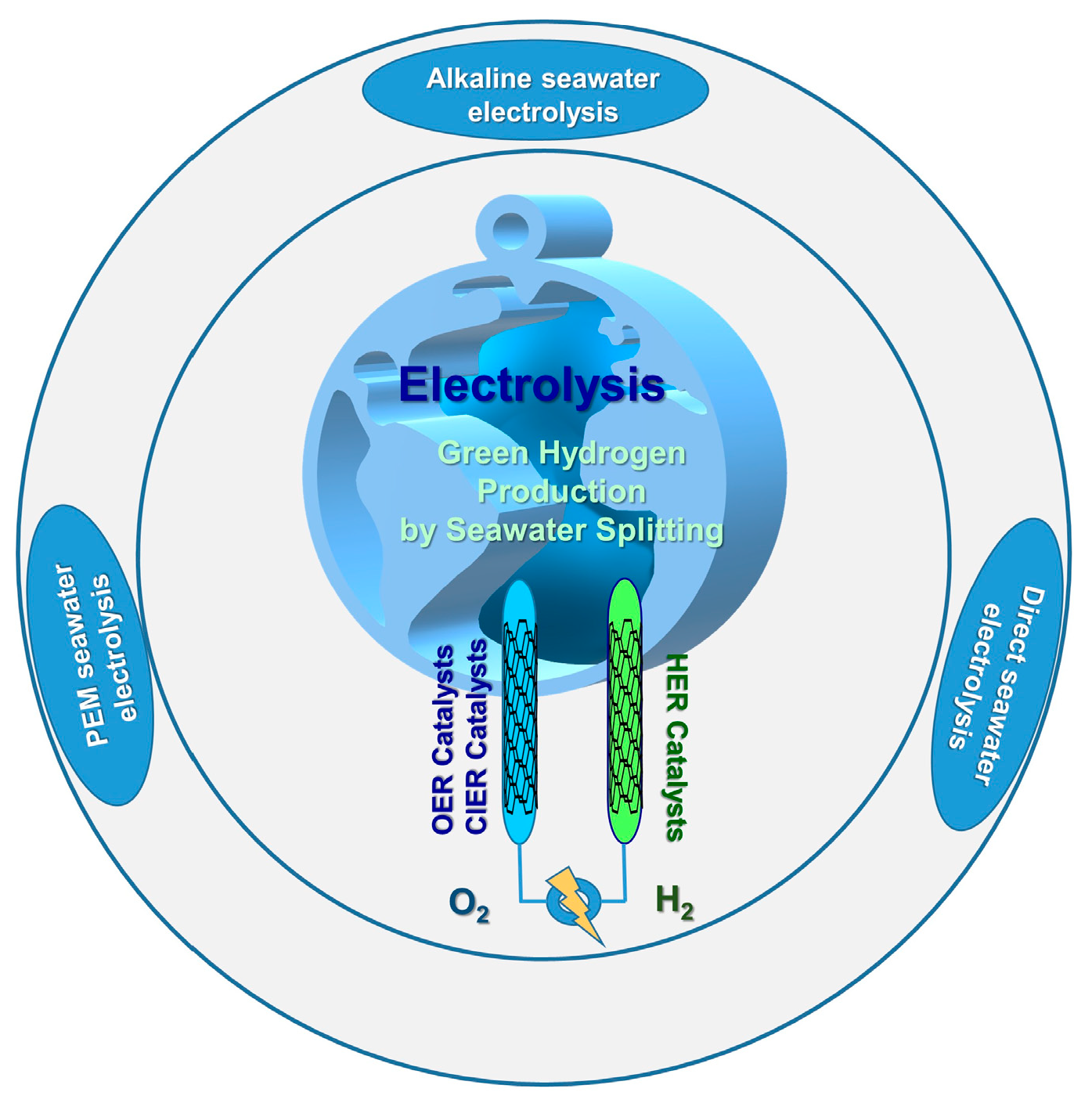
4. Electrochemical Green Hydrogen Production by Seawater Splitting
4.1. MOF-Based Approaches to Prevent Chloride-Induced Corrosion and Undesired Side Reactions During Seawater Electrolysis
4.1.1. Enhanced Electrostatic Repulsion Force
4.1.2. Design of MOF Electrocatalysts
4.1.3. Functionalizing MOFs with Lewis Acids
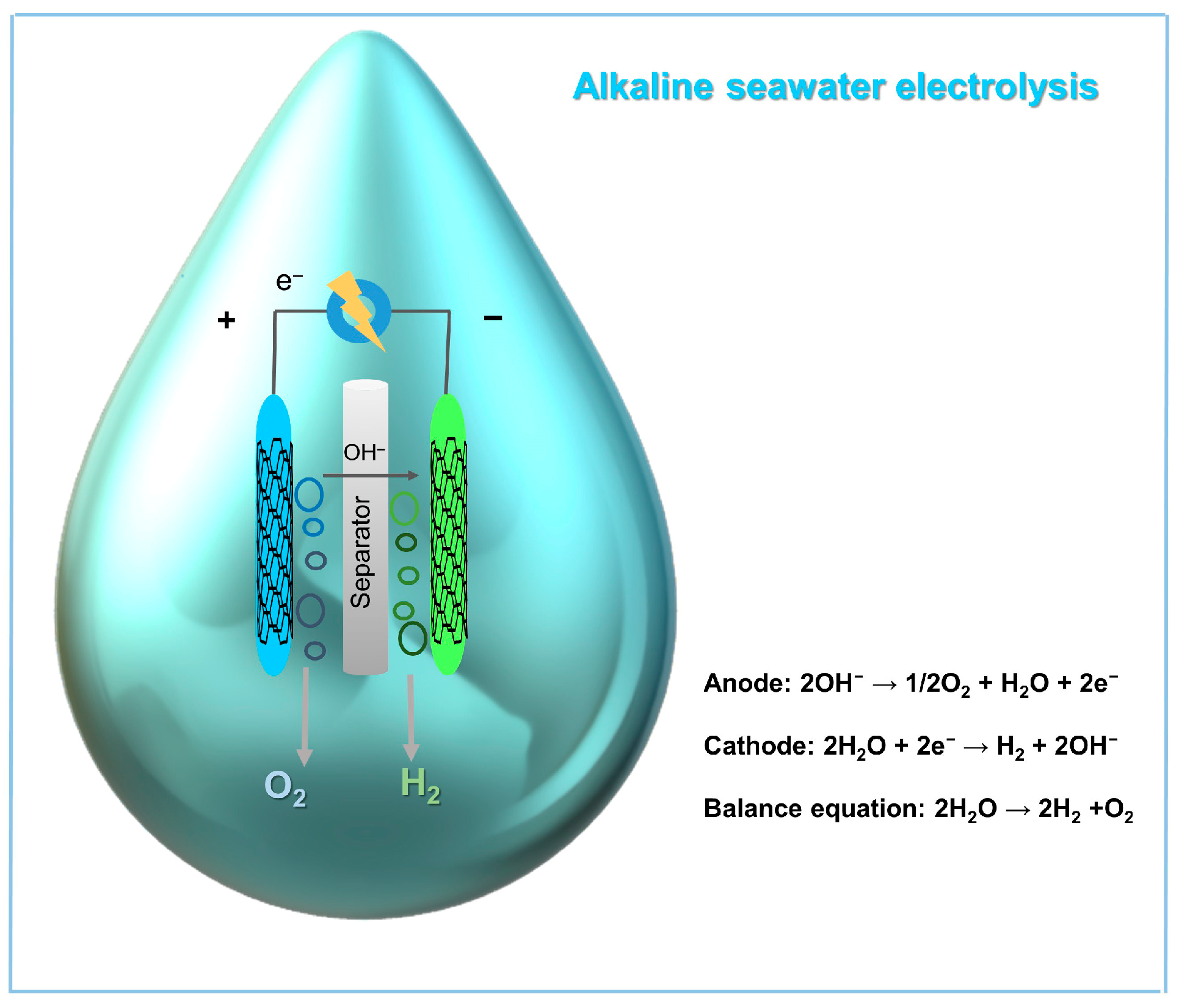
4.2. MOF-Based Electrocatalysts for Alkaline Seawater Electrolysis
4.3. MOF-Based Electrocatalysts for Proton Exchange Membrane Seawater Electrolysis
4.4. MOF-Based Electrocatalysts for Direct Seawater Electrolysis
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEM | Anion Exchange Membrane |
| AI | Artificial Intelligence |
| ASWE | Alkali Seawater Electrolysis |
| BDC | 1,4-Benzenedicarboxylate |
| BPM | Bipolar Membrane |
| CER | Chlorine Evolution Reaction |
| CF | Copper Foam |
| DFT | Density-Functional Theory |
| DSWE | Direct Seawater Electrolysis |
| HER | Hydrogen Evolution Reaction |
| LDH | Layered Double Hydroxide |
| MOFs | Metal–Organic Frameworks |
| NF | Nickel Foam |
| OER | Oxygen Evolution Reaction |
| ORR | Oxygen Reduction Reaction |
| PEM | Proton Exchange Membrane |
| PVDF | Polyvinylidene Fluoride |
| SHE | Satandart Hydrogen Electrode |
| ZIF | Zeolitic Imidazolate Framework |
References
- Gao, X.; Yang, Z.; Li, D.; Wang, J.; Li, X.; Liu, Q.; Feng, L.; Lv, S.; Xing, M.; Suo, L.; et al. Scalable synthesis of ultrathin P,O–doped carbon composite CoP nanosheets for efficient electrocatalytic hydrogen evolution in natural seawater. J. Power Sources 2025, 643, 237069. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189 Pt A, 113941. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Gao, F.Y.; Yu, P.C.; Gao, M.R. Seawater electrolysis technologies for green hydrogen production: Challenges and opportunities. Curr. Opin. Chem. Eng. 2022, 36, 100827. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Khosravi, M.; Thomas, H.; Peeters, L.; Holland, K.; Hosseini, T.; Carter, L.; Bayatsarmadi, B.; Hou, Y. Sustainable seawater electrolysis: Evaluating environmental impacts and technological development opportunities. Sustain. Energy Fuels 2025, 9, 4238–4261. [Google Scholar] [CrossRef]
- Zaman, N.; Iqbal, N.; Tayyaba Noor, T. Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review. Arab. J. Chem. 2022, 15, 103906. [Google Scholar] [CrossRef]
- Haq, T.; Haik, Y. Strategies of Anode Design for Seawater Electrolysis: Recent Development and Future Perspective. Small Sci. 2022, 2, 2200030. [Google Scholar] [CrossRef]
- Li, M.; Chu, G.; Gao, J.; Ye, X.; Hou, M.; Guo, S.; Li, Y.; Zhou, Z.; Yang, L.; Briois, P. Electrochemical deposition of bimetallic sulfides on novel BDD electrode for bifunctional alkaline seawater electrolysis. Sci. Rep. 2025, 15, 2862. [Google Scholar] [CrossRef]
- Mandal, R.; Dadhich, B.K.; Bandyopadhyay, D.; Stein, P.; Neyman, A.; Bar-Ziv, R.; Bar-Sadan, M. Tailoring Cu-based nanostructures via sulfur doping and precursor selection for efficient bifunctional water splitting electrocatalysis. J. Solid State Electrochem. 2025, 1–10. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent progresses in electrocatalysts for water electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef]
- Oraby, M.; Shawqi, A. Green Hydrogen Production Directly from Seawater with No Corrosion Using a Nonmetallic Electrode: A Novel Solution and a Proof of Concept. Int. J. Energy Res. 2024, 2024, 5576626. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Yang, G.; Jiao, Y.; Dong, Y.; Tian, C.; Yan, H.; Fu, H. MXene-Assisted NiFe sulfides for high-performance anion exchange membrane seawater electrolysis. Nat. Commun. 2025, 16, 1319. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.; Ge, A.; Liu, L.; Faria, J.L.; Xu, G.; Zhu, M. Direct seawater splitting for hydrogen production: Recent advances in materials synthesis and technological innovation. Green Energy Environ. 2025, 10, 11–33. [Google Scholar] [CrossRef]
- Melchers, R.E. Microbiological and abiotic processes in modelling longer-term marine corrosion of steel. Bioelectrochemistry 2014, 97, 89–96. [Google Scholar] [CrossRef]
- Rasitha, T.; Krishna, N.G.; Anandkumar, B.; Vanithakumari, S.; Philip, J. A comprehensive review on anticorrosive/antifouling superhydrophobic coatings: Fabrication, assessment, applications, challenges and future perspectives. Adv. Colloid Interface Sci. 2024, 324, 103090–103131. [Google Scholar] [CrossRef]
- Rowsell, J.L.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Micro Mesopor. Mat. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Remya, V.; Kurian, M. Synthesis and catalytic applications of metal-organic frameworks: A review on recent literature. Int. Nano Lett. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal-organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, X.; Zhu, G.; Song, D.; Wang, J. Research on chitosan-encapsulated functionalized MOF composites and the enhanced integrated anti-corrosion and anti-fouling coatings. Colloids Surf. A Physicochem. Eng. Asp. 2025, 723, 137389. [Google Scholar] [CrossRef]
- Niu, X.; Tang, Q.; He, B.; Yang, P. Robust and stable ruthenium alloy electrocatalysts for hydrogen evolution by seawater splitting. Electrochim. Acta 2016, 208, 180–187. [Google Scholar] [CrossRef]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Ayyub, M.M.; Chhetri, M.; Gupta, U.; Roy, A.; Rao, C.N.R. Photochemical and photoelectrochemical hydrogen generation by splitting seawater. Chemistry 2018, 24, 18455–18462. [Google Scholar] [CrossRef]
- Simamora, A.J.; Hsiung, T.L.; Chang, F.C.; Yang, T.C.; Liao, C.Y.; Wang, H.P. Photocatalytic splitting of seawater and degradation of methylene blue on CuO/nano TiO2. Int. J. Hydrogen Energy 2012, 37, 13855–13858. [Google Scholar] [CrossRef]
- Jiang, N.; Meng, H.M.; Song, L.J.; Yu, H.Y. Study on Ni–Fe–C cathode for hydrogen evolution from seawater electrolysis. Int. J. Hydrogen Energy 2010, 35, 8056–8062. [Google Scholar] [CrossRef]
- Song, L.J.; Meng, H.M. Effect of carbon content on Ni–Fe–C electrodes for hydrogen evolution reaction in seawater. Int. J. Hydrogen Energy 2010, 35, 10060–10066. [Google Scholar] [CrossRef]
- Yang, T.C.; Chang, F.C.; Wang, H.P.; Wei, Y.L.; Jou, C.J. Photocatalytic splitting of seawater effected by (Ni-ZnO)@C nanoreactors. Mar. Pollut. Bull. 2014, 85, 696–699. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef]
- Chang, C.J.; Huang, K.L.; Chen, J.K.; Chu, K.W.; Hsu, M.H. Improved photocatalytic hydrogen production of ZnO/ZnS based photocatalysts by Ce doping. J. Taiwan Inst. Chem. Eng. 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Li, Y.; He, F.; Peng, S.; Lu, G.; Li, S. Photocatalytic H2 evolution from NaCl saltwater over ZnS1−x−0.5yOx(OH)y–ZnO under visible light irradiation. Int. J. Hydrogen Energy 2011, 36, 10565–10573. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Peng, S.; Lu, G.; Li, S. Modification of ZnS1−x−0.5yOx(OH)y–ZnO photocatalyst with NiS for enhanced visible-light-driven hydrogen generation from seawater. Int. J. Hydrogen Energy 2013, 38, 15976–15984. [Google Scholar] [CrossRef]
- Ji, S.M.; Jun, H.; Jang, J.S.; Son, H.C.; Borse, P.H.; Lee, J.S. Photocatalytic hydrogen production from natural seawater. J. Photochem. Photobiol. Chem. 2007, 189, 141–144. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Wu, C.X.; Feng, X.J.; Tan, H.Q.; Yan, L.K.; Liu, Y.; Kang, Z.H.; Wang, E.B.; Li, Y.G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal–Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chem. Int. Ed. 2012, 51, 3364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.L.; Nguyen, T.T.; Nguyen, K.D.; Phan, N.T.S. Metal–organic framework MOF-199 as an efficient heterogeneous catalyst for the aza-Michael reaction. Appl. Catal. A Gen. 2012, 425–426, 44. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reactio. J. Am. Chem. Soc. 2012, 134, 6707. [Google Scholar] [CrossRef]
- Wen, L.L.; Wang, F.; Feng, J.; Lv, K.L.; Wang, C.G.; Li, D.F. Two New Metal−Organic Frameworks with Mixed Ligands of Carboxylate and Bipyridine: Synthesis, Crystal Structure, and Sensing for Methanol. Cryst. Growth Des. 2009, 9, 3581. [Google Scholar] [CrossRef]
- Luo, F.; Che, Y.X.; Zheng, J.M. Trinuclear Cobalt Based Porous Coordination Polymers Showing Unique Topological and Magnetic Variety upon Different Dicarboxylate-like Ligands. Cryst. Growth Des. 2009, 9, 1066–1070. [Google Scholar] [CrossRef]
- Deffo, G.; Tamo, A.K.; Fotsop, C.G.; Tchoumi, H.H.B.; Talla, D.E.N.; Wabo, C.G.; Deussi, M.C.N.; Temgoua, R.C.T.; Doungmo, G.; Njanja, E.; et al. Metal-organic framework-based materials: From synthesis and characterization routes to electrochemical sensing applications. Coord. Chem. Rev. 2025, 536, 216680. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Liu, L.; Yang, X.; Xu, X. Electrochemical investigation of a new Cu-MOF and its electrocatalytic activity towards H2O2 oxidation in alkaline solution. Electrochem. Commun. 2013, 33, 131–134. [Google Scholar] [CrossRef]
- Mehmood, R.A.; Aslam, A.A.; Iqbal, M.J.; Sajid, A.H.; Hamza, A.; Tahira, H.F.; Islam, I.U.; Yabalak, E. A review on hydrogen production using decorated metal-organic frameworks by electrocatalytic and photocatalytic water splitting. Fuel 2025, 387, 134416. [Google Scholar] [CrossRef]
- Hu, E.; Yao, Y.; Cui, Y.; Qian, G. Strategies for the enhanced water splitting activity over metal–organic frameworks-based electrocatalysts and photocatalysts. Mater. Today Nano 2021, 15, 100124. [Google Scholar] [CrossRef]
- Qiu, Y.; Jia, Q.; Yan, S.; Liu, B.; Liu, J.; Ji, X. Favorable amorphous–crystalline iron oxyhydroxide phase boundaries for boosted alkaline water oxidation. ChemSusChem 2020, 13, 4911–4915. [Google Scholar] [CrossRef]
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Z.; Sun, A.; Zhang, X.; Ji, X.; Liu, J. Electrochemical in situ self-Healing of porous nanosheets Based on the phase Reconstruction of carbonate Hydroxide to layered double Hydroxides with unsaturated coordination metal Sites for high-performance water oxidation. ACS Sustain. Chem. Eng. 2022, 10, 16417–16426. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, A.; Zhang, X.; Liu, X.; Wang, Y.; Liu, J. Interfacial stress induced by the adaptive construction of hydrangea-like heterojunctions based on in situ electrochemical phase reconfiguration for highly efficient oxygen evolution reaction at high current density. J. Mater. Chem. 2022, 10, 23580–23589. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, X.; Han, H.; Liu, Z.; Liu, J.; Ji, X. Advantageous metal-atom-escape towards super-hydrophilic interfaces assembly for efficient overall water splitting. J. Power Sources 2021, 499, 229941. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal–organic frameworks as platforms for catalytic applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. The postsynthetic renaissance in porous solids. J. Am. Chem. Soc. 2017, 139, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Rafiq, K.; Najam, T.; Hussain, E.; Sohail, M.; Abid, M.Z.; Mahmood, A.; Javed, M.S.; Shah, S.S.A. Metal-organic frameworks for electrocatalytic water-splitting: Beyond the pyrolysis. Int. J. Hydrogen Energy 2023, 48, 35075–35111. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, Z.; Zhang, C.; Chen, W. Advanced Electrocatalysts Based on Metal–Organic Frameworks. ACS Omega 2020, 5, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Guo, W.; Tabassum, H.; Zou, R. Metal-Organic Framework-Based Nanomaterials for Electrocatalysis. Adv. Energy Mater. 2016, 6, 1600423. [Google Scholar] [CrossRef]
- Gupta, A.; Puri, N. Recent advances in hydrogen production using metal organic frameworks and their composites. Int. J. Hydrogen Energy 2024, 78, 303–324. [Google Scholar] [CrossRef]
- Chen, X.M.; Tong, M.L. Solvothermal in Situ Metal/Ligand Reactions: A New Bridge between Coordination Chemistry and Organic Synthetic Chemistry. Acc. Chem. Res. 2007, 40, 162–170. [Google Scholar] [CrossRef]
- Paz, F.A.A.; Klinowski, J.; Vilela, S.M.F.; Tomé, J.P.C.; Cavaleiro, J.A.S.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar] [CrossRef]
- Kushwaha, A.; Kumar, A. MOFs and MOF derivatives for electrocatalytic hydrogen evolution reaction: Designing strategies, syntheses and future prospects. Int. J. Hydrogen Energy 2025, 150, 150148. [Google Scholar] [CrossRef]
- Vidales, A.G.; Mathias Semai, M. Platinum nanoparticles supported on nickel-molybdenum-oxide for efficient hydrogen production via acidic water electrolysis. J. Mol. Struct. 2023, 1290, 135956. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.; Wu, Z.Y.; Xiong, Y.; Xu, Q.; Yu, S.H.; Jiang, H.L. From Bimetallic Metal-Organic Framework to Porous Carbon: High Surface Area and Multicomponent Active Dopants for Excellent Electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef]
- Li, F.L.; Shao, Q.; Huang, X.; Lang, J.P. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1888. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Lin, C.Y.; Dou, S.; Feng, S.; Chen, D.; Liu, D.; Huo, J.; Xia, Z.; Wang, S. Creating coordinatively unsaturated metal sites in metal-organic-frameworks as efficient electrocatalysts for the oxygen evolution reaction: Insights into the active centers. Nano Energy 2017, 41, 417–425. [Google Scholar] [CrossRef]
- Qin, J.; Hu, S.; Zhang, X.; Hong, M.; Du, C.; Chen, J. Hydrangea shaped bimetallic NiRu MOFs directly catalyzing highly-efficient alkaline freshwater/seawater overall splitting based on electronic structure and oxygen vacancy modulation. Fuel 2024, 371 Pt B, 132025. [Google Scholar] [CrossRef]
- Sun, F.; Qin, J.; Wang, Z.; Yu, M.; Wu, X.; Sun, X.; Qiu, J. Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 2021, 12, 4182. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Zheng, L.; Song, X.; Yan, Y.; Li, J.; Tian, S.; Wang, M.; Peng, M.; Yin, Z.; et al. Construction of 3D hollow NiCo-layered double hydroxide nanostructures for high-performance industrial overall seawater electrolysis. Nano Res. 2024, 17, 9472–9482. [Google Scholar] [CrossRef]
- Sanati, S.; Abazari, R.; Kirillov, A.M. Bimetallic NiCo Metal–Organic Frameworks with High Stability and Performance Toward Electrocatalytic Oxidation of Urea in Seawater. Inorg. Chem. 2024, 63, 15813–15820. [Google Scholar] [CrossRef]
- Xin, Y.; Shen, K.; Guo, T.; Chen, L.; Li, Y. Coupling Hydrazine Oxidation with Seawater Electrolysis for Energy-Saving Hydrogen Production over Bifunctional CoNC Nanoarray Electrocatalysts. Small 2023, 19, 2300019. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, X.; He, L.; Zheng, Y.; Pang, J.; Wang, L.; Jiang, R.; Hou, J.; Guo, X.; Chen, L. Structural and Electronic Modulation of Iron-Based Bimetallic Metal–Organic Framework Bifunctional Electrocatalysts for Efficient Overall Water Splitting in Alkaline and Seawater Environment. ACS Appl. Mater. Interfaces 2022, 14, 46374–46385. [Google Scholar] [CrossRef]
- Xiao, M.; Wu, C.; Zhu, J.; Zhang, C.; Li, Y.; Lyu, J.; Zeng, W.; Li, H.; Chen, L.; Mu, S. In situ generated layered NiFe-LDH/MOF heterostructure nanosheet arrays with abundant defects for efficient alkaline and seawater oxidation. Nano Res. 2023, 16, 8945–8952. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Cai, Z.; Yang, C.; Sun, S.; Wang, X.; Zhang, M.; Yue, M.; Zheng, D.; Farouk, A.; et al. Ir nanoparticles decorated NiFe metal–organic framework as a highly efficient and stable heterostructure electrocatalyst for overall seawater splitting. J. Mater. Chem. A 2024, 12, 31121–31126. [Google Scholar] [CrossRef]
- Zhai, X.; Yu, Q.; Liu, G.; Bi, J.; Zhang, Y.; Chi, J.; Lai, J.; Yang, B.; Wang, L. Hierarchical microsphere MOF arrays with ultralow Ir doping for efficient hydrogen evolution coupled with hydrazine oxidation in seawater. J. Mater. Chem. A 2021, 9, 27424–27433. [Google Scholar] [CrossRef]
- Jazaa, Y.; Abdulkareem, R.; Fiallos, L.M.F.; Saraswat, S.K.; Abdullaev, S.; Castillo, R.M.T.; Rao, D.P.; Mahmoud, Z.H.; Rajhi, A.A. Aniline-Naphthylamine Copolymer Integrated with Aluminum Terephthalate-Based Metal Organic Framework for Efficient Hydrogen Evolution From Seawater. J. Mater. Eng. Perform. 2025, 34, 960–967. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Nguyen, L.; Le, N.; Chhetri, K.; Acharya, D.; Teke, S.; Saud, S.; Nguyen, D.B.; Kim, S.J.; Mok, Y.S. Cyanide Functionalization and Oxygen Vacancy Creation in Ni-Fe Nano Petals Sprinkled with MIL-88A Derived Metal Oxide Nano Droplets for Bifunctional Alkaline Seawater Electrolysis. Small 2025, 21, 2410027. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Z.; Meng, X. Low-Pt supported on MOF-derived Ni(OH)2 with highly-efficiently electrocatalytic seawater splitting at high current density. Appl. Catal. B Environ. 2023, 331, 122703. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Tran, T.T.N.; Truong, T.K.; Le, T.A.; Le, T.N.M.; Nguyen, L.H.T.; Nguyen, C.C.; Tran, N.Q. MOF-Templated Synthesis of Three-Dimensional B-Doped NiCoP Hollow Nanorod Arrays for Highly Efficient and Stable Natural Seawater Splitting. ACS Appl. Energy Mater. 2023, 6, 10713–10722. [Google Scholar] [CrossRef]
- Wu, Y.; Du, X.; Zhang, X. Fe-doped CoMoO4/Ni3S2 grown on Ni foam as a multifunctional electrode for electrocatalytic seawater and urea splitting. J. Alloys Compd. 2025, 1030, 180927. [Google Scholar] [CrossRef]
- Pushkareva, I.V.; Pushkarev, A.S.; Grigoriev, S.A.; Modisha, P.; Bessarabov, D.G. Comparative study of anion exchange membranes for low-cost water electrolysis. Int. J. Hydrogen Energy 2020, 45, 26070–26079. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Pandiyan, A.; Uthayakumar, A.; Subrayan, R.; Cha, S.W.; Moorthy, S.B.K. Review of solid oxide electrolysis cells: A clean energy strategy for hydrogen generation. Nanomater. Energy 2019, 8, 2–22. [Google Scholar] [CrossRef]
- Rezk, H.; Olabi, A.G.; Abdelkareem, M.A.; Alahmer, A.; Sayed, E.T. Maximizing green hydrogen production from water electrocatalysis: Modeling and optimization. J. Mar. Sci. Eng. 2023, 11, 617. [Google Scholar] [CrossRef]
- Alinejad, Z.; Parham, N.; Tawalbeh, M.; Al-Othman, A.; Almomani, F. Progress in green hydrogen production and innovative materials for fuel cells: A pathway towards sustainable energy solutions. Int. J. Hydrogen Energy 2025, 140, 1078–1094. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Gaffney, O.; Steffen, W. The Anthropocene equation. The Anthropocene equation. Anthr. Rev. 2017, 4, 53–61. [Google Scholar]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Panigrahy, B.; Narayan, K.; Rao, B.R. Green hydrogen production by water electrolysis: A renewable energy perspective. Mater. Today Proc. 2022, 67 Pt 8, 1310–1314. [Google Scholar] [CrossRef]
- Aziz, M.A.D.; Hisham, S.M.; Sazali, N.; Junaidi, A.; Kadirgama, K. Recent Advances in Nanostructured Electrocatalysts for Seawater Electrolysis: Towards Sustainable Hydrogen Production. Int. J. Mod. Manuf. Technol. 2025, 9, 146–159. [Google Scholar] [CrossRef]
- Khandekar, R.V.; Yadav, J.B. Metal–Organic Framework as Electrocatalyst in Electrochemical Water Splitting. In Electrocatalytic Materials; Springer: Cham, Switzerland, 2024; pp. 447–497. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Zhang, M.; Wang, Y.; Cao, Y.; Kim, D.H.; Liu, Y.; Lin, Z. Strategies Toward High Selectivity, Activity, and Stability of Single-Atom Catalysts. Small 2024, 20, 2308213. [Google Scholar] [CrossRef]
- Kasani, A.; Maric, R.; Bonville, L.; Bliznakov, S. Catalysts for direct seawater electrolysis: Current status and future prospectives. ChemElectroChem 2024, 11, e202300743. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Topalov, A.A.; Overmeere, Q.V.; Cherevko, S.; Chen, X.X.; Ventosa, E.; Schuhmann, W.; Mayrhofer, K.J.J. Rational design of the electrode morphology for oxygen evolution–enhancing the performance for catalytic water oxidation. Rsc Adv. 2014, 4, 9579–9587. [Google Scholar] [CrossRef]
- Gomaa, F.A.; Nada, A.A.; Gomaa, H.E.M.; El-Maghrabi, H.H. Advancing Green Hydrogen: Innovations and Challenges in Seawater Electrolysis for Sustainable Energy Production. J. Environ. Chem. Eng. 2025, 13, 115644. [Google Scholar] [CrossRef]
- Omeiza, L.A.; Abdalla, A.M.; Wei, B.; Dhanasekaran, A.; Subramanian, Y.; Afroze, S.; Reza, M.S.; Bakar, S.A.; Azad, A.K. Nanostructured electrocatalysts for advanced applications in fuel cells. Energies 2023, 16, 1876. [Google Scholar] [CrossRef]
- Jiao, L.; Sun, J.; Li, Z.; Meng, X. Recent advances in electrocatalysts for seawater splitting in hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 29685–29697. [Google Scholar] [CrossRef]
- Din, M.A.U.; Krishnan, M.R.; Edreese, H.; Alsharaeh, E.H. Design strategies for cost-effective high-performance electrocatalysts in seawater electrolysis to produce hydrogen. J. Energy Chem. 2025, 102, 497–515. [Google Scholar] [CrossRef]
- Tran, N.Q.; Truong, T.T.; Tran, T.T.N.; Truong, T.-K.; Yu, J.; Nguyen, T.D.; Le, T.A.; Nguyen, C.C.; Nguyen, L.H.T.; Vu, N.H.; et al. Multi-metallic Metal–Organic Framework Nanosheets with 3D Flower-like Nanostructure-Based Natural Seawater Splitting toward Stable Industrial-Scale Current Density. ACS Sustain. Chem. Eng. 2024, 12, 1038–1050. [Google Scholar] [CrossRef]
- Deng, P.-J.; Xue, R.; Lu, J.; Tsiakaras, P. Strategies for Designing Anti-Chlorine Corrosion Catalysts in Seawater Splitting. Adv. Energy Mater. 2025, 15, 2405749. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design Criteria, Operating Conditions, and Nickel–Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem 2016, 9, 962. [Google Scholar] [CrossRef]
- Yao, D.; Liu, C.; Zhang, Y.; Wang, S.; Nie, Y.; Qiao, M.; Zhu, D. Modulating selectivity and stability of the direct seawater electrolysis for sustainable green hydrogen production. Mater. Today Catal. 2025, 8, 100089. [Google Scholar] [CrossRef]
- Le, T.A.; Hai, N.D.; Tran, T.T.N.; Trinh, K.T.L.; Tran, N.Q. Electrically conductive metal–organic framework-based electrocatalysts: From synthesis strategies to catalytic applications. Chem. Commun. 2025, 61, 13543–13560. [Google Scholar] [CrossRef]
- Asghari, E.; Abdullah, M.I.; Foroughi, F.; Lamb, J.J.; Pollet, B.G. Advances, opportunities, and challenges of hydrogen and oxygen production from seawater electrolysis: An electrocatalysis perspective. Curr. Opin. Electrochem. 2022, 31, 100879. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Chen, H.; Yang, Q.; Liu, H.; Bao, S.; Tian, Z.; Slavcheva, E.; Lu, Z. Progress in Anode Stability Improvement for Seawater Electrolysis to Produce Hydrogen. Adv. Mater. 2024, 36, 2311322. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, Y.; Yang, T.; Jiang, L. Recent advances in electrocatalysts for seawater splitting. Nano Mater. Sci. 2023, 5, 101–116. [Google Scholar] [CrossRef]
- Yuan, R.; Liao, C.; Cao, L.; Li, D.; Sun, S.; Wang, G.; Li, G.; Xie, J.; Shao, Z. Highly Efficiency Seawater Electrolysis Guided by Coordinating Catalysis of Oxygen Evolution Reaction. Adv. Funct. Mater. 2025, e08413. [Google Scholar] [CrossRef]
- Ma, T.; Xu, W.; Li, B.; Chen, X.; Zhao, J.; Wan, S.; Jiang, K.; Zhang, S.; Wang, Z.; Tian, Z.; et al. The critical role of additive sulfate for stable alkaline seawater ox-idation on Ni-based electrode. Angew. Chem. Int. Edit. 2021, 60, 22740–22744. [Google Scholar] [CrossRef]
- Li, S.; Qiu, X.; An, X.; Li, E.; Li, X.; Wang, G.; Li, P.; Shi, C.; Liu, Y.; Guan, G. Metal-organic framework derived spinel tricobalt tetroxide with trifle iridium sites for near-pH-neutral seawater electrolysis. Chem. Eng. J. 2024, 491, 151924. [Google Scholar] [CrossRef]
- Patila, S.A.; Khotd, A.C.; Chavan, V.D.; Rabani, I.; Kim, D.; Jung, J.; Im, H.; Shrestha, N.K. Electrostatically robust CoFeOF nanosheet against chloride for green-H2 production in alkaline seawater electrolysis. Chem. Eng. J. 2024, 480, 146545. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, W.; Zhou, X.; Yuan, J.; Zhang, X.; Wang, L.; Li, J.; Meng, X.; Sun, F.; Jihui Gao, J.; et al. The Corrosive Cl−–Induced Rapid Surface Reconstruction of Amorphous NiFeCoP Enables Efficient Seawater Splitting. ACS Catal. 2024, 14, 18322–18332. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, L.; Sun, W.; Chen, Y.; Yu, H.; Li, K.; Jia, B.; Zhang, L.; Ma, T. Electrocatalytic Water Splitting: From Harsh and Mild Conditions to Natural Seawater. Small 2021, 18, 2105830. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–662911. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Z.; Yu, E.; Ye, H.; Li, Z.; Guo, X.; Zhou, D.; Wang, C.; Sha, Q.; et al. A Review of Hydrogen Production via Seawater Electrolysis: Current Status and Challenges. Catalysts 2024, 14, 691. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Yang, Z.; Li, B.; Wang, Q.; Kuliiev, R.; Orlovskaya, N.; Gu, M.; Du, Y.; Wang, G.; et al. Dual-doping and synergism toward high-performance seawater electrolysis. Adv. Mater. 2021, 33, 2101425. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, J.; Wan, H.; Chen, F.; Lin, Y.; Xu, X.; Ma, R.E.; Sasaki, T. Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation. J. Energy Chem. 2019, 32, 93–104. [Google Scholar] [CrossRef]
- Gu, Y.; Park, D.H.; Kim, M.H.; Byeon, J.H.; Lim, D.M.; Park, S.H.; Kim, J.H.; Jang, J.S.; Park, K.W. NiFe layered double hydroxides synthesized based on solvent properties as anode catalysts for enhanced oxygen evolution reaction. Chem. Eng. J. 2024, 480, 147789. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A., III; Chen, S.; Ren, Z. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nat. Comm. 2018, 9, 2551. [Google Scholar] [CrossRef]
- You, B.; Zhang, Y.; Jiao, Y.; Davey, K.; Qiao, S.Z. Negative charging of transition-metal phosphides via strong electronic coupling for destabilization of alkaline water. Angew. Chem. 2019, 131, 11922–11926. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Yu, Y.; Zhang, B. Recent advances in nanostructured transition metal phosphides: Synthesis and energy-related applications. Energy Environ. Sci. 2020, 13, 4564–4582. [Google Scholar] [CrossRef]
- Shetty, B.; Puranam-Rajashekar, S.; Aralikatti, G.H.; Nagaraj, P.; Kumar, L.H.; Tengli, P.N.; Thakur, M.S.; Sridhar, V.; Krishnappa, M. Seawater electrolysis: A critical review on fundamentals, recent progress, and future perspectives on sustainable hydrogen generation. Next Energy 2025, 9, 100407. [Google Scholar] [CrossRef]
- Li, J.; Fu, G.; Sheng, X.; Li, G.; Chen, H.; Shu, K.; Dong, Y.; Wang, T.; Yida Deng, Y. A comprehensive review on catalysts for seawater electrolysis. Adv. Powder Mater. 2024, 3, 100227. [Google Scholar] [CrossRef]
- Deng, X.; Tüysüz, H. Cobalt-Oxide-Based Materials as Water Oxidation Catalyst: Recent Progress and Challenges. ACS Catal. 2014, 4, 3701–3714. [Google Scholar] [CrossRef]
- Anwar, F.; Khan, S.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Jang, D.; Cho, H.S.; Lee, S.; Park, M.; Kim, S.; Park, H.; Kang, S. Investigation of the operation characteristics and optimization of an alkaline water electrolysis system at high temperature and a high current density. J. Clean. Prod. 2023, 424, 138862. [Google Scholar] [CrossRef]
- Dash, S.; Singh, K.A.; Jose, S.; Wilson, D.V.H.; Elangovan, D.; Surapraraju, S.K.; Natarajan, S.K. Advances in green hydrogen production through alkaline water electrolysis: A comprehensive review. Int. J. Hydrogen Energy 2024, 83, 614–629. [Google Scholar] [CrossRef]
- Fathima, T.K.S.; Ghosh, A.; Ramaprabhu, S. Efficient, chlorine-free, and durable alkaline seawater electrolysis enabled by MOF-derived nanocatalysts. Int. J. Hydrogen Energy 2024, 90, 546–556. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.; Yang, O.; Yuan, W.; Liu, Y.; He, L.; Hu, Y.; Zhao, Z.; Zhou, L.; Wang, J.; et al. Self-Powered Seawater Electrolysis Based on a Triboelectric Nanogenerator for Hydrogen Production. ACS Nano 2022, 16, 15286–15296. [Google Scholar] [CrossRef]
- Li, W.; Guo, B.; Zhang, K.; Chen, X.; Zhang, H.; Chen, W.; Chen, H.; Li, H.; Feng, X. Ru-regulated electronic structure CoNi-MOF nanosheets advance water electrolysis kinetics in alkaline and seawater media. J. Colloid Interface Sci. 2024, 668, 181–189. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, X.; Min, Y.; Xu, O.; Li, Q. An in situ grown NiFe-based MOF for efficient oxygen evolution in alkaline seawater at high current densities. New J. Chem. 2025, 49, 2665–2673. [Google Scholar] [CrossRef]
- Thangasamy, P.; Vo, T.G.; Venkatramanan, R.; Ng, Y.T.; Gao, J.; Liu, Y. Bimetallic Ni–Co-MOF Nanostructures for Seawater Electrolysis: Unveiling the Mechanism of the Oxygen Evolution Reaction Using Impedance Spectroscopy. Inorg. Chem. 2025, 64, 5586–5597. [Google Scholar] [CrossRef]
- Bao, Y.; Ru, H.; Wang, Y.; Zhang, K.; Yu, R.; Wu, Q.; Yu, A.; Li, D.S.; Sun, C.; Li, W.; et al. Hetero MOF-On-MOF of Ni-BDC/NH2-MIL-88B(Fe) Enables Efficient Electrochemical Seawater Oxidation. Adv. Funct. Mater. 2024, 34, 2314611. [Google Scholar] [CrossRef]
- Na, G.; Zheng, H.; Chen, M.; Sun, H.; Wu, Y.; Li, D.; Chen, Y.; Zhao, B.; Zhao, B.; Zhou, T.; et al. In-situ Pt reduction induced topological transformation of NiFe-MOF for industrial seawater splitting. Chin. J. Catal. 2025, 72, 211–221. [Google Scholar] [CrossRef]
- Tran, N.Q.; Le, Q.M.; Tran, T.T.N.; Truong, T.K.; Yu, J.; Peng, L.; Le, T.A.; Doan, T.L.H.; Phan, T.B. Boosting Urea-Assisted Natural Seawater Electrolysis in 3D Leaf-Like Metal–Organic Framework Nanosheet Arrays Using Metal Node Engineering. ACS Appl. Mater. Interfaces 2024, 16, 28625–28637. [Google Scholar] [CrossRef]
- Tran, T.T.N.; Le, T.A.; Dinh, N.T.T.; Hai, N.D.; Truong, T.K.; Yu, J.; Peng, L.; Nguyen, C.C.; Tran, N.Q. Crystalline Ru-Decorated MOF-Derived Amorphous CoMo-LDH Nanosheet Arrays as Bifunctional Catalysts for Overall Natural Seawater Electrolysis. ACS Appl. Mater. Interfaces 2024, 16, 53675–53687. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S.; Feng, Y.; Wang, Y.; Xiong, J.; Zhao, Y. Two-dimensional MOF derived NiFe-VOx with high catalytic activity and corrosion resistance for seawater splitting. Appl. Catal. A-Gen. 2025, 705, 120448. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Liu, X.; Yu, X.; Li, W.; Pei, C.; Park, H.S.; Kim, J.K.; Pang, H. Interface-Driven Catalytic Enhancements in Nitrogen-Doped Carbon Immobilized CoNi2S4@ReS2/CC Heterostructures for Optimized Hydrogen and Oxygen Evolution in Alkaline Seawater-Splitting. Adv. Sci. 2025, 12, 2413245. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, S.; Devarayapalli, K.C.; Kim, B.; Lim, Y.; Lee, D.S. MXene-boosted MOF-derived hierarchical porous C, N-doped In2O3/Gd2MoO6 heterostructures with rich oxygen vacancies enable highly efficient bifunctional electrocatalysts for water/seawater electrolysis. J. Mater. Sci. Technol. 2026, 247, 130–148. [Google Scholar] [CrossRef]
- Li, G.; Feng, S.; Li, J.; Deng, P.; Tian, X.; Wang, C.; Hua, Y. P-Ni4Mo Catalyst for Seawater Electrolysis with High Current Density and Durability. Chin. J. Struct. Chem. 2022, 41, 2207068–2207073. [Google Scholar] [CrossRef]
- Hancke, R.; Bujlo, P.; Holm, T.; Ulleberg, Ø. High-pressure PEM water electrolyser performance up to 180 bar differential pressure. J. Power Sources 2024, 601, 234271. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo-fermentation of lignocellulosic biomass Biomass. Biomass Conv. Bioref. 2023, 13, 8465–8483. [Google Scholar] [CrossRef]
- Rahim, A.H.A.; Salami, A.; Kamarudin, S.K.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 30, 56–65. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Compañ, V. A deep insight into different acidic additives as doping agents for enhancing proton conductivity on polybenzimidazole membranes. Polymers 2020, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Kamaroddin, M.F.A.; Sabli, N.; Nia, P.M.; Abdullah, T.A.T.; Abdullah, L.C.; Izhar, S.; Ripin, A.; Ahmad, A. Phosphoric acid doped composite proton exchange membrane for hydrogen production in medium-temperature copper chloride electrolysis. Int. J. Hydrogen Energy 2020, 45, 22209–22222. [Google Scholar] [CrossRef]
- Liu, R.T.; Xu, Z.L.; Li, F.M.; Chen, F.Y.; Yu, J.Y.; Yan, Y.; Chen, Y.; Xia, B.Y. Recent advances in proton exchange membrane water electrolysis. Chem. Soc. Rev. 2023, 52, 5652–5683. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, J.; Zhao, P.; Tolj, I.; Li, S.; Tu, Z. Structure-property relationship analysis of metal-organic frameworks(MOFs) doped proton exchange membrane(PEM). Int. J. Hydrogen Energy 2025, 141, 811–822. [Google Scholar] [CrossRef]
- Maiti, S.K.; Ali, N.; Maiti, T.K.; Suhag, A.; Ray, M.; Chattopadhyay, S. Metal-organic framework catalyst influencing hydrogen production in bipolar membrane water electrolyzer. Int. J. Hydrogen Energy 2024, 80, 1062–1074. [Google Scholar] [CrossRef]
- Du, J.; Li, Z.; Wang, L.; Ding, Y.; Ye, W.; Yang, W.; Sun, L. Anion Exchange Membrane Seawater Electrolysis at 1.0 A cm−2 With an Anode Catalyst Stable for 9000 H. Adv. Sci. 2025, 12, 2416661. [Google Scholar] [CrossRef]
- Li, P.; Zhao, S.; Huang, Y.; Huang, Q.; Xi, B.; An, X.; Xiong, S. Corrosion Resistant Multilayered Electrode Comprising Ni3N Nanoarray Overcoated with NiFe-Phytate Complex for Boosted Oxygen Evolution in Seawater Electrolysis. Adv. Energy Mater. 2024, 14, 2303360. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, X.; Wang, Z.; Wang, J.; Shi, H.; Wang, C.; Bai, Z.; Gao, Y.; Zhu, C.; et al. Hollow Bimetallic Cobalt-Based Selenide Polyhedrons for Efficient Hydrogen Production in Practical PEM Electrolysis. ACS Sustain. Chem. Eng. 2024, 12, 10020–10032. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, S.; Sharma, J.; Upadhyay, P.; Kulshrestha, V. Polyvinylidene fluoride-based modified membranes for hydrogen generation by direct seawater electrolysis and proton exchange membrane fuel cells. J. Mater. Chem. A 2024, 12, 29854–29868. [Google Scholar] [CrossRef]
- Malek, A.; Lu, X.; Shearing, P.R.; Brett, D.J.L.; He, G. Strategic comparison of membrane-assisted and membrane-less water electrolyzers and their potential application in direct seawater splitting (DSS). Green Energy Environ. 2023, 8, 989–1005. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Yu, K.; Feng, Y.; Zhu, Z. 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting. Nanoscale 2020, 12, 6176–6187. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Erami, R.S.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Marin, D.H.; Perryman, J.T.; Hubert, M.A.; Lindquist, G.A.; Chen, L.; Aleman, A.M.; Kamat, G.A.; Niemann, V.A.; Stevens, M.S.; Regmi, Y.N.; et al. Hydrogen production with seawater-resilient bipolar membrane electrolyzers. Joule 2023, 7, 765–781. [Google Scholar] [CrossRef]
- Yu, L.; Ning, M.; Wang, Y.; Ren, Z. Direct seawater electrolysis for hydrogen production. Nat. Rev. Mater. 2025, 1–17. [Google Scholar] [CrossRef]
- Han, J.H.; Jwa, E.; Lee, H.; Kim, E.J.; Nam, J.Y.; Hwang, K.S.; Jeong, N.; Choi, J.; Kim, H.; Jeung, Y.C.; et al. Direct seawater electrolysis via synergistic acidification by inorganic precipitation and proton flux from bipolar membrane. J. Chem. Eng. 2022, 429, 132383. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, C.; Wang, P.; Zeng, W.; Zhu, J.; Li, Y.; Peng, W.; Liu, Q.; Xu, H.; Zhao, Y.; et al. Polymetallic phosphides evolved from MOF and LDH dual-precursors for robust oxygen evolution reaction in alkaline and seawater media. Mater. Today Phys. 2022, 24, 100684. [Google Scholar] [CrossRef]
- Khan, M.W.; Loomba, S.; Haris, M.; Tran, K.; Gbadamasi, S.; Xu, K.; Mohiuddin, M.; Nettem, V.; Jannat, A.; Taylor, P.D.; et al. Unveiling rare ionic bonds in dissimilar 2D materials for selective ampere-level oxygen evolution reaction in seawater. EES Catal. 2025, 3, 712–722. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Han, Y.; Kim, Y.; Lee, S.; Yang, J.; Choi, S.M.; Li, O.L. Plasma-Engineered 2D Ni Nanoplates as Advanced Oxygen Evolution Reaction Electrocatalysts for Direct Seawater Electrolysis. ACS Appl. Energy Mater. 2025, 8, 1101–1111. [Google Scholar] [CrossRef]
- Sun, W.; Hu, J.; Xu, Z.; Wang, W.; Lou, Y.; Chen, J. Ru Doping and Phytic Acid Etching Synergistically Regulate Mof Active Sites for Efficient Water Splitting. Available online: https://ssrn.com/abstract=5344548 (accessed on 8 July 2025).
- Xin, H.; Shen, Z.; Li, X.; Fang, J.; Sun, H.; Deng, C.; Zhou, L.; Kuang, Y. Direct 2400 h Seawater Electrolysis Catalyzed by Pt-Loaded Nanoarray Sheets. Catalysts 2025, 15, 634. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Liang, F.; Lv, Y.; Guo, J. Enhanced Corrosion Resistance and Stability in Seawater Electrolysis via In Situ Generated CeO2 Layer on the Ce–Co(OH)2@FeOOH Electrode, Sustaining 2500 h at 2 A cm–2. ACS Appl. Mater. Interfaces 2025, 17, 43011–43019. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, G.; Fernando Patolsky, F. Routes to Avoiding Chlorine Evolution in Seawater Electrolysis: Recent Perspective and Future Directions. ACS Mater. Lett. 2024, 6, 3202–3217. [Google Scholar] [CrossRef]
- Kaur, R.; Gaur, A.; Pundir, V.; Sharma, J.; Bera, C.; Bagchi, V. Electronic Reallocation in MOF-Derived Co4N–Ni3N Heterostructure Renders Chlorine-Free Overall Seawater Splitting under Large Current Density. Energy Fuels 2024, 38, 11137–11147. [Google Scholar] [CrossRef]
- Li, X.; Afsar, N.U.; Chen, X.; Wu, Y.; Chen, Y.; Shao, F.; Song, J.; Yao, S.; Xia, R.; Qian, J.; et al. Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability. Membranes 2022, 12, 601. [Google Scholar] [CrossRef]
- Xu, T.; Shehzad, M.A.; Wang, X.; Wu, B.; Ge, L.; Xu, T. Engineering Leaf-Like UiO-66-SO3H Membranes for Selective Transport of Cations. Nano-Micro Lett. 2020, 12, 51. [Google Scholar] [CrossRef]
- Li, K.; Tao, Z.; Ma, X.; Wu, J.; Wu, T.; Guo, G.; Qi, Y.; Yu, J.; Zheng, J.; Xue, J. The application and research progress of d-band center theory in the field of water electrolysis. Int. J. Hydrogen Energy 2025, 132, 183–211. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, S.; Wang, J.; Zhong, W. Modulating the D-Band Center of Electrocatalysts for Enhanced Water Splitting. Chem. Eur. J. 2024, 30, e202402725. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Q.; Wu, L.; Cheng, L.; Huang, K.; Chen, J.; Yao, X. Optimal Electrocatalyst Design Strategies for Acidic Oxygen Evolution. Optimal Electrocatalyst Design Strategies for Acidic Oxygen Evolution. Adv. Sci. 2024, 11, 2401975. [Google Scholar] [CrossRef]
- Hai, G.; Jia, X.; Zhang, K.; Liu, X.; Wu, Z.; Wang, G. High-performance oxygen evolution catalyst using two-dimensional ultrathin metal-organic frameworks nanosheets. Nano Energy 2018, 44, 345–352. [Google Scholar] [CrossRef]
- Wen, D.; Xie, D.; Huang, B.; Huang, Q.; Lin, D.; Xu, C.; Xie, F.; Wang, G.; Guo, W. Tuning the d-band states of NiFe-MOFs by combining early and late transition metals for enhanced electrocatalytic oxygen evolution. CrystEngComm 2024, 26, 1613–1619. [Google Scholar] [CrossRef]
- Musho, T.; Li, J.; Wu, N. Band gap modulation of functionalized metal-organic frameworks. Phys. Chem. Chem. Phys. 2014, 16, 23646–23653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Y.; Ni, B.; Ding, K.; Chen, W.; Wu, K.; Huang, X.; Zhang, Y. Effects of ligand functionalization on the photocatalytic properties of titanium-based MOF: A density functional theory study. AIP Adv. 2018, 8, 035012. [Google Scholar] [CrossRef]
- Mukoyoshi, M.; Kitagawa, H. Nanoparticle/metal–organic framework hyb rid catalysts: Elucidating the role of the MOF. Chem. Commun. 2022, 58, 10757–10767. [Google Scholar] [CrossRef]
- Rinawati, M.; Wang, Y.X.; Chen, K.Y.; Yeh, M.H. Designing a spontaneously deriving NiFe-LDH from bimetallic MOF-74 as an electrocatalyst for oxygen evolution reaction in alkaline solution. Chem. Eng. J. 2021, 423, 130204. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, Z.; Fei Qiao, F.; Gai, H.; Qiu, S.; Zhang, C.; Wang, X.; Chen, Z.; Jiang, H.; Huang, M. Unraveling volcano trend in OER of metal–organic frameworks with asymmetric configuration through energy band engineering. Appl. Catal. B Environ. 2024, 353, 124089. [Google Scholar] [CrossRef]





| MOF-Based Electrocatalysts | Results Obtained | Ref. |
|---|---|---|
| NiRu-PTA/NF | Have been used directly for alkaline seawater electrolysis as a bifunctional HER/OER electrocatalyst. At a very low overpotential of 16 mV and 298 mV, respectively, the NiRu-PTA/NF, a self-supporting electrode, has efficiently catalyzed both the HER and OER to produce 10 mA cm−2 for the HER and 20 mA cm−2 for the OER. Furthermore, following a 200-h chronopotentiometry test, the NiRu-PTA/NF//NiRu-PTA/NF integrated water electrolysis cell with NiRu-PTA/NF as both cathode and anode in a minimal cell configuration demonstrated excellent long-term stability with an unattenuated cell voltage and a very low cell voltage of 1.54 V to drive the current density of 10 mA cm−2. | [63] |
| NiCo/MXene | The hybrid seawater electrolyzer has completely eliminated the chlorine risks to cell performance in neutral or alkaline seawater by enabling hydrogen production at ultralow cell voltages of 0.7–1.0 V. Meanwhile, stable seawater electrolysis for 140 h at 500 mA cm–2 with high Faradaic efficiency can produce hydrogen at an intense rate of 9.2 mol h–1 gcat–1. Compared to seawater electrolyzers, the electricity cost has been significantly decreased by 30–52% at a high current density of 500 mA cm–2. | [64] |
| NiCo-LDH nanostructures | The electrocatalyst has demonstrated low overpotentials of 356 mV for HER and 433 mV for OER at 400 mA·cm−2 in alkaline seawater, which is significantly better than the majority of documented non-noble metal catalysts. With remarkably low cell voltages of 1.56 and 1.89 V at current densities of 10 and 400 mA·cm−2, respectively, the derived CF electrode loading of CCH@NiCo LDH has demonstrated exceptional performance as anodes and cathodes for total alkaline seawater electrolysis. Furthermore, in alkaline seawater, the strong stability of 100 h has also been shown at levels exceeding 200 mA·cm−2. | [65] |
| NiCo/MOF | Ni0.15Co0.85-MOF material has revealed exceptional electrocatalytic performance, as evidenced by low values of overpotential (1.33 V vs. RHE at 10 mA cm–2), TOF (0.47 s–1), and Tafel slope (125 mV dec–1). At a 40 mA cm–2 current density, Ni0.15Co0.85-MOF also has shown excellent stability during the 72 h tests. | [66] |
| CC@CoNC | Using the optimized CC@CoNC as both cathode and anode, the hydrazine-assisted water electrolysis system has been successfully completed. To reach 200 mA cm−2, it requires only an ultra-low cell voltage of 0.557 V and an electricity usage of 1.22 kW h per cubic meter of H2. Additionally, in the hydrazine-assisted seawater electrolysis system for H2 production, the optimized CC@CoNC has demonstrated much enhanced stability, operating steadily for more than 40 h at ≈10 mA cm−2. | [67] |
| CdFe-BDC | The electrocatalyst has possessed outstanding overpotentials of 290 mV at 100 mA cm–2 and 148 mV at 10 mA cm–2, respectively. HER and OER have demonstrated the lowest performance. In real seawater media, the entire water splitting performance needs 1.68 V to reach a current density of 10 mA cm–2, and at ambient alkaline conditions, it has produced H2 and O2 at competitive rates of 6.4 and 3.1 μL s–1, respectively. | [68] |
| NiFe-LDH/MOF | Seawater oxidation has required just 235 and 307 mV OER overpotentials to reach current densities of 20 and 100 mA·cm−2, respectively, with nearly no attenuation for a 100-h stability test at 20 mA·cm−2. | [69] |
| Ir@NiFe-MOF/NF | With overpotentials of 445 and 233 mV and a current density of 1000 mA cm−2, the material has shown remarkable alkaline seawater OER and HER characteristics. It also outperforms other previously reported bifunctional electrocatalysts, operating steadily for 400 h in alkaline seawater and requiring just 2.11 V to drive 250 mA cm−2 in a membrane electrode device. | [70] |
| MIL-(IrNiFe)@ NF | The electrocatalyst has worked really well and lasts a long time when helping to split water into hydrogen and oxygen in seawater. It only has needed a little bit of power (1.9 volts) to produce a lot of hydrogen. | [71] |
| αNPANI/MIL53(Al) | Scientists have found that adding both naphthylamine and a MOF to PANI helped it produce a lot of hydrogen better. They also have tested how well this new material has worked for at least 48 h without breaking down. | [72] |
| FeNi-LDH/MIL-88A | The electrocatalyst has worked really well, producing a good amount of energy. The electrocatalyst has also been tested for a long time—more than 200 h—and has been found to have stayed strong and worked just as well the whole time, even when using seawater. | [73] |
| Pt2/Ni(OH)2/NF | The catalyst has shown an excellent HER performance, requiring only 283 mV at 1000 mA cm−2 and high stability for 200 h. | [74] |
| 3D B-NiCoP hollow nanorod | In alkaline natural seawater electrolytes, 3D B-NiCoP hollow nanorod arrays have been reported to be capable of driving a cathodic current density of 10 mA cm–2 at an overpotential of 98 mV. In seawater electrolytes, the electrocatalyst has exhibited outstanding long-term stability for over 85 h at a high current density of 113 mA cm–2. Furthermore, in natural seawater, the 3D B-NiCoP hollow nanorod array electrocatalyst has demonstrated exceptional HER activity and stability. | [75] |
| MOF-Based Electrocatalysts | Results Obtained | Ref. |
|---|---|---|
| (Ni@ZIF67)-(Ni–Co–CoO@C) | In comparison to the ZIF67-derived nanocomposites, the material has demonstrated good HER activity and significantly increased OER activity. In alkaline seawater, the NZ700 catalyst has demonstrated the best OER activity (η10 = 281 mV versus RHE) among the catalysts, whereas the NZ500 catalyst has shown the best HER activity (η50 = 196 mV vs. RHE). | [124] |
| NiCoP-MOF | A NiCoP-MOF catalyst based on carbon paper has been used to electrolyze natural seawater for the acid-free in situ generation of hydrogen or alkaline additives due to its strong electrocatalytic activity (overpotential = 166 mV and Tafel slope = 181.2 mV dec–1). | [125] |
| Ru@CoNi-MOF | At a current density of 10 mA/cm2, the catalyst has needed an overpotential below 47 and 279 mV in order to reach OER and HER, respectively. Notably, Ru@CoNi-MOF’s mass activity for OER and HER was 25.9 and 10.6 mA mg−1, respectively, almost 15.2 and 8.8 times greater than Ni-MOF’s. | [126] |
| NiFe-based MOF | The material has demonstrated remarkable OER performance in alkaline seawater settings, with an η40 of 285 mV, in addition to a surprisingly low overpotential (η200) of just 286 mV at a current density of 200 mA cm−2 in 1 M KOH solution. Furthermore, after responding for 100 h at a high current density of 200 mA cm−2 in alkaline seawater, NFN-MOF/NF only showed 2.3% and 4.8% chronopotentiometric degradation. | [127] |
| Ni–Co-MOF | Because of their high voltammetric charge density and enhanced electrochemically accessible active surface, the electrodes have shown remarkable performance and endurance in ASWE. By focusing on kinetic parameters, electrochemical impedance spectroscopy analysis has examined the kinetics of the water oxidation reaction in the presence of Cl– ions (at concentrations ranging from 0.5 M to 3.5 M). The results have indicated that the chemical process following the initial electron transfer was the step that determines the rate. | [128] |
| Pt2/Ni(OH)2/NF | In seawater splitting, the catalyst has demonstrated exceptional catalytic activity. The overpotential was higher than the commercial 20% Pt/C, measuring 19 mV at 10 mA cm−2. A seawater electrolyzer using a Pt2/Ni(OH)2/NF cathode catalyst and an AEM has a cell voltage of just 1.46 V at 10 mA cm−2. At a current density of 200 mA cm−2, the energy consumption for generating 1 m3 H2 was 3.8 kW h, which was less than that of NF = |NF (4.3 kW h). | [74] |
| Ni-BDC/NH2-MIL-88B(Fe) | A long stability of 200 h and low overpotentials of 232 and 299 mV at 100 mA cm−2 in seawater solutions have been demonstrated by this effective OER electrocatalyst for extremely efficient seawater electrolysis. | [129] |
| Pt/T-NiFe-BDC | In alkaline seawater, the material has demonstrated competitive HER activity, achieving ultralow overpotentials of 158 and 266 mV at 500 and 1000 mA cm–2 with exceptional stability and quick kinetics. In a 500-h continuous test at 500 mA cm–2, an asymmetric water electrolyzer using Pt/T-NiFe-BDC as the cathode demonstrated no attenuation and only needed a voltage of 1.89 V to generate an industrial density of 1000 mA cm–2. | [130] |
| NH2–NiCoFe-MIL-101 | In electrolytes based on natural seawater, the bifunctional electrode has demonstrated exceptional stability and catalytic activity. Remarkably, the NH2-NiCoFe-MIL-101 two-electrode urea-assisted alkaline natural seawater electrolysis cell required only 1.56 mV to produce 100 mA cm–2, which is significantly less than the 1.78 V required for alkaline natural seawater electrolysis cells. It also has shown excellent long-term stability at a current density of 80 mA cm–2 for 80 h. | [131] |
| Ru-CoMo-LDH | Ru-CoMo-LDH∥Pt/C has demonstrated excellent electrochemical performance (i.e., overpotentials of 1.5545 and 1.731 V to generate current densities of 10 and 200 mA cm–2, respectively) and great stability in a comprehensive water splitting test conducted in natural seawater. | [132] |
| NiFe-VOx/NF | According to electrochemical tests, in alkaline natural seawater, the improved NiFe4-VOx/NF have needed only 285 mV overvoltage to reach a current density of 100 mA cm−2. Additionally, in alkaline natural seawater, the catalyst has functioned steadily at high current density for at least 100 h. | [133] |
| NC-CoNi2S4@ReS2/CC | In comparison to the control samples, thhe material has shown lesser overpotentials of 87 and 253 mV for OER and HER at 10 mA cm−2, as well as a lower Tafel slope and Rct. A 56-h CA test and 1000 cycles of cyclic voltammetry have been used to verify the higher catalytic stability. Furthermore, because of the sulfur particles’ resistance to corrosion at the interface, NC-CoNi2S4@ReS2/CC has demonstrated remarkable electrocatalytic activity in both alkaline and saltwater electrolytes. | [134] |
| 4-GInMx@NF | The material has exhibited outstanding performance, achieving low HER overpotentials (η) of 110 and 104 mV with Tafel slopes of 76 and 83 mV/dec at current density (J) of 10 mA/cm−2 in natural seawater. | [135] |
| P-Ni4Mo/CF | In alkaline seawater, the catalyst has demonstrated significant HER performance and stability, with an overpotential as low as 260 mV at a current density of 100 mA cm−2. At an overpotential of 551 mV, the P-Ni4Mo/CF achieved 1.0 A cm−2, which was marginally less than that of the Pt/C catalyst (453 mV). Furthermore, following more than 200 h of durability testing, P-Ni4Mo/CF has shown strong durability with virtually no activity loss. | [136] |
| MOF-Based Electrocatalysts | Results Obtained | Ref. |
|---|---|---|
| BPM Fe- MOF | Over the MEA containing 1.0 mg∙cm−2 of Fe-MOF, the greatest hydrogen production rate of 1.45 L∙h−1 has been recorded at 500 mA∙cm−2, which has been observed over the different current density range of 0–500 mA∙cm−2. | [146] |
| NiFe-LHD | The material has demonstrated exceptional long-term stability over 9000 h under 1.0 A cm−2 in alkaline natural seawater, as well as an industrial-level current density of 1.0 A cm−2 at overpotentials of 200 and 220 mV in alkaline simulated (1 M KOH + 0.5 M NaCl) and natural (1 M KOH + seawater) seawater, respectively. | [147] |
| NF/Ni3N@NiFe-PA | The NF/Ni3N@NiFe-PA has exhibited notable OER activity in seawater thanks to its regulated electronic state caused by the synergism between Ni and Fe species and enhanced proton-coupled electron transfer through accelerated proton movement with the help of phytic acid, which has been incorporated as a proton transfer relay, and has enhanced mass transfer provided by special superhydrophilic and superaerophobic properties. | [148] |
| Ni0.1Co0.9Se2/ NCHP | At a current density of 10 mA cm–2 in 0.5 M H2SO4, the material has demonstrated a low overpotential of 89.8 mV and a tiny Tafel slope of just 48.3 mV dec–1. Additionally, it might have shown remarkable qualities in both 0.1 M PBS and 1.0 M KOH environments at the same time. With Ni0.1Co0.9Se2/NCHP as the cathode, the PEM electrolyzer has demonstrated exceptional stability (500 mA cm–2@100 h) and a notably high critical current density (1.83 V@500 mA cm–2). | [149] |
| PVDF-modified PEM | A PEM with a high sulfonic acid density that has been modified by PVDF has been created. In liquid water electrolysis, vapor-phase water electrolysis, and direct seawater, the membrane containing 25 weight percent Nafion™/sulfonated Quino-PVDF (also known as QuinoCEM-0.25) has demonstrated good performance. It has achieved maximum current densities of 130, 480, and 240 mA cm−2 over a cell voltage of 1.8 V at 80 °C, respectively. | [150] |
| MOF-Based Electrocatalysts | Results Obtained | Ref. |
|---|---|---|
| S-Ni-MOF/Fe-MOF | The material has achieved low overpotentials of 281 and 279 mV at 100 mA cm−2 current density and has remained stable for at least 100 h. | [157] |
| (Cr2O3–CoOx) | At 500 mA cm−2, the direct electrolysis of actual seawater that has not been acidified nor alkalized produced results that have been stable for 100 h. Lewis acid-modified electrodes (Cr2O3–CoOx) in a flow electrolyzer for natural seawater have demonstrated the industrially necessary current density of 1.0 A cm−2 at 1.87 V and 60 °C. | [158] |
| Fe2P–NiCoP | In seawater media, the material has demonstrated outstanding OER catalytic activity and stability. With a Faraday efficiency of about 100%, the electrolyzer has attained a current density of 10 mA cm−2, Pt/C || Fe2P–NiCoP only needs the ultra-low voltage in saltwater media (1.525 V). | [159] |
| MOF/Fe2O3 | At an overpotential of 410 mV, the material has achieved a current density of 1 A cm−2, which was approximately 200 percent greater than that of IrO2 that is employed in commercial settings. Because of its selective anodic reaction and anti-corrosive operating mode, the heterostructured catalyst has demonstrated long-lasting performance against chlorine corrosion for over 350 h at a higher current density of ∼1.5 A cm−2. | [160] |
| 2D Ni Nanoplates | Compared to RuO2, Ni nanoplates have demonstrated significantly lower cell voltages of 267 and 393 mV at current densities of 500 and 1000 mA cm–2. Interestingly, a durability test at 100 mA cm–2 revealed that the cell voltage changed very little over the course of 90 h. | [161] |
| RuFe-MOF-PA60 | This material has outlasted commercial Pt/C||IrO2 systems in terms of durability and has shown stable operation for 70 h at an industrial current density of 50 mA cm−2. The catalyst outperformed the majority of MOF-based bifunctional systems, achieving ultralow overpotentials of 255 mV (OER) and 70 mV (HER) at 10 mA cm−2. | [162] |
| Pt@FeCoNi phosphide nanosheet arrays | The material has outperformed conventional Pt/C by accomplish an ultralow overpotential of 17 mV at −10 mA cm−2. It has also steadily delivered industrial-level current densities up to 2000 A m−2 for more than 2400 h with low energy consumption (4.16 kWh/Nm3 H2) and no voltage deterioration. | [163] |
| Ce–Co(OH)2@FeOOH | The system has shown exceptional stability, sustaining electrolysis for 400 h in high-salinity conditions (2 M NaCl) and for 2500 h at 2 A cm–2. | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsacheva, I.; Yazici, M.S.; Mahadi, A.H.; Uzunoglu, A.; Uzun, D. Metal–Organic Frameworks for Seawater Electrolysis and Hydrogen Production: A Review. Electrochem 2025, 6, 37. https://doi.org/10.3390/electrochem6040037
Tsacheva I, Yazici MS, Mahadi AH, Uzunoglu A, Uzun D. Metal–Organic Frameworks for Seawater Electrolysis and Hydrogen Production: A Review. Electrochem. 2025; 6(4):37. https://doi.org/10.3390/electrochem6040037
Chicago/Turabian StyleTsacheva, Ivelina, Mehmet Suha Yazici, Abdul Hanif Mahadi, Aytekin Uzunoglu, and Dzhamal Uzun. 2025. "Metal–Organic Frameworks for Seawater Electrolysis and Hydrogen Production: A Review" Electrochem 6, no. 4: 37. https://doi.org/10.3390/electrochem6040037
APA StyleTsacheva, I., Yazici, M. S., Mahadi, A. H., Uzunoglu, A., & Uzun, D. (2025). Metal–Organic Frameworks for Seawater Electrolysis and Hydrogen Production: A Review. Electrochem, 6(4), 37. https://doi.org/10.3390/electrochem6040037









