Study on the Corrosion Resistance and Application of Nano-Y2O3/Al2O3-Modified Anchor Rod Coatings Based on Electrodeposition Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
2.3. Corrosion Resistance Test
2.3.1. Electrochemical Test
2.3.2. Immersion Accelerated Corrosion Experiment
2.4. Microhardness Test
2.5. Contact Angle Text
2.6. Friction Performance Test
3. Results and Discussion
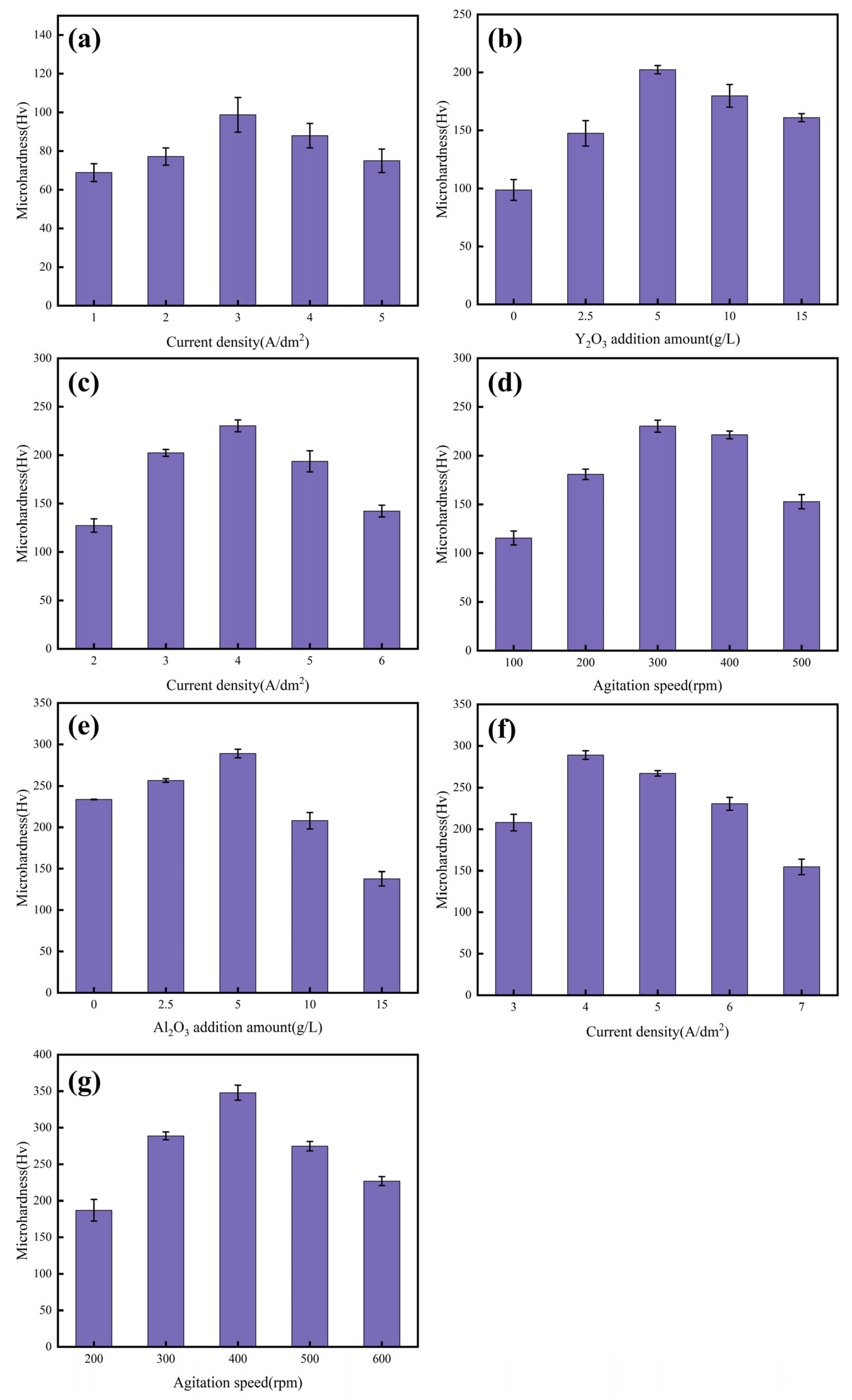
3.1. Preparation of Coating
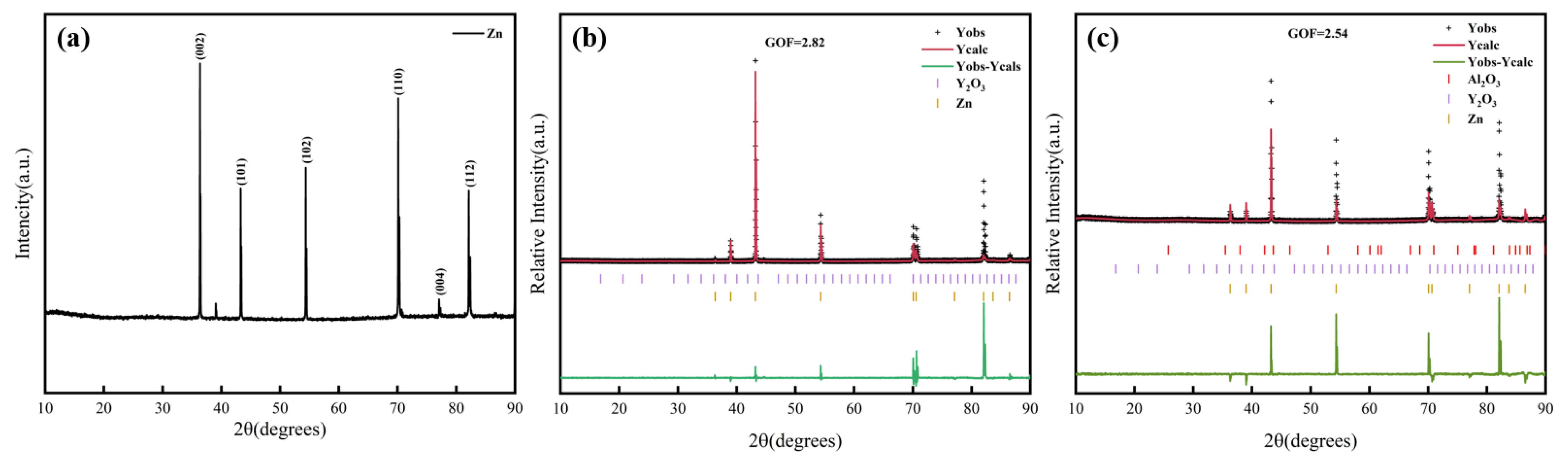
3.2. X-Ray Diffraction (XRD)
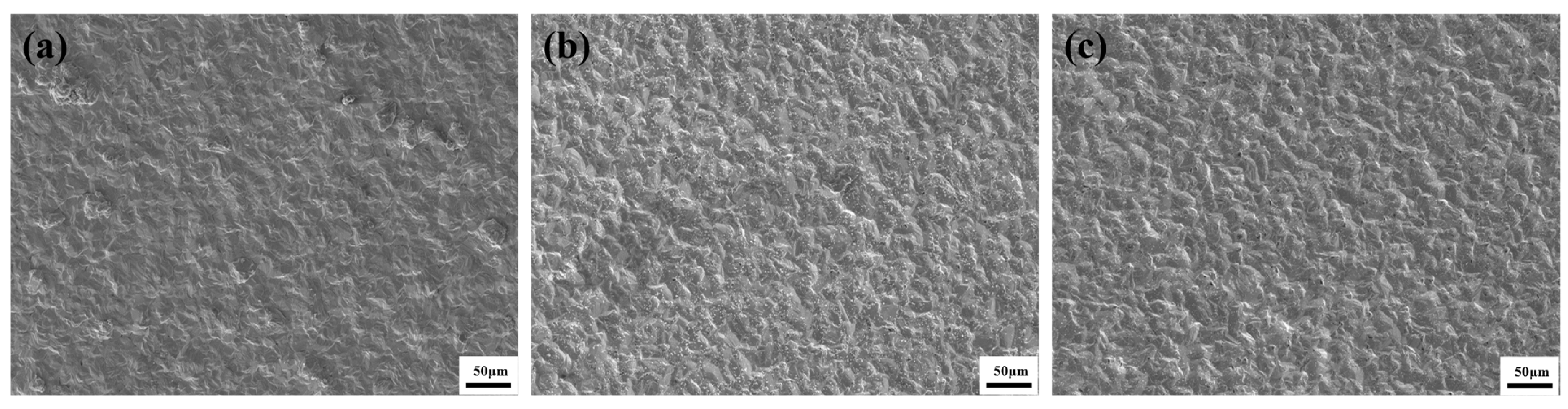
3.3. Surface Morphology of Composite Coatings
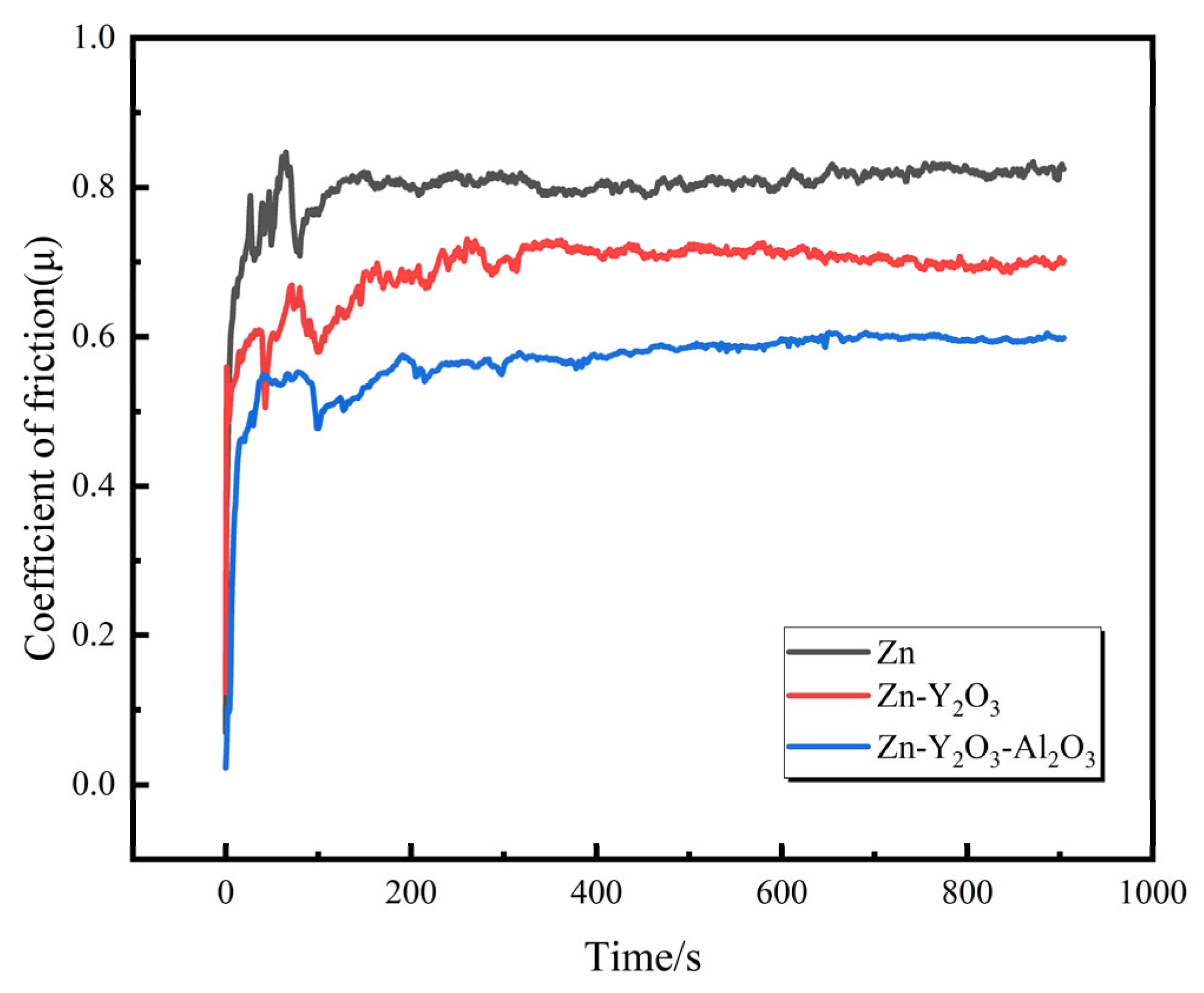
3.4. Wear Resistance Test
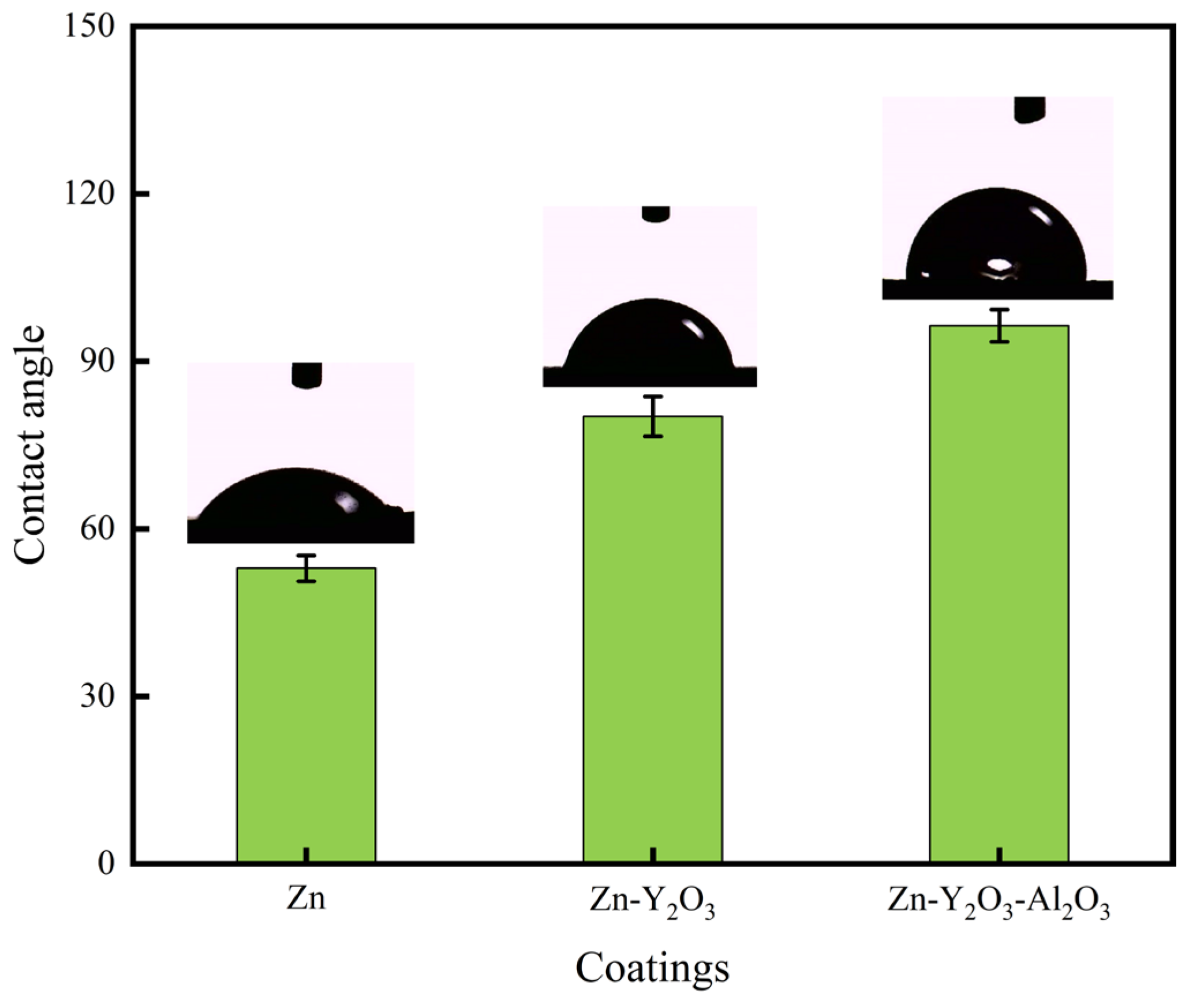
3.5. Contact Angle Text
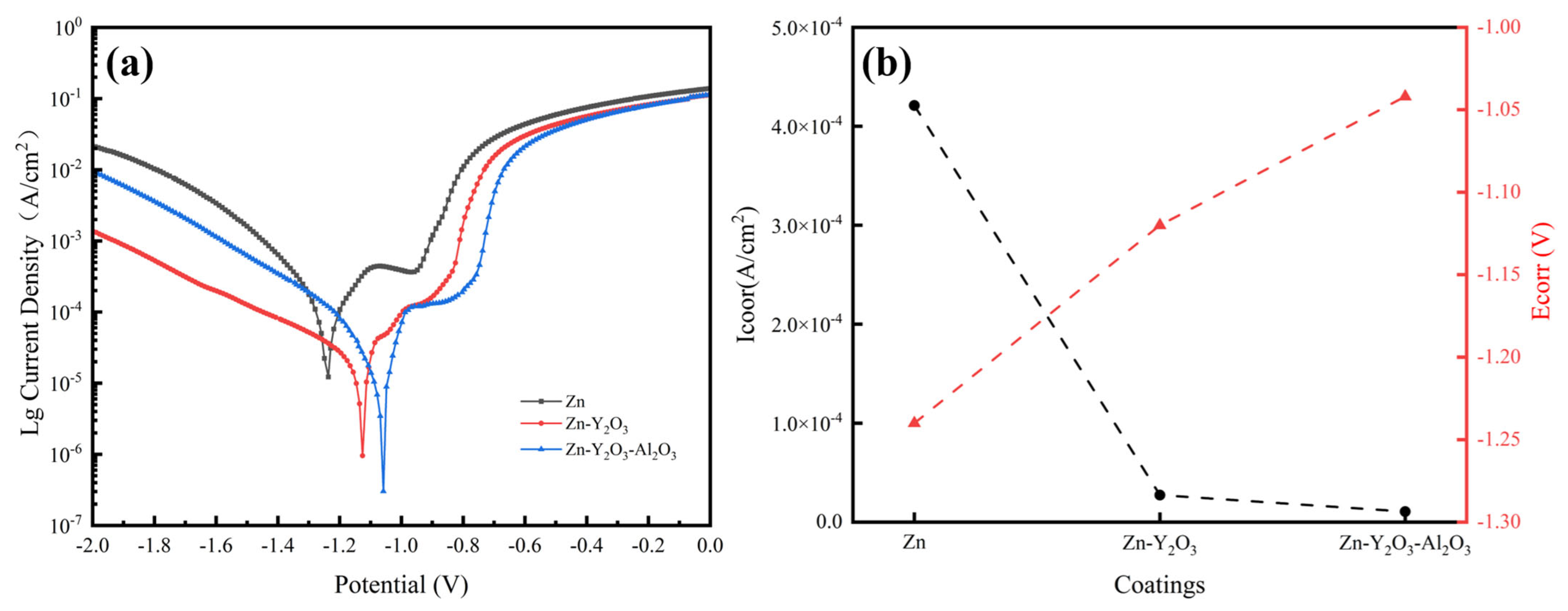
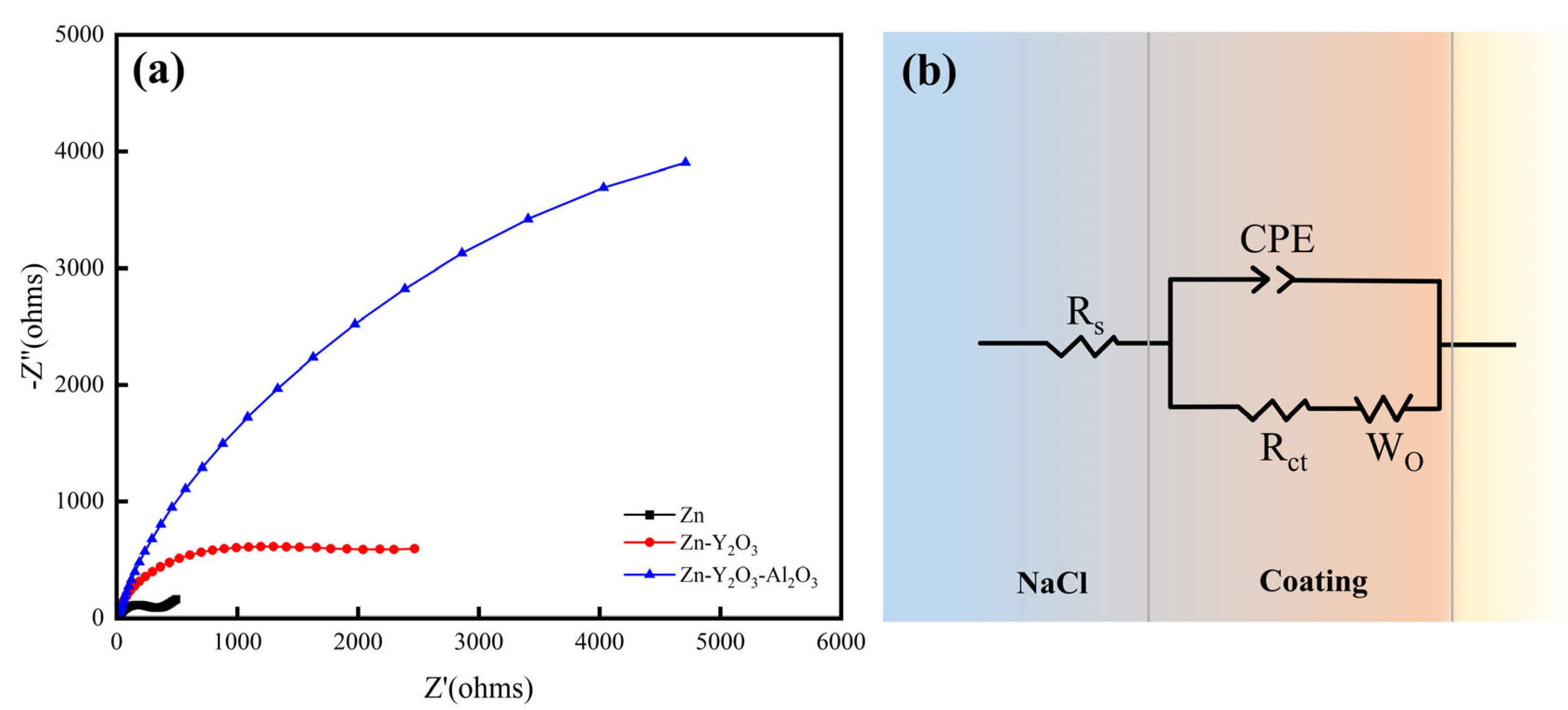
3.6. Electrochemical Test
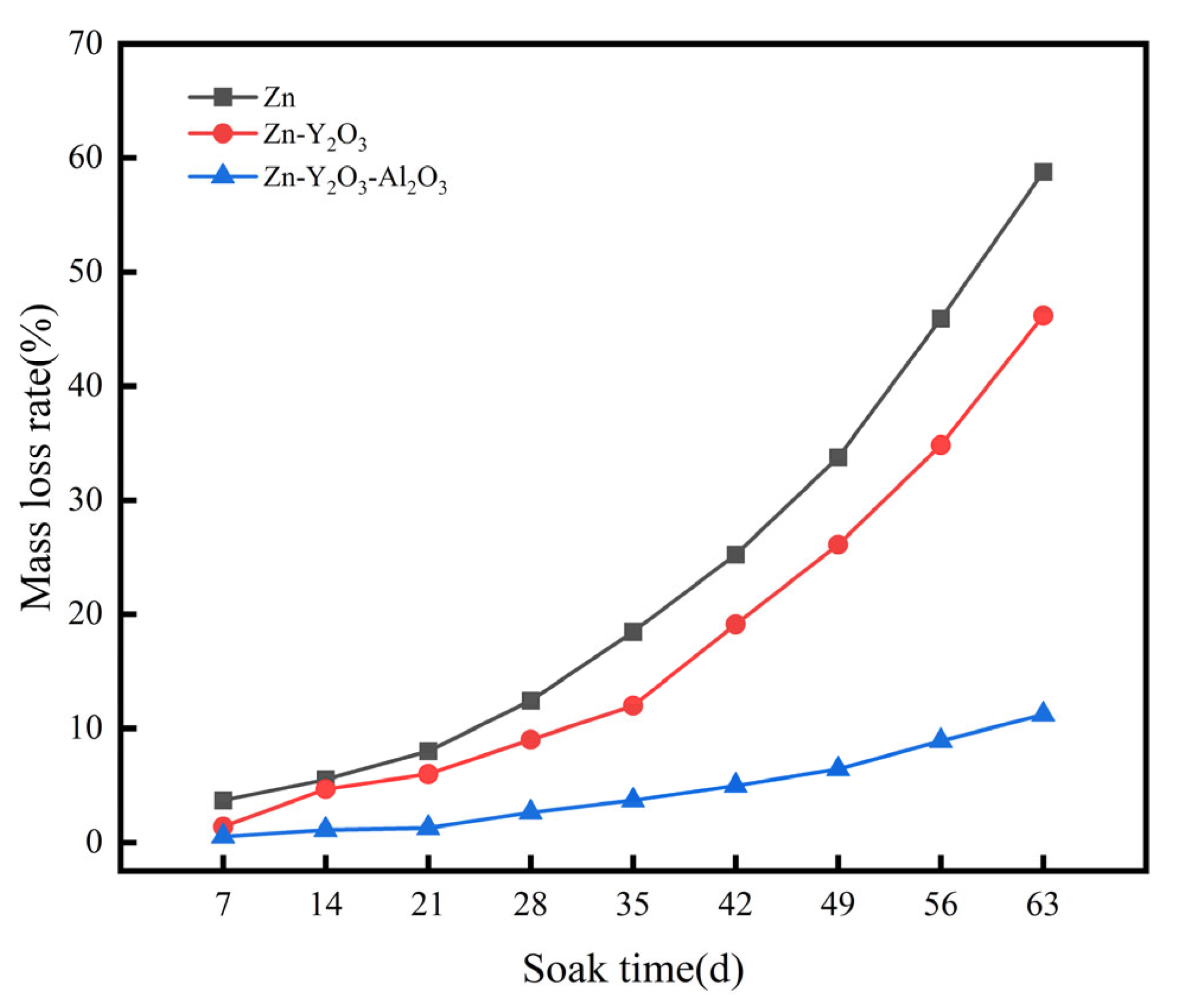
3.7. Immersion Accelerated Corrosion Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FSEM | Field Emission Scanning Electron Microscope |
| EIS | Electrochemical impedance spectroscopy |
References
- Zhang, J.; Zhuo, Q.-S.; Yang, S.; Yang, T.; Wang, B.; Bai, W.-Y.; Wu, J.-J.; He, Y.-F.; Li, H.-R. Study on Antiweathering Support Technology of Thin Spray-On Liners in Shallowly Buried Coal Seam Roadway under Corrosion Condition. Shock Vib. 2022, 2022, 7065650. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Z.; You, H.; Xu, H.; Bai, L.; Yang, S.; Zhang, M.; Shi, Z. Field Pull-Out Test and Analysis of Fiberglass Anchors in Sanshandao Gold Mine. Geofluids 2022, 2022, 5160876. [Google Scholar] [CrossRef]
- Chu, H.; Li, G.; Liu, Z.; Liu, X.; Wu, Y.; Yang, S. Multi-Level Support Technology and Application of Deep Roadway Surrounding Rock in the Suncun Coal Mine, China. Materials 2022, 15, 8665. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, H.; Zou, J.; Lin, N.; Ma, Y.; Wang, Z.; Tian, W.; Yao, X.; Han, P.; Wang, Z.; et al. Effect of adding Lanthanum (La3+) on surface performance of Ni-P electroless plating coatings on RB400 support anchor rod steel. Int. J. Electrochem. Sci. 2016, 11, 3269–3284. [Google Scholar] [CrossRef]
- Aritan, A.E.; Can, M.F. The corrosion effect on supports used in underground mining operations generated by low-rank salt-bearing coals: The Central Anatolia case. Arab. J. Geosci. 2019, 12, 200. [Google Scholar] [CrossRef]
- Wu, S.; Northover, M.; Craig, P.; Canbulat, I.; Hagan, P.C.; Saydam, S. Environmental influence on mesh corrosion in underground coal mines. Int. J. Min. Reclam. Environ. 2018, 32, 519–535. [Google Scholar] [CrossRef]
- Ekolu, S.O.; Diop, S.; Azene, F. Potentiodynamic polarization study of the corrosion characteristics of acid mine drainage. In Construction Materials and Structures; IOS Press: Amsterdam, The Netherlands, 2014; pp. 1436–1441. [Google Scholar] [CrossRef]
- Zhang, Y.; An, G.; Mao, J.; Chen, A.; Wang, Q.; Qing, S.; Zhang, H.; Xiao, Y.; Mao, J.; Liu, W.; et al. A safe and environmental-friendly solid acid with sustained release, high etching, and low corrosion. Geoenergy Sci. Eng. 2024, 237, 212785. [Google Scholar] [CrossRef]
- Pan, J.; Ma, Y.; Zhang, L.; Ning, Z.; Zhang, Y.; Xi, X. Effect of Chemical Corrosion on Rock Fracture Behavior in Coastal Deep Mines: Insights from Mechanical and Acoustic Characteristics. J. Mar. Sci. Eng. 2024, 12, 869. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, G.; Han, Z.; Ji, Y.; Zhang, Y.; Zhang, C.; Luo, H.; Lu, Y.; Cao, C.; Xu, Z.; et al. Research on the influence of major ions in weakly alkaline mine water on anchor cable corrosion and protection technique. Case Stud. Constr. Mater. 2024, 21, e03584. [Google Scholar] [CrossRef]
- Weber, J.Z. Corrosion of Rock Anchors in Illinois Coal Basin Mines. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2013. [Google Scholar]
- Liu, H.; Zhou, G.; Ji, Y.; Rong, X. Research on the corrosion behavior of underground cables influenced by different ions. J. Constr. Steel Res. 2025, 226, 109194. [Google Scholar] [CrossRef]
- Wei, X.; Lu, S.; Ding, J.; Zheng, S.; Chen, Z.; Lu, J.; Liu, Z.; Yin, P.; Du, N.; Yang, W.; et al. Enhanced corrosion resistance of a novel periodic multilayered Si/(Si, N)-DLC coating against simulated coal mine water. Ceram. Int. 2024, 50, 49385–49399. [Google Scholar] [CrossRef]
- Moloto, A.; Seshweni, M.H.E.; Aribo, S.; Falodun, O.E.; Olalemi, O.T.; Ige, O.O.; Olubambi, P.A. Application of Raman spectroscopy and X-ray diffraction to study the erosion -corrosion of UNS S32205 in mine water. Mater. Today Proc. 2020, 28, 1273–1277. [Google Scholar] [CrossRef]
- Wu, S.; Guo, J.; Shi, G.; Li, J.; Lu, C. Laboratory-Based Investigation into Stress Corrosion Cracking of Cable Bolts. Materials 2019, 12, 2146. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-K.; Chien, S.-H.; Li, H.; Monnell, J.D.; Dzombak, D.A.; Vidic, R.D. Corrosion Control when Using Passively Treated Abandoned Mine Drainage as Alternative Makeup Water for Cooling Systems. Water Environ. Res. 2011, 83, 807–814. [Google Scholar] [CrossRef]
- Hadjigeorgiou, J.; Savguira, Y.; Thorpe, S.J. Comparative Susceptibility to Corrosion of Coated Expandable Bolts. Rock. Mech. Rock. Eng. 2019, 52, 2665–2680. [Google Scholar] [CrossRef]
- Ma, K.J.; Stankus, J.; Faulkner, D. Development and evaluation of corrosion resistant coating for expandable rock bolt against highly corrosive ground conditions. Int. J. Min. Sci. Technol. 2018, 28, 145–151. [Google Scholar] [CrossRef]
- Meikle, T.; Tadolini, S.C.; Sainsbury, B.A.; Bolton, J. Laboratory and field testing of bolting systems subjected to highly corrosive environments. Int. J. Min. Sci. Technol. 2017, 27, 101–106. [Google Scholar] [CrossRef]
- Zellele, D.M.; Yar-Mukhamedova, G.S.; Rutkowska-Gorczyca, M. A Review on Properties of Electrodeposited Nickel Composite Coatings: Ni-Al2O3, Ni-SiC, Ni-ZrO2, Ni-TiO2 and Ni-WC. Materials 2024, 17, 5715. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.D.; Olivier, M.G.; To, T.X.H. A review: Hydrotalcite layers as protective coatings on zinc and zinc alloys. Vietnam. J. Sci. Technol. 2024, 62, 1047–1064. [Google Scholar]
- Popoola, A.P.I.; Fayomi, O.S. Performance Evaluation of Zinc Deposited Mild Steel in Chloride Medium. Int. J. Electrochem. Sci. 2011, 6, 3254–3263. [Google Scholar]
- Basavanna, S.; Naik, Y.A. Electrochemical studies of Zn-Ni alloy coatings from acid chloride bath. J. Appl. Electrochem. 2009, 39, 1975–1982. [Google Scholar] [CrossRef]
- Dan, A.; Bijalwan, P.K.; Pathak, A.S.; Bhagat, A.N. A review on physical vapor deposition-based metallic coatings on steel as an alternative to conventional galvanized coatings. J. Coat. Technol. Res. 2022, 19, 403–438. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids. Appl. Sci. 2020, 10, 5352. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Cao, W. Recent advances in spinel-based protective coatings produced by electrochemical method on metallic interconnects for solid oxide fuel cells. Int. J. Hydrog. Energy 2024, 50, 977–991. [Google Scholar] [CrossRef]
- Tian, S.; Feng, Y.; Zheng, Z.; He, Z. TiO2-Based Photocatalytic Coatings on Glass Substrates for Environmental Applications. Coatings 2023, 13, 1472. [Google Scholar] [CrossRef]
- Zhang, Q.; He, L.; Hao, C.; Zhao, Z.; Lu, Y.; Wang, L. Influence of Y2O3 content on the properties of Ni-Y2O3-MgO nanocomposite coatings prepared by pulse electrodeposition. Int. J. Electrochem. Sci. 2024, 19, 100769. [Google Scholar] [CrossRef]
- Jia, Z.-W.; Sun, W.-C.; Guo, F.; Dong, Y.-R.; Liu, X.-J. Microstructure, friction and corrosion resistance properties of a Ni-Co-Al2O3 composite coating. RSC Adv. 2018, 8, 12138–12145. [Google Scholar] [CrossRef]
- Roventi, G.; Giuliani, G.; Pisani, M.; Bellezze, T. Electrodeposition of Zn-Ni-ZrO2, Zn-Ni-Al2O3 and Zn-Ni-SiC Nanocomposite Coatings from an Alkaline Bath. Int. J. Electrochem. Sci. 2017, 12, 663–678. [Google Scholar] [CrossRef]
- Kallappa, D.; Venkatarangaiah, V.T. Synthesis of CeO2 doped ZnO nanoparticles and their application in Zn-composite coating on mild steel. Arab. J. Chem. 2020, 13, 2309–2317. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, A.P.I.; Fayomi, O.S.I. The effect of nanoparticulate loading on the fabrication and characterization of multi-doped Zn-Al2O3-Cr2O3 hybrid coatings on mild steel. J. Adv. Manuf. Technol. 2017, 90, 2443–2452. [Google Scholar] [CrossRef]
- Kanyane, L.R.; Gandazha, K.; Fayomi, O.S.I.; Popoola, A.P.I. Microstructural evolution and mechanical properties of Zn-Ni composite coating with Y2O3 as a dopant. Procedia Manuf. 2019, 35, 814–819. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, A.P.I.; Fayomi, O.S.I.; Loto, C.A. Multifaceted incorporation of Zn-Al2O3/Cr2O3/SiO2 nanocomposite coatings: Anti-corrosion, tribological, and thermal stability. J. Adv. Manuf. Technol. 2016, 82, 1335–1341. [Google Scholar] [CrossRef]
- Vathsala, K.; Venkatesha, T.V. Zn-ZrO2 nanocomposite coatings: Elecrodeposition and evaluation of corrosion resistance. Appl. Surf. Sci. 2011, 257, 8929–8936. [Google Scholar] [CrossRef]
- Wu, T.; Ma, M.; Ding, K.; Nan, X.; Wang, Z.; Wei, X.; Zhu, X. Effect of Y2O3 nanoparticles on the microstructure and corrosion resistance of electrodeposited Ni-Mo-Y2O3 nanocomposite coatings. Int. J. Electrochem. Sci. 2023, 18, 100095. [Google Scholar] [CrossRef]
- Praveen, B.M.; Venkatesha, T.V. Electrodeposition and properties of Zn-nanosized TiO2 composite coatings. Appl. Surf. Sci. 2008, 254, 2418–2424. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Chandrashekarappa, M.P.G.; Kulkarni, R.M.; Pimenov, D.Y.; Giasin, K. The Effect of Zn and Zn-WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. Materials 2021, 14, 2253. [Google Scholar] [CrossRef]
- Shen, X.; Sheng, J.; Zhang, Q.; Xu, Q.; Cheng, D. The Corrosion Behavior of Zn/Graphene Oxide Composite Coatings Fabricated by Direct Current Electrodeposition. J. Mater. Eng. Perform. 2018, 27, 3750–3761. [Google Scholar] [CrossRef]
- Alagi, P.; Ghorpade, R.; Choi, Y.J.; Patil, U.; Kim, I.; Baik, J.H.; Hong, S.C. Carbon Dioxide-Based Polyols as Sustainable Feedstock of Thermoplastic Polyurethane for Corrosion-Resistant Metal Coating. ACS Sustain. Chem. Eng. 2017, 5, 3871–3881. [Google Scholar] [CrossRef]
- Jin, W.; Xiao, S.; Kou, Q.; Ding, D.; Zhang, J.; Fang, X.; Ge, C.; Zhong, C.; Zhu, H.; Haarberg, G.M. Preparation of diboride coatings by electrophoretic deposition in nanoparticle-containing molten inorganic salts. Mater. Lett. 2022, 306, 130908. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Microstructure and corrosion behaviours of composite coatings on S355 offshore steel prepared by laser cladding combined with micro-arc oxidation. Appl. Surf. Sci. 2019, 497, 143703. [Google Scholar] [CrossRef]
- Ren, A.; Kang, M.; Fu, X. Corrosion behaviour of Ni/WC-MoS2 composite coatings prepared by jet electrodeposition with different MoS2 doping concentrations. Appl. Surf. Sci. 2023, 613, 155905. [Google Scholar] [CrossRef]



| Composition | Concentration (g/L) | Parameters | |
|---|---|---|---|
| ZnCl2 | 150 (±0.01) | Temperature (°C) | 25 (±2) |
| KCl | 50 (±0.01) | pH | 3.8 (±0.1) |
| H3BO3 | 30 (±0.01) | Plating time (min) | 45 |
| Y2O3 | 0–15 (±0.01) | ||
| Al2O3 | 0–15 (±0.01) |
| Sample | Rs (Ω cm2) | Q (μΩ−1 cm2Sn) | n | ZWo (μΩ−1 cm2Sn) | Rct (Ω cm2) |
|---|---|---|---|---|---|
| Zn | 8.05 | 8.17 × 10−5 | 0.74 | 5.00 × 10−3 | 322.1 |
| Zn-Y2O3 | 6.89 | 1.60 × 10−5 | 0.89 | 1.21 × 10−3 | 1299.0 |
| Zn-Y2O3-Al2O3 | 5.18 | 1.01 × 10−5 | 0.90 | 1.10 × 10−4 | 5947.0 |
| Salt Ions and Heavy Metals | Concentration (mg/L) |
|---|---|
| SO42− | 1170 |
| Cl− | 342 |
| NO3− | 67 |
| Na+ | 102 |
| Ca2+ | 27.13 |
| Mg2+ | 10.54 |
| Fe | 16.47 |
| Pb | 0.14 |
| As | 0.0512 |
| Cd | 0.0126 |
| Cr | 0.016 |
| Mn | 1.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Qiu, F. Study on the Corrosion Resistance and Application of Nano-Y2O3/Al2O3-Modified Anchor Rod Coatings Based on Electrodeposition Method. Electrochem 2025, 6, 14. https://doi.org/10.3390/electrochem6020014
Feng X, Qiu F. Study on the Corrosion Resistance and Application of Nano-Y2O3/Al2O3-Modified Anchor Rod Coatings Based on Electrodeposition Method. Electrochem. 2025; 6(2):14. https://doi.org/10.3390/electrochem6020014
Chicago/Turabian StyleFeng, Xiujuan, and Falong Qiu. 2025. "Study on the Corrosion Resistance and Application of Nano-Y2O3/Al2O3-Modified Anchor Rod Coatings Based on Electrodeposition Method" Electrochem 6, no. 2: 14. https://doi.org/10.3390/electrochem6020014
APA StyleFeng, X., & Qiu, F. (2025). Study on the Corrosion Resistance and Application of Nano-Y2O3/Al2O3-Modified Anchor Rod Coatings Based on Electrodeposition Method. Electrochem, 6(2), 14. https://doi.org/10.3390/electrochem6020014






