Abstract
In this study, an electrochemical sensor for the monitoring of Hg (II) at trace levels by using differential pulse anodic stripping voltammetry has been reported. Basically the electrochemical sensor is a Phanerochaete chrysosporium-based carbon paste electrode. Here, Phanerochaete chrysosporium has played a new vital role in electrochemical detection of heavy metal apart from its known contribution in their removal. Optimal voltammetric response was observed at −0.7 V deposition potential l, 5% biomass concentration ratio (w/w), and neutral pH conditions with 12 min as the accumulation time. Selectivity was evaluated in the presence of different interfering cations. Linear range was observed for 5–50 µgL−1 of metal concentration with a detection limit of 4.4 µgL−1. The equivalence of new and reference analytical methods was statistically assessed in mercury samples collected from chlor-alkali industrial effluent by correlation of results (Pearson’s product-moment correlation), weighted Deming regression analysis, paired comparison test, relative standard deviation (RSD), median relative error (MRE), root mean square error (RMSE), and predicted residual sum of square (PRESS). This work presented a simple, efficient, and promising analytical tool in trace level detection of Hg (II), as compared to previously reported carbon paste electrodes based on biological material.
1. Introduction
During the last few decades, mercury has received a constant attention from international environmental protection agencies, being the most toxic global pollutant [1]. Recently, a new legally binding global treaty titled ‘The Minamata Convention on Mercury’ was signed on 10 October 2013 that aims to reduce mercury emission [2]. This shows that the environment protection agencies have performed a consistent effort to study the emission sources and global effects of mercury on humans. As a result, now we are able to understand that mercury has bio accumulated in the food chains and water resources at an alarming level [3]. In Pakistan, the natural water resources are heavily contaminated by mercury beyond the WHO and Pakistan Environmental protection agency (Pak-EPA) permissible limit of 1 µgL−1 [4]. This situation has developed despite the presence of drafted legal frameworks, laws, and waste management plans designed specifically for mercury removal. In fact, overburden of population, unchecked disposal of industrial effluent, and municipal waste into water reservoirs has resulted in elevated levels of mercury in our surrounding [5]. Mercury exists in three chemical states viz., elemental, inorganic, and organic. This study is focused on inorganic mercury that exhibits two different forms. Among these two inorganic species, Hg (II) is more common in natural water resources due to its inherent stability and more solubility than Hg (I) [6].
In addition to the natural emission of mercury from mineral deposits, forest fires, and volcanoes in Pakistan, it is also anthropogenically added by industry of chlor-alkali, bulb, fluorescent lamps, and medical devices [7]. Mercury pollution results in toxicological effects of brain damage, paralysis, blindness, and chromosome breakage [8]. Hence, there is an utmost need to detect mercury at trace levels for its control and regulatory purposes.
Compared with the benchmark methods (atomic spectrometry) of mercury analysis, electrochemical measurements are more sensitive and have no limitations of expensive instrumentation, well-trained operators, or prerequisite of sample preparation [9]. In electrochemical analysis, stripping voltammetry has, in particular, attracted significant interest for trace analysis of heavy metals due to its excellent sensitivity, short analysis time, low power consumption, and cheap equipment [10]. A combination of pre concentration and stripping steps in stripping voltammetry has made it a reliable method for trace level detection of mercury. Efficient pre concentration of target species onto a certain substrate has a great role in stripping analysis. Many solid electrodes have been reported in the literature for stripping analysis of mercury [11]. However, fouling of the surface or passivation is a common problem to these electrodes [12] and rigorous mechanical or electrochemical pretreatment procedures are adopted to achieve reproducibility [13].
Modified carbon paste electrodes have proved themselves an effective alternate of solid electrodes, as they have wide potential range, robustness, stable response, low ohmic resistance, and chemical inertness. Moreover, the problem of passivation in this case is simply solved by renewal of their surface [14]. Readily renewable surfaces and ease of modification are the two features that gave an edge to modified carbon paste electrodes over other electrodes. Modification can be achieved through chemical or biological agents. Biologically modified carbon paste electrodes are considered environmentally friendly, as they are based on materials of biological origin. Microorganisms, such as fungi, can be potent source of modification in carbon paste electrodes, as they are a rich source of functional moieties that can serve as ligand sites for attachment of heavy metal ions [15]. Moreover, it is easy and economical to have large scale production of microbial biomass, which greatly simplify the fabrication process and enhance the performance of electrode. [16] As adsorption media, white rot fungi have played a considerable role in heavy metal removal [17] and among white rot fungi, maximum work had been reported on Phanerochaete chrysosporium mycelium. It had also been employed in case of Hg (II) adsorption removal studies [18,19]. However, until this point, biosorption capacity of any fungal species had not been utilized in electroanalytical detection of Hg (II).
In the present study, dead biomass of Phanerochaete chrysosporium was used in preparation of modified carbon paste electrode, which was later employed for the stripping analysis of Hg (II). Various stripping parameters, such as deposition potential, biomass-to-carbon ratio, pH of accumulating medium (analyte solution), and accumulation time were optimized on standard Hg (II) solution. Interference studies were also carried out in the presence of alkali (Na+, K+), alkaline earth (Ca2+, Mg2+), and other heavy metal ions (Fe3+, Mn2+, Ni2+, Pb2+, Cd2+, Cu2+, As3+).
High correlation coefficient value does not indicate the adequacy of new methods, as there could be systematic difference between the new and reference methods [20]. Paired comparison test can be misleading if there exists a proportional or mixed proportional-additive error in the results of two methods [21]. Likewise, poor repeatability of results can provide slope and intercept values of 1 and 0 in regression analysis due to wide confidence intervals [22]. Thus, proper statistical treatment of data generated by new and reference methods is required, as a single statistical parameter would be insufficient and can disguise the method differences.
2. Experimental
2.1. Reagents and Solutions
An amount of 1 mgL−1 stock solution of Hg (II) was prepared from its chloride salt (Merck, Rahway, NJ, USA) and working solutions were prepared on daily basis by the dilution of stock solution. Analytical grade graphite powder (Alfa Aesar, Haverhill, MA, USA) and mineral oil (MP, Biomedicals, Santa Ana, CA, USA) were used in preparation of modified carbon paste electrodes. The pH was adjusted using 0.1 M HCl and/or 0.1 M NaOH solutions and measured by Eutech pH 2700 (Singapore) instrument. All the other chemicals (HCl, HNO3, H2SO4, KCl, NaCl, CaCl2, MgCl2, FeCl3, MnCl2, CdCl2, Pb(NO3)2, NiCl2, As2O3, CuCl2) used were also of analytical grade.
2.2. Microorganism’s Culture Conditions
The nutrient medium of Phanerochaete chrysosporium (ATTC 24725) was prepared and autoclaved, according to a method reported in the literature [23]. Erlenmeyer flasks containing 100 mL of nutrient media were inoculated with 1 × 107 conidia and left on a shaker (110 rpm) for 15 days at 35 °C. Later, it was harvested, washed with double distilled water, and dried in oven at 90 °C for 24 h. The dried biomass was then grinded and sieved through mesh of 250 µm.
2.3. Preparation of Modified Carbon Paste Electrode
Graphite powder (67.25–63% w/w) and mineral oil (32.25–27% w/w) were the principal components of modified carbon paste electrodes while Phanerochaete chrysosporium (0.5–10% w/w) acted as modifier. All of these three components were mixed in varying amounts until a homogenized paste was obtained. Then, it was packed into the plastic electrode body (i.d 5 mm), as described in our previous work [24]. Surface of the prepared modified electrode was smoothened with a shiny weighing paper. Unmodified carbon paste electrode was prepared in similar way without the addition of modifier.
2.4. Apparatus and Voltammetric Procedure
Differential pulse anodic stripping (DPASV) and cyclic voltammetric (CV) studies were carried out with a Potentiostat/Galvanostat (Reference 600 TM, Gamry, Munich, Germany) instrument equipped with Gamry Framework and Echem Analyst software. This was three electrode-based cell system in which modified carbon paste electrode, platinum wire, saturated calomel electrode were taken as working, auxiliary, and reference electrodes. Stripping analysis was performed by dipping the modified carbon paste electrode in 10 µgL−1 of standard Hg (II) solution under specific pH conditions for a predetermined accumulation time at a particular deposition potential. After this, the modified carbon paste electrode was taken out, washed with double distilled water and dipped in stripping medium (0.1 M HCl), where an anodic scan was run in the range of −0.7 to +0.6 V with scan rate of 100 mV/s. Initially different parameters, such as deposition potential (−0.3 to −0.8 V), biomass concentration (0.5–10%), pH of analyte/accumulating solution (2–12), and accumulation time (2–20 min), were optimized on standard analyte solution. In addition to these parameters, linear range, limit of detection, and interference studies were also evaluated. In the end, Hg (II) analysis in real samples was carried out under these optimized experimental parameters.
In order to cross validate efficiency of current method, inductively coupled plasma optical emission spectrometric (ICP-OES) technique was taken as the reference method. ICP-OES used was a Perkin Elmer Optima DV 2100 model with axial viewing configuration.
2.5. Surface Characterization of Modified Carbon Paste Electrode
FTIR spectra (Alpha Bruker, Ettlingen, Germany) of unloaded and Hg (II)-loaded modified carbon paste was taken in the range of 4000–400 wavenumber cm−1, and data were evaluated with OPUS 5.5 software. Sample was directly placed on the ATR accessory of instrument. Later, respective spectrum was recorded by irradiating samples with infrared beam at an angle of 45°.
2.6. Collection of Mercury Samples from Chlor-Alkali Industrial Effluent
Twenty mercury samples were collected from a local chlor-alkali industry at different discharge points. These samples were collected in properly capped plastic bottles and kept refrigerated until further use. Mercury concentration was determined in each sample with current voltammetric method and ICP-OES independently.
2.7. Statistical Comparison of New and Reference Method
Results of new and reference methods run independently on the same sample were compared statistically by correlation of results (Pearson’s product–moment correlation) followed by regression analysis and paired comparison test. Additional statistical metrics, such as relative standard deviation (RSD), median relative error (MRE), root mean square error (RMSE), and predicted residual sum of square (PRESS), were calculated by applying their standard equations and used to assess the agreement of results between the new and reference methods for individual samples.
All the data were statistically analyzed by IBM SPSS Statistics (version 20©) and MedCalc (version 15.8©) software.
3. Results and Discussion
3.1. Surface Characterization of Unloaded and Loaded Carbon PASTE Electrode
The FTIR spectrum (Figure 1, spectrum a) revealed the complex nature of Phanerochaete chrysosporium-based carbon paste electrode. The absorption bands at 3262.93 cm−1, 1647.86 cm−1, and 1018 cm−1 can be attributed to –OH group, C=O stretch in carboxyl or in amide I and amide II groups and C-O stretch of ether group [25]. In the case of Hg (II)-loaded modified carbon paste electrode (Figure 1, spectrum b), the peak at 3262.93 cm−1 disappeared while other peaks at 1647.86 cm−1 and 1018 cm−1 observed red shifts to 1721.30 cm−1 and 1123.7 cm−1, respectively. This clearly indicated the participation of these functional moieties in the binding of Hg (II). Asymmetric stretch of aliphatic chains (-CH) gave their peak at 2921.30 cm−1 in an unloaded electrode and its intensity remained almost unchanged in a loaded one (2920.19 cm−1), showing that it did not participate towards the adherence of analyte ions.
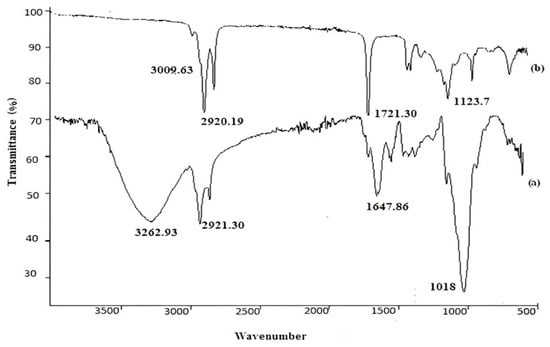
Figure 1.
FTIR spectra of unloaded (a) and Hg (II)-loaded (b) Phanerochaete chrysosporium-based working electrode.
3.2. Electrochemical Characterization of Modified Carbon Paste Electrode
In our reported study [26], detailed cyclic voltammetric mechanism of modified and unmodified carbon paste electrodes with 5 mM K3 [Fe (CN)6] in 0.1 M KCl solution within the range of −0.2 to 0.6 V with a scan rate 100 mV/s has been discussed. Modified carbon paste electrode gave a more pronounced anodic (253.9 mV) and cathodic peak (108.1 mV), as compared to unmodified one [26]. This is due to the fact that the addition of a modifier to the carbon paste electrode reduces the electrical resistance and facilitates the charge transfer mechanism. Further, it also contributes to a large effective electrochemical surface area of modified electrode (0.2844 cm2), in comparison to an unmodified one (0.1248 cm2).
3.3. Stripping Procedure and Effect of Stripping Electrolyte
In the pre concentration step, Hg (II) is deposited on the surface of electrode as Hg (0) by applying a cathodic deposition potential (−0.7 V) for a fixed accumulation time period. This was followed by an equilibrium time of 60 s. Then, an anodic scan was run in the range of −0.7 to +0.6 V that stripped off or re oxidized Hg (0) back to Hg (II), thus, giving an anodic peak at 0.5 V (Figure 2). On the other hand, the unmodified carbon paste electrode gave no peak due to a lack of ability to bind mercury ions (Figure 2). Similar findings had been reported in the literature, with respect to unmodified and modified carbon paste electrodes [27]. The area under the anodic peak is equivalent to the current intensity.
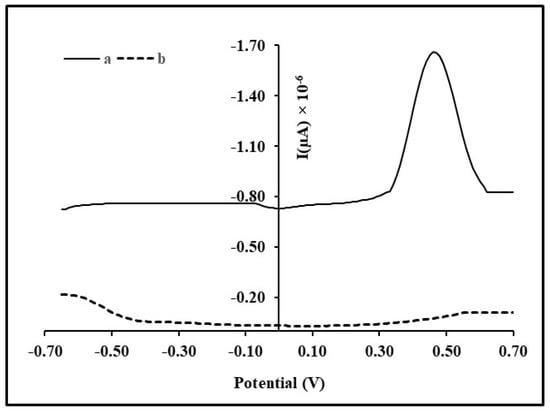
Figure 2.
Stripping peak for Hg (II) at modified (a) and unmodified (b) carbon paste electrodes using differential pulse anodic stripping voltammetry.
During the accumulation step, Hg (II) is reduced to Hg (0) and deposited on the surface of electrode at cathodic deposition potential. Afterwards, this accumulated Hg (II) must be stripped off in a stripping electrolyte in order to have a voltammetric response. Various electrolytes at a concentration of 0.1 M were analyzed for this purpose (Table 1). Acidic media (HNO3, H2SO4, HCl) gave a better current response for the Hg (II) oxidation peak than the neutral medium of KCl. Among the acidic media, HCl has been proven to be the most efficient electrolyte, as already cited in the literature. [28]. In stripping analysis of Hg (II), HCl is particularly preferred as it has been shown to enhance the intensity of stripping peak, decrease the background current, and thus improve the sensitivity [28]. H2SO4 gave the lowest current intensity value. This is due to the fact that HCl and HNO3 provided chloride [29] and nitrate [30] ion that could form complexes with mercury and facilitate oxidation in stripping step. On the other hand, the bulky nature and high dissociation energy of the sulphate ion caused low peak current values in the case of H2SO4. Thus, 0.1 M HCl was chosen as the stripping electrolyte for further experiments.

Table 1.
Effect of different stripping electrolytes (0.1 M concentration) on peak current intensity for 10 µg L−1 Hg (II) solution at a deposition potential of −0.7 V and 12 min accumulation time.
3.4. Optimization of Stripping Parameters
Hg (II) should be successfully adsorbed on the surface of electrode during the accumulation step prior to the electrochemical determination. Various operating parameters that significantly affect the adsorption process were optimized to ensure maximum adsorption of Hg (II).
Deposition potential is an important parameter, as it determines that potential at which the maximum number of Hg (II) ions will be reduced and deposited on the surface of carbon paste electrode. It was studied in the range of −0.3 to −0.8 V (Figure 3a). The stripping peak current value steadily increased as the deposition potential moved towards more negative values. Maximum response was observed at −0.7 V. Thus, −0.7 V was chosen as the optimum deposition potential value.
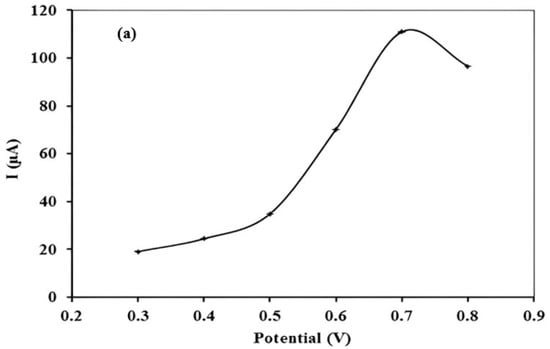
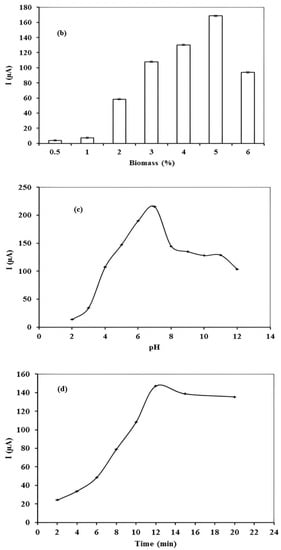
Figure 3.
(a) Effect of deposition potential on stripping peak current value of 10 µgL−1 Hg (II) at pH 7 with accumulation time of 12 min (b) Effect of biomass concentration (%) in modified carbon paste for 10 µgL−1 Hg (II) at pH 7 with deposition potential −0.7 V and accumulation time of 12 min (c) Effect of pH of accumulating medium on current value of 10 µgL−1 Hg (II) stripping peak at deposition potential −0.7 V, accumulation time of 12 min, and biomass concentration 5% (d) Effect of accumulation time on stripping peak current value of 10 µgL−1 Hg (II) with pH 7, deposition potential −0.7 V, and biomass concentration 5%.
The concentration of biomass also played a determinate role in the performance of the modified carbon paste electrode. The ratio of Phanerochaete chrysosporium biomass varied between the range of 0.5–10% and response was shown in Figure 3b. An increased current value was observed up to 5% of biomass ratio. This may be due to the increased number of binding sites on the surface of the electrode while further increases in biomass to the modified carbon paste decreased the current value and a current overload appeared at a concentration ratio greater than 6%. This decrease is probably due to a decrease in the conductive area of the electrode [31]. Thus, 5% w/w ratio of biomass was taken for further experimental work. Basically, in modified carbon paste electrode, graphite powder acts as the conducting material, while a modifier further improves the efficiency of working electrodes to chemically accumulate the adsorbed metal ions and ultimately facilitate the electrochemical determination.
The sensitivity of the voltammetric procedure was also influenced by the pH of the accumulating medium. Stripping peak current was studied as a function of pH value over the range of 2–12. In acidic medium, the current value was found to increase steadily until it attained a maximum value at pH 7 (Figure 3c). With regard to this behavior, pH 7 was chosen as optimum pH value for the accumulating medium. In alkaline medium, Hg (II) exists as anionic hydroxyl species [32] and functional moieties (hydroxyl, amide I, and amide II) on the surface of loaded electrode deprotonates and acquire negative charge. This creates repulsive forces and hinders the attachment of analyte. On the other hand, cationic species [HgOH]+ in acidic and neutral media [33] facilitates attachment, thus, increasing the current value.
The efficiency of the procedure was also defined by the time given to the Hg (II) ions to get accumulated on the surface of electrode. Accumulation time in the range of 2–20 min was studied. Accumulation time–peak current profile was given in Figure 3d and it can be seen that with an increase in accumulation time, more Hg (II) ions become accumulated, causing a significant enhancement in peak current value. A maximum response was obtained at 12 min, and thus it was chosen as the optimum accumulation time value. At 12 min, equilibrium has been established due to saturation of the electrode’s surface [34].
3.5. Analytical Performance
The analytical application of modified carbon paste electrode was studied over the linear range of 5–50 µgL−1 metal concentration, as shown in Figure 4. The value for the limit of detection was 4.4 µgL−1, as calculated by using the formula; CLOD = 3Sb/m, where Sb is the standard deviation of blank signals and m (µA/µgL−1) is the slope of the calibration curve [35]. Reported sensor-depicted LOD value of 4.4 µg/L. This value is a bit higher than the WHO recommended limit of 1–2 µg/L. However, this detection method can play significant role with respect to the concentration level of mercuric ions reported in different water reservoirs of Pakistan [36,37].
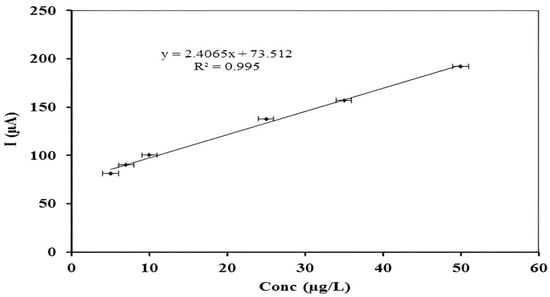
Figure 4.
Calibration plot showing linear range for Hg (II) concentration at optimized stripping parameters.
A comparison of the current method with other modified carbon paste electrode-based electrochemical sensors is illustrated in Table 2. In the literature, water hyacinth leaves [38] and vegetable waste [39]-based biologically modified carbon paste electrodes have been reported for Hg (II) analysis with the detection limit of 195 µgL−1 and 57.75 µgL−1, respectively.

Table 2.
Comparison of current voltammetric method with other modified carbon paste electrodes applied for stripping analysis of mercury.
Other cited studies in Table 2 with low detection limits are based on chemically modified electrodes. Reported publication fabricated chemically modified electrodes, utilizing MWCNTs with 3H-spiro[isobenzofuran-1,6′-pyrrolo [2,3-d]pyrimidine]-2′,3,4′,5′-tetraones (3HSIPPT) [1], Dipenylcarbazone (DPC)/ethylene glycol dimethacrylate (EGDMA) [3], N rich covalent organic framework [8], doped zinc nano fibers coated with mercaptosuccinic acid/pyridine dicarboxylic acid [40], etc. This is due to the fact that synthetic chemicals have been considered as modifiers that exhibit selective response towards the Hg (II), leading to more sensitive analysis.
In comparison, our reported work is based on biologically modified carbon paste electrodes. These are less selective, as compared to chemically modified examples. However, these are based on low cost and environmentally friendly material.
3.6. Interference Studies
The selectivity of the modified carbon paste electrode towards the analyte was studied in the presence of alkali (Na+, K+), alkaline earth (Ca2+, Mg2+), and heavy metal ions (Fe3+, Mn2+, Ni2+, Pb2+, Cd2+, Cu2+, As3+). Anions were not included as part of this study, as they do not undergo direct electrochemical reduction easily [41].
Equally, proportional solutions of alkali and alkaline earth metals were prepared for this study. A decrease of 4.7% in stripping peak current was observed at 300 mgL−1 concentrations of alkali and alkaline earth metals (Table 3). As the concentration was increased from 300 mgL−1 to 2400 mgL−1, there was a proportional decrease from 4.7% to 33.38% in current values. This shows that increasing concentration of alkali and alkaline earth metals suppress the peak current value. The decrease in current intensity can be attributed to two reasons. First, increased ionic strength suppressed the electrostatic attraction between the Hg (II) and functional moieties of modified electrodes. Second, at higher concentrations, Ca2+ and Mg2+ are known to form hydrated complexes on the surface of electrodes, thus, hampering the attachment of Hg (II) [42].

Table 3.
Interference study of Hg (II) with different concentrations of alkali and alkaline earth metal ions. The conditions applied were: biomass concentration 5%; Hg (II) concentration 10 µgL−1; alkali and alkaline earth metals concentration 300–2400 mgL−1; pH 7; stripping media 0.1 M HCl; and deposition potential −0.7 V.
It is illustrated from Figure 5 that the current value for Hg (II) practically remains unaffected in the presence of heavy metal ions (Fe3+, Mn2+, Ni2+, Pb2+, Cd2+, As3+), except for Cu (II), which has been found to increase the current intensity of anodic peak for Hg (II). This can be explained on the basis of the Pearson concept of hard and soft acid base nature. According to the Pearson concept, Hg (II), Cu (II), and Cd (II) are soft natured metal ions. Owing to similarity in nature, Cu (II) and Hg (II) combine covalently rather than electrostatically with the binding sites. This same nature accounts for the enhancement in stripping peak current value of Hg (II) [43]. However, the soft nature of Cd (II) did not significantly alter the peak current value of Hg (II), as Cd (II) deposited and reduced at much higher negative deposition potential [44].
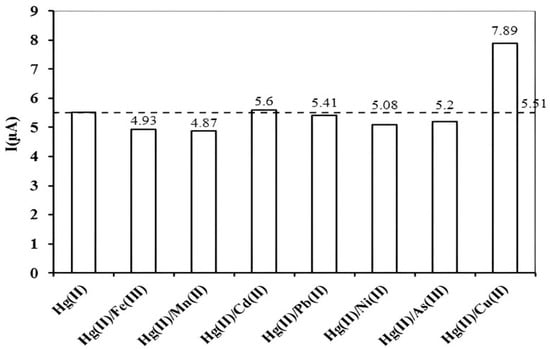
Figure 5.
Interference studies of Hg (II) with binary systems of metal cations (Fe3+, Mn2+, Ni2+, Pb2+, Cd2+, Cu2+, As3+) at concentration ratio of 1:20; operating conditions were: pH 7, deposition potential −0.7 V, biomass concentration 5%, accumulation time 12 min, concentration of Hg (II) (10 µgL−1), and concentration of interfering ions (200 µgL−1 each).
3.7. Statistical Comparison of New and Reference Method
The new method is considered as a suitable alternative to the reference method only if within an assay (replicates) RSD ≤ 3%, Pearson coefficient r > 0.95, slope, and intercept from regression analysis does not differ from 1 and 0, respectively, and paired test proved to be non-significant with low relative (−10 to +10%) and absolute errors (≤2%), along with PRESS r2 > 0.95 [45].
In this study, regression analysis was performed by weighted Deming linear regression with specified analytical error ratio as the Pearson coefficient r > 0.99 (Table S1), and paired data was compared by paired t test, as data were found to be normally distributed by skewness, kurtosis, and Shapiro–Wilk method.
New voltammetric method for Hg (II) analysis was tightly correlated with the reference method (Pearson coefficient r > 0.99), the weighted Deming regression slope and intercept value was not significantly different from 1 and 0, respectively (Figure S1). Overall no significant difference was observed between the predictive and measured result (t (19) = −0.49, p ˃ 0.05). Greater predictive performance was indicated by lower relative (−1.75 to 0.02%) and absolute error (0.12) and high PRESS r2 value (0.99). The RSD values for the new and reference method was found to be 0.19% and 0.17%, respectively. Higher relative standard deviation value for the new method might be due to lack of surface homogeneity. However, RSD values for both the methods are less than 3%, which indicates the repeatability between the replicate measurements.
4. Conclusions
In this study, Phanerochaete chrysosporium-based modified carbon paste electrode has been fabricated and employed for the stripping analysis of Hg (II). Initially different parameters were optimized on standard solutions, and then these conditions are considered for analysis in real samples collected from an industrial effluent. In the end, results of real samples were compared against a standard method using weighted Deming regression analysis and other statistical parameters. It can be concluded that this is a simple, cost effective, and suitable substitute for the analytical detection of Hg (II) at low levels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/electrochem3040049/s1, Figure S1: Weighted Deming regression plot between the new voltammetric and reference method; Table S1: Statistical comparison of new voltammetric method against a reference method for Hg (II) analysis in chlor-alkali industrial effluent.
Author Contributions
Conceptualization, M.M.A. and U.F.; methodology, M.Z.; software, M.Z.; validation, M.M.A., U.F. and M.Z.; formal analysis, M.Z.; investigation, M.Z.; resources, M.M.A.; data curation, U.F. and M.Z.; writing—original draft preparation, M.Z.; writing—review and editing, M.M.A., U.F. and M.Z.; visualization, U.F. and M.Z.; supervision, M.M.A.; project administration, M.M.A.; funding acquisition, M.M.A. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Higher Education Commission, Pakistan under Indigenous PhD Fellowship, Batch VII, Phase I.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fathinezhad, M.; Tarighat, A.M.; Dastan, D. Chemometrics heavy metal content clusters using electrochemical data of modified carbon paste electrode. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100307. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, W.; Pi, S.; Cheng, Y.; Xie, Q. Anodic stripping voltammetry analysis of mercury(II) on a pyridine-Au/pyridine/glassy carbon electrode. Sens. Actuators B Chem. 2020, 317, 128202. [Google Scholar] [CrossRef]
- Fadillah, G.; Inayatussholeha, N.E.; Mukarom, A.N.; Rattyananda, S.B.; Wicaksono, P.W.; Fatimah, I.; Saleh, A.T. Ion imprinted-carbon paste electrode as electrochemical sensor for ultra-trace recognizing speciation of mercury. Results Chem. 2022, 4, 100489. [Google Scholar] [CrossRef]
- Azizullah, A.; Khattak, K.N.M.; Richter, P.; Häder, P.D. Water pollution in Pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479. [Google Scholar] [CrossRef]
- Nordberg, F.G.; Fowler, A.B.; Nordberg, M. Handbook on the Toxicology of Metals; Elsevier: London, UK, 2014. [Google Scholar]
- Hua, K.; Xu, L.X.; Luo, P.Z.; Fang, D.; Bao, R.; Yi, H.J. Effective removal of mercury ions in aqueous solutions: A review. Curr. Nanosci. 2020, 16, 363. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M.; Saeed, A.; Farooq, U. Electrochemical determination of inorganic mercury and arsenic—A review. Biosens. Bioelectron. 2015, 74, 895. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Y. Rapid determination of mercuryions in environmental water based on an N-rich ccvalent organic framework potential sensor. Int. J. Chem. Eng. 2022, 2022, 3112316. [Google Scholar] [CrossRef]
- Gao, C.; Huang, J.X. Voltammetric determination of mercury(II). TrAC Trends Anal. Chem. 2013, 51, 1. [Google Scholar] [CrossRef]
- Kiliç, D.H.; Deveci, S.; Dönmez, B.K.; Çetinkaya, E.; Karadağ, S.; Doğu, M. Application of stripping voltammetry method for the analysis of available copper, zinc and manganese contents in soil samples. Inter. J. Environ. Anal. Chem. 2018, 98, 308. [Google Scholar] [CrossRef]
- Yerga, M.D.; González-García, B.M.; Costa-García, A. Electrochemical determination of mercury: A review. Talanta 2013, 116, 1091. [Google Scholar] [CrossRef]
- Barek, J. How to improve the performance of electrochemical sensors via minimization of electrode passivation. Chemosensors 2021, 9, 12. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, D.; Song, D.; Gong, J. Layered titanate nanosheets as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury(II). Sens. Actuators B Chem. 2013, 181, 432. [Google Scholar] [CrossRef]
- Michalkiewicz, S.; Agata Skorupa, A.; Magdalena Jakubczyk, M. Carbon materials in electroanalysis of preservatives: A review. Materials 2021, 14, 7630. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Nazır, H.; Dönmez, G. Using of Rhizopus arrhizus as a sensor modifying component for determination of Pb(II) in aqueous media by voltammetry. Biores. Technol. 2010, 101, 7551. [Google Scholar] [CrossRef]
- D’souza, F.S. Microbial Biosensors. Biosens. Bioelectron. 2001, 16, 337. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzyme Micro. Technol. 2003, 32, 78. [Google Scholar] [CrossRef]
- Saglam, A.; Yalcinkaya, Y.; Denizli, A.; Arica, M.; Genc, O.; Bektas, S. Biosorption of mercury by carboxymethylcellulose and immobilized Phanerochaete chrysosporium. Microchem. J. 2002, 71, 73. [Google Scholar] [CrossRef]
- Sağlam, N.; Say, R.; Denizli, S.; Patır, M.; Arıca, Y. Biosorption of inorganic mercury and alkylmercury species on to Phanerochaete chrysosporium mycelium. Process Biochem. 1999, 34, 725. [Google Scholar] [CrossRef]
- Sheiner, B.L.; Beal, L.S. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 1981, 9, 503. [Google Scholar] [CrossRef]
- Linnet, K. Necessary sample size for method comparison studies based on regression analysis. Clinical Chem. 1999, 45, 882. [Google Scholar] [CrossRef]
- Lynch, M.J.; Barbano, M.D. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A. Biosorption of reactive dye by loofa sponge-immobilized fungal biomass of Phanerochaete chrysosporium. Process Biochem. 2007, 42, 1160. [Google Scholar] [CrossRef]
- Zaib, M.; Saeed, A.; Hussain, I.; Athar, M.M.; Iqbal, M. Voltammetric detection of As(III) with Porphyridium cruentum based modified carbon paste electrode biosensor. Biosens. Bioelectron. 2014, 62, 242. [Google Scholar] [CrossRef] [PubMed]
- Bashardoost, R.; Vahabzadeh, F.; Shokrollahzadeh, S.; Monazzami, R.A. Sorption performance of live and heat inactivated loofa immobilized Phanerochaete chrysosporium in mercury removal from aqueous solution. Iran. J. Chem. Chem. Eng. 2010, 29, 79. [Google Scholar]
- Zaib, M.; Athar, M. Electrochemical evaluation of Phanerocheaete chrysosporium based carbon paste electrode with potassium ferricyanide redox system. Int. J. Electrochem. Sci. 2015, 10, 6690. [Google Scholar]
- Cesarino, I.; Marino, G.; Matos, R.J.; Cavalheiro, G.T.E. Evaluation of a carbon paste electrode modified with organofunctionalised SBA-15 nanostructured silica in the simultaneous determination of divalent lead, copper and mercury ions. Talanta 2008, 75, 15. [Google Scholar] [CrossRef]
- Bernalte, E.; Sánchez, M.C.; Gil, P.E. Determination of mercury in ambient water samples by anodic stripping voltammetry on screen-printed gold electrodes. Anal. Chim. Acta 2011, 689, 60. [Google Scholar] [CrossRef]
- Giacomino, A.; Abollino, O.; Malandrino, M.; Mentasti, E. Parameters affecting the determination of mercury by anodic stripping voltammetry using a gold electrode. Talanta 2008, 75, 266. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Zemanian, S.T.; Fryxell, E.G. Voltammetric detection of lead(II) and mercury(II) using a carbon paste electrode modified with thiol self-assembled monolayer on mesoporous silica (SAMMS). Analyst 2003, 128, 467. [Google Scholar] [CrossRef]
- Popa, E.D.; Mureseanu, M.; Tanase, G.I. Organofunctionalized mesoporous silica carbon paste electrode for simultaneously determination of copper, lead and cadmium. Rev. Chim. 2012, 63, 507. [Google Scholar]
- Khani, H.; Rofouei, K.M.; Arab, P.; Gupta, K.V.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion (II). J. Hazard. Mater. 2010, 183, 402. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Aroua, K.M.; Yusoff, R. Potentiometric determination of trace amounts of mercury (II) in water sample using a new modified palm shell activated carbon paste electrode based on Kryptofix 5. Am. J. Anal. Chem. 2012, 3, 859. [Google Scholar] [CrossRef]
- Alpat, S.; Alpat, K.S.; Çadırcı, H.B.; Yaşa, I.; Telefoncu, A. A novel microbial biosensor based on Circinella sp. modified carbon paste electrode and its voltammetric application. Sens. Actuators B Chem. 2008, 134, 175. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.A.; Hefnawy, M.M.; Almaged, A.; Belal, F.F. Development of square-wave adsorptive stripping voltammetric method for determination of acebutolol in pharmaceutical formulations and biological fluids. Chem. Cent. J. 2012, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Junaid, M.; Aslam, M.W.; Ali, K.; Rasool, A.; Zhang, H. A review on the status of mercury pollution in Pakistan: Sources and impacts. Arch. Environ. Contam. Toxicol. 2019, 76, 519. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Shah, A.I.; Tulcan, S.X.R.; Rashid, W.; Sillanpaa, M. Contamination, exposure, and health risk assessment of Hg in Pakistan: A review. Environ. Pollut. 2022, 301, 118995. [Google Scholar] [CrossRef]
- Rajawat, S.D.; Srivastava, S.; Satsangee, P.S. Electrochemical determination of mercury at trace levels using Eichhornia crassipes modified carbon paste electrode. Int. J. Electrochem. Sci. 2012, 7, 11456. [Google Scholar]
- Devnani, H.; Satsangee, P.S. Voltammetric trace determination of mercury using plant refuse modified carbon paste electrodes. Environ. Monitor. Assess. 2013, 185, 9333. [Google Scholar] [CrossRef]
- Rotake, D.; Goswami, P.P.; Singh, G.S. Ultraselective, ultrasensitive, point-of-care electrochemical sensor for detection of Hg(II) ions with electrospun-InZnO nanofibers. J. Electroanal. Chem. 2022, 915, 116350. [Google Scholar] [CrossRef]
- Salimi, A.; Noorbakhash, A.; Karonian, S.F. Amperometric detection of nitrite, iodate and periodate on glassy carbon electrode modified with thionin and multi-wall carbon nanotubes. Int. J. Electrochem. Sci. 2006, 1, 435. [Google Scholar]
- Cheng, C.; Wang, J.; Yang, X.; Li, A.; Philippe, C. Adsorption of Ni(II) and Cd(II) from water by novel chelating sponge and the effect of alkali-earth metal ions on the adsorption. J. Hazard. Mater. 2014, 264, 332. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Lodeiro, P.; Rey-Castro, C.; Vilariño, T.; De Vicente, S.E.M. Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga Cystoseira baccata. Water Res. 2005, 39, 3199. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, S.D.; Kumar, N.; Satsangee, P.S. Trace determination of cadmium in water using anodic stripping voltammetry at a carbon paste electrode modified with coconut shell powder. J. Anal. Sci. Technol. 2014, 5, 19. [Google Scholar] [CrossRef]
- Oftedal, T.O.; Eisert, R.; Barrell, K.G. Comparison of analytical and predictive methods for water, protein, fat, sugar, and gross energy in marine mammal milk. J. Dairy Sci. 2014, 97, 4713. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).