1. Introduction
With the breakthrough experiments made by Jaroslav Heyrovský in the 1920s, the dropping mercury electrode has become the most important working electrode for the ultratrace determination of metals in aqueous samples by voltammetric measurements [
1]. Mercury has many advantages such as a high overpotential for molecular hydrogen formation, enabling measurements at very negative potentials. It is ideally polarizable and has given the entire field of voltammetry with (liquid) mercury drop electrodes the name polarography. Moreover, with every new drop a perfectly new surface is formed avoiding the typical deactivation observed for solid state electrodes after several uses, and finally, the formation of amalgams makes the reduction of many metal species thermodynamically more favorable. Unfortunately, mercury is highly toxic. It is a liquid at room temperature and has a fairly high vapor pressure for a metal. Exposure to mercury or mercury vapor can cause severe damages to the brain, kidneys, and lungs. It is also responsible for severe diseases such as acrodynia, Hunter–Russell syndrome, and Minamata disease [
2]. Unfortunately, there are nearly no treatments for acute and long-term mercury poisoning. Emissions, and the use and disposal of mercury is therefore strictly regulated in most countries. E.g., the European Union limited or even banned the usage of mercury in several applications and devices [
3]. Moreover, the Minamata Convention on Mercury, ratified by 140 countries, comprises an international treaty to protect human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds [
4].
As a consequence, the voltammetric community is looking desperately for non-, or at least less, toxic alternatives that can replace mercury in most voltammetric applications. Many tested materials failed due to a low cathodic potential limit, high background noise, lack of reproducibility or multiple peak formation in the voltammogram. More than two decades ago, bismuth coated electrodes were considered for the first time to determine zinc, cadmium, and lead by anodic stripping voltammetry [
5,
6]. Bismuth films on glassy carbon and carbon fiber electrodes, respectively, have been successfully tested, which are formed in situ together with the enrichment of the analyte in the film electrode. Bismuth provides an accessible potential window between approximately −0.2 and −1.2 V (versus Ag/AgCl), which is restricted by hydrogen formation on the cathodic side and by bismuth oxidation on the anodic side. Due to this broad potential window very similar to mercury, a simultaneous determination of five to six elements is possible. The first results were very promising, since the measurements provided well defined, sharp, and well separated current peaks in the differential pulse mode [
5,
7]. Bismuth and its compounds are in general much less toxic to humans than other heavy metals [
8]. This is explained by the low solubility of bismuth salts. Studies indicate that it does not bioaccumulate, or at least to a much lesser extent than other metals. Bismuth therefore seems to be a very promising and much more environmentally friendly alternative to mercury with comparable electrochemical properties. In the following years, more studies have been published concerning not only the voltammetric determination of zinc, cadmium, and lead [
9,
10], but also of other heavy metals such as nickel and cobalt [
11,
12,
13]. The latter two species were measured by adsorptive stripping voltammetry (adSV), i.e., by complexation with dimethylglyoxime or nioxime and enrichment in the bismuth film under cathodic potentials, and subsequent stripping under more anodic potentials. However, the in situ deposition of the bismuth film under adsorptive stripping conditions turned out to be challenging. The adSV determination of nickel and cobalt is usually performed in an ammonia buffered solution, but bismuth suffers from hydrolysis in weakly alkaline media where dissoluble bismuth hydroxide precipitates. To prevent precipitation, the bismuth film deposition was performed ex situ in an acetate buffered bismuth(II) solution. By using tartrate as stabilizing agent, Korolczuk et al. successfully managed the in situ bismuth film formation and adSV measurement [
11].
Platinum metals are emitted into the environment by exhaust catalysts used nowadays in nearly every motorized vehicle [
14,
15]. With the increasing usage of polymer electrolyte membrane (PEM) electrolyzers and fuel cells with platinum as electrocatalyst, new possible sources for platinum emissions are evolving [
16,
17,
18,
19,
20]. Exposure to platinum compounds may cause eyes, nose, or throat irritation as well as respiratory and skin allergies. Hence, environmental monitoring measures of platinum group metals are advisable. Moreover, since platinum group metals are quite expensive, recycling is of high importance.
A further and increasingly relevant field of application for platinum analysis methods is the investigation of electrolyzer and fuel cell aging due to platinum dissolution. Platinum electrocatalysts typically used in low-temperature PEM fuel cells suffer from oxidation, which is accompanied by a reduction of electrochemically active surface area (ECSA) and finally by a loss of activity [
17,
21]. It has been reported that the cathode potential can locally increase up to 1.5 V, in particular during the start-up of the fuel cell with still insufficient gas supply [
20]. A very recent X-ray photoelectron spectroscopy investigation has shown that at potentials above the platinum oxidation potential of approximately 1.0 V
, a Pt-PtO
interface—also called 2D oxide, surface oxide, or oxide monolayer—is formed with contributions of both Pt
and Pt
species [
22]. It has been speculated that the formed oxide layer is likely hydrous. A (surface) platinum(II) hydroxide could be formed by oxidation of platinum under humid conditions at high potentials:
Under dryconditions this hydroxide could be transformed into a (surface) platinum(II) oxide:
In addition, a subsequent oxidation to a (surface) platinum(IV) oxide is also comprehensible:
The thus formed platinum(II) or platinum(IV) ions can diffuse via the surface from the catalyst layer to the interface with the PEM of the fuel cell [
23]. This effect causes a dissolution of the catalytically active platinum nanoparticles and hence a reduction of the ECSA, which severely limits the performance and lifetime of the entire fuel cell [
24]. Moreover , molecular hydrogen from the anode crossing the membrane (“H
crossover”) can reduce Pt
or Pt
again to elemental platinum, which can subsequently redissipate or agglomerate to new but now wrongly placed platinum nanoparticles [
20]. By this way, a characteristic “platinum band” is formed at the interface between catalyst-coated membrane and PEM. Moreover, platinum and platinum nanoparticles can migrate into the PEM, where it can catalyze severe deactivation reactions such as oxidation of water to hydroxyl radicals that can chemically destroy the membrane. Alternatively, not reduced platinum ions can dissolve in the product water and finally leave the fuel cell with the humid exhaust gas stream [
16,
25,
26,
27,
28].
Therefore, online or mobile analysis of platinum in this product water during fuel cell operation can give direct insights into the current ageing state of a fuel cell and can help to make predictions of the end of its life. For all these reasons, sensitive, selective, and cost-efficient analytical methods are needed that ideally can be used in online measurements. Highly sensitive and selective, but also very expensive and bulky methods such as inductively coupled plasma - mass spectrometry (ICP-MS) are not useful for such applications. Instead, voltammetric methods can be considered as promising due to their rather low costs, compactness, and high sensitivities for many redox-active elements.
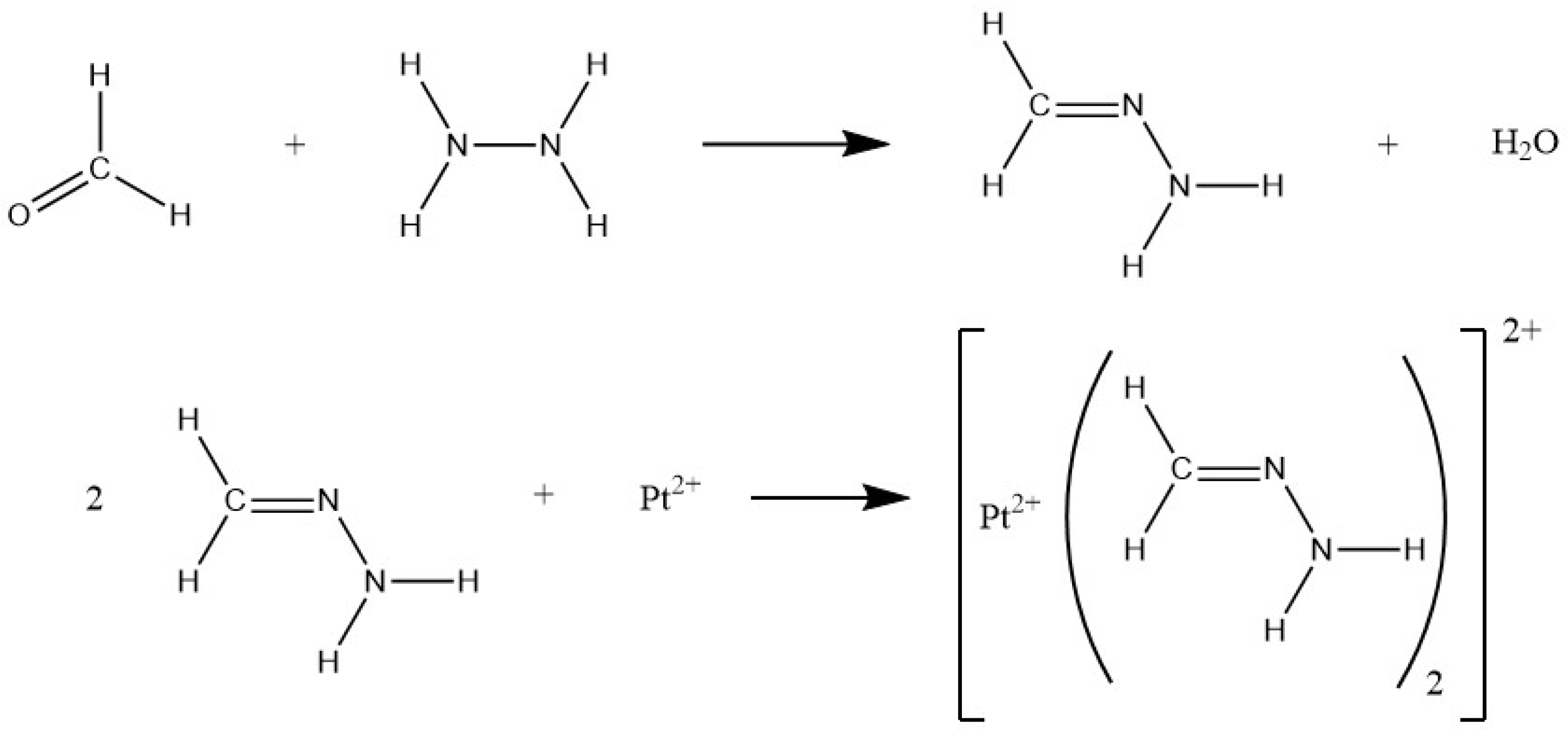
As for the determination of platinum group elements, the adSV method at a hanging mercury drop electrode (HMDE) exhibits superior limits of detection and quantitation in the low or even sub-nanogram per liter range [
29,
30]. The measuring principle of the detection of platinum at an HMDE is usually based on the formation of a platinum complex, which accumulates in the mercury electrode at cathodic potentials. Upon subsequent scanning to even more negative potentials, enriched platinum catalyzes the reduction of protons to molecular hydrogen by lowering mercury’s hydrogen overpotential. This resulting current flow is proportional to the amount of enriched platinum and its total concentration in the measurement solution. For platinum complexation, formaldehyde and hydrazine are added to the electrolyte forming a platinum(II) formazone complex following the reactions depicted in
Figure 1 and according to Ref. [
30].
As for the determination of platinum group metals, stripping voltammetry using the peak of the selective electrooxidation of lead from a lead–platinum–group metal electrolytic deposit on a graphite electrode has been tested as mercury electrode alternative [
31,
32]. Unfortunately, the heavy-metal lead is itself a toxic element, too. Alternatively, co-precipation of platinum(IV) with copper or silver on graphite electrodes impregnated with polyethylene in combination with stripping voltammetry has been tested with limited success [
33]. Bismuth films on glassy carbon exhibit a much more promising potential for the sensitive determination of platinum and rhodium with reported limits of detection in the ppt range [
34,
35]. Comparable to the HMDE, platinum can be determined on bismuth films by applying the adSV method. Usually, dimethylglyoxime (fully protonated form: H
DMG; half-deprotonated form HDMG
) is used as complexing agent. In an ammonia buffer, the following reactions are reported to take place at the working electrode [
34]:
Again, the current due to the formation of hydrogen is proportional to the concentration of accumulated platinum, which is proportional to its total concentration in the measurement solution.
The present publication deals with the validation of bismuth films deposited ex situ on glassy carbon solid state electrodes and on screen-printed glassy carbon electrodes for the determination of platinum in aqueous samples. The latter comprises for the first time a complete three-electrode on-chip approach that can be perfectly used in portable and online applications. The results are compared with the performance of an HMDE, and the methods were tested and discussed in terms of their applicability for the measurement of platinum in the product water of a real PEM fuel cell stack upon applying different load cycles.
2. Materials and Methods
2.1. Instrumentation
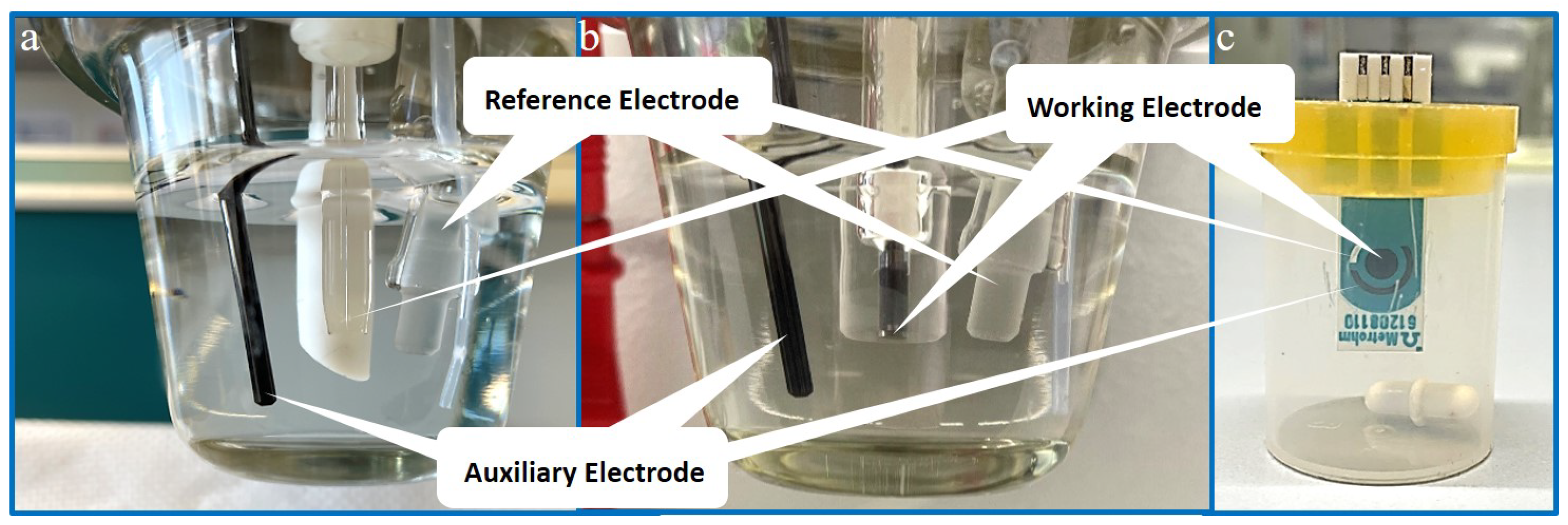
HMDE measurements were performed with a 797 VA Computrace voltammeter (Metrohm, Herisau, Switzerland), equipped with a multimode electrode filled with liquid mercury as working electrode, a glassy carbon electrode as auxiliary electrode, and an Ag/AgCl (3 M KCl) reference electrode.
As for measurements with a bismuth film electrode, a 663 VA Stand voltammeter (Metrohm, Herisau, Switzerland) connected to a PGSTAT204 potentiostat (Metrohm, Herisau, Switzerland) via the interface IME663 (Metrohm, Herisau, Switzerland) was used. The setup contained a three-electrode system consisting of an Ag/AgCl (3 M KCl) reference electrode, a glassy carbon auxiliary electrode, and a glassy carbon solid state electrode (GCE) as working electrode. The working electrode was coated with a bismuth film prior to Pt analysis, resulting in a bismuth film electrode (BiFE).
The 910 PSTAT mini setup (Metrohm, Herisau, Switzerland) was used for measurements with the bismuth film on screen-printed electrode (BiSPE). The electrode applied was a screen-printed DropSense electrode (Metrohm, Herisau, Switzerland) with a silver reference electrode, a carbon auxiliary electrode, and a 4 mm diameter glassy carbon working electrode on one chip.
All voltammetric measurements were performed in differential pulse adsorption stripping voltammetry (DPAdSV) mode and all given potentials were set against an Ag/AgCl (3 M KCl) reference electrode.
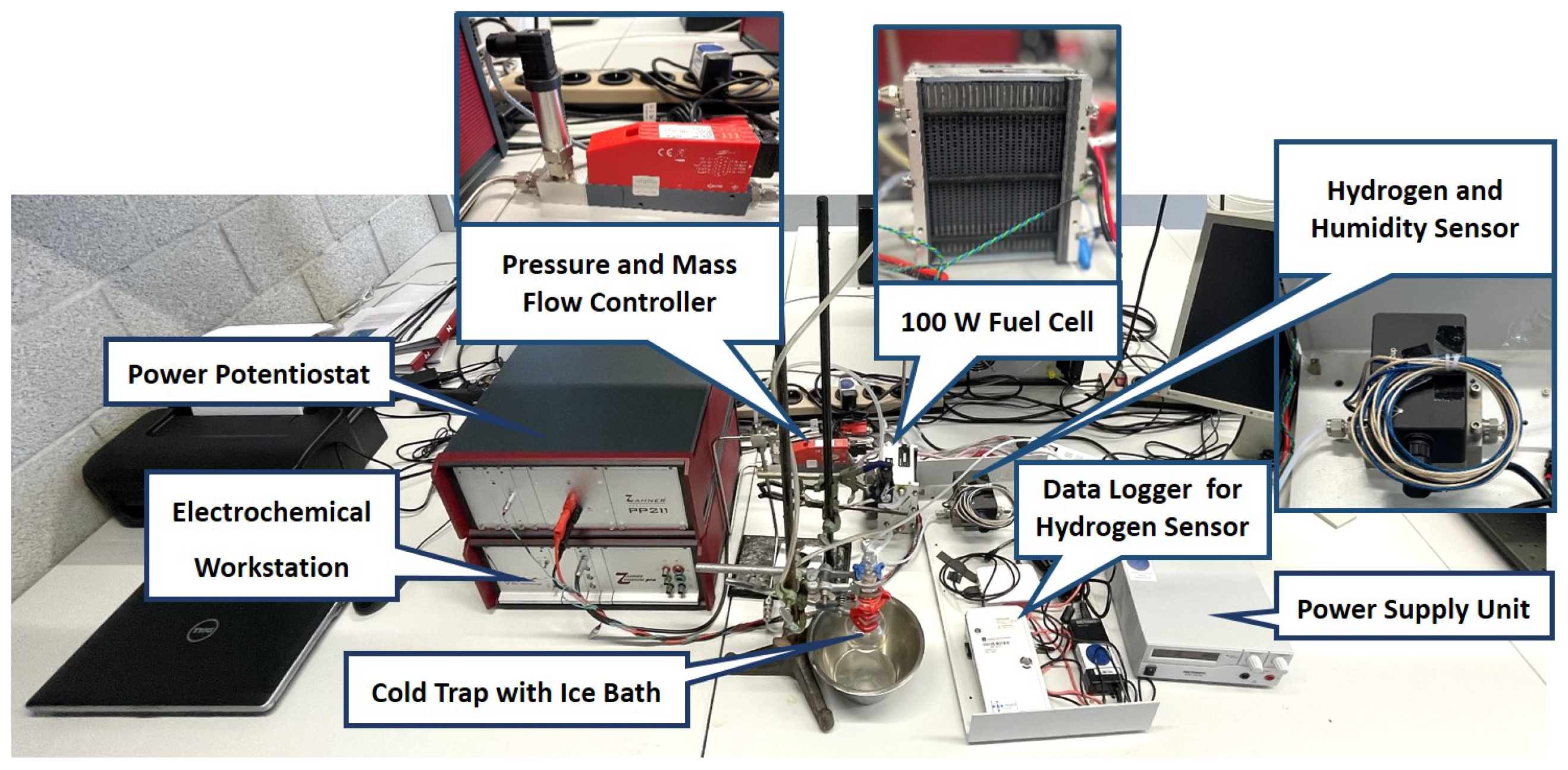
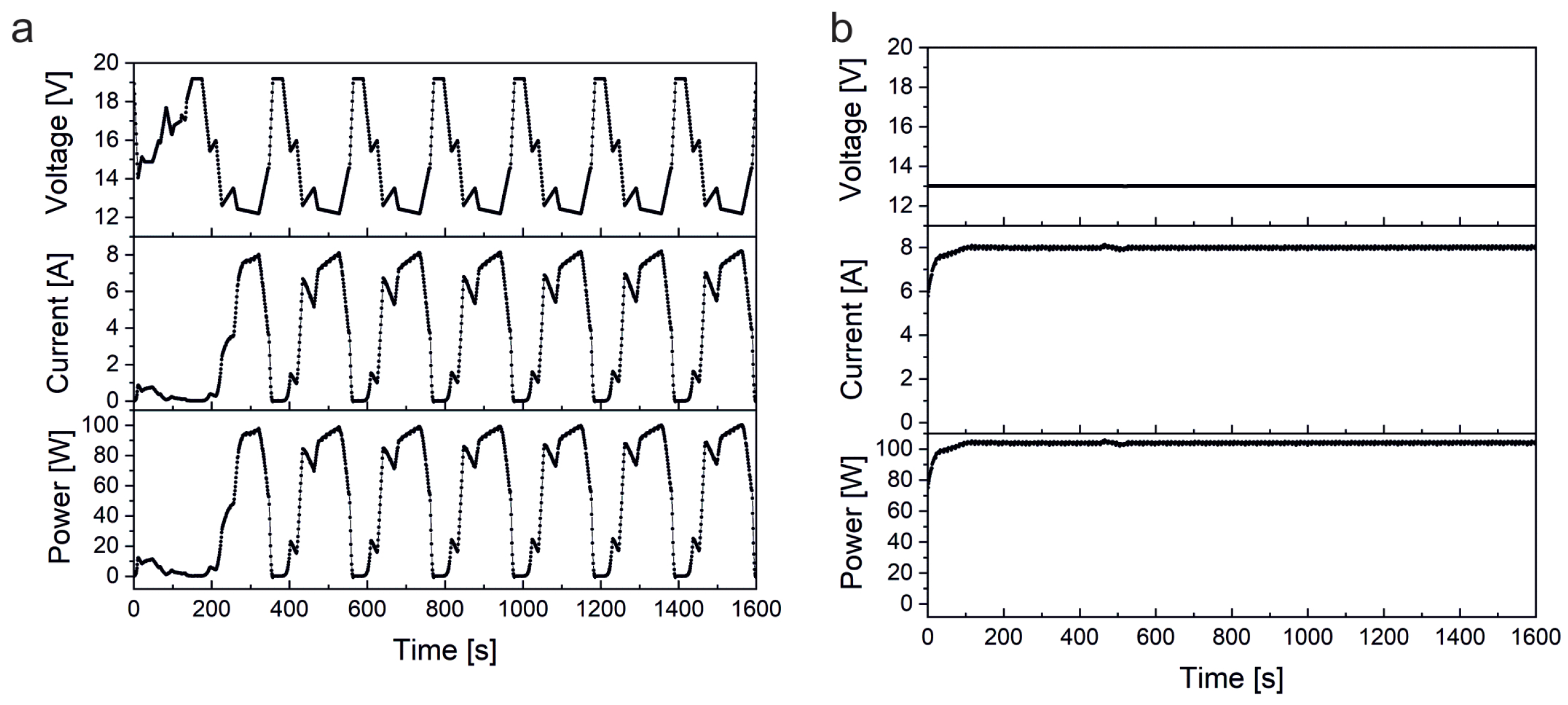
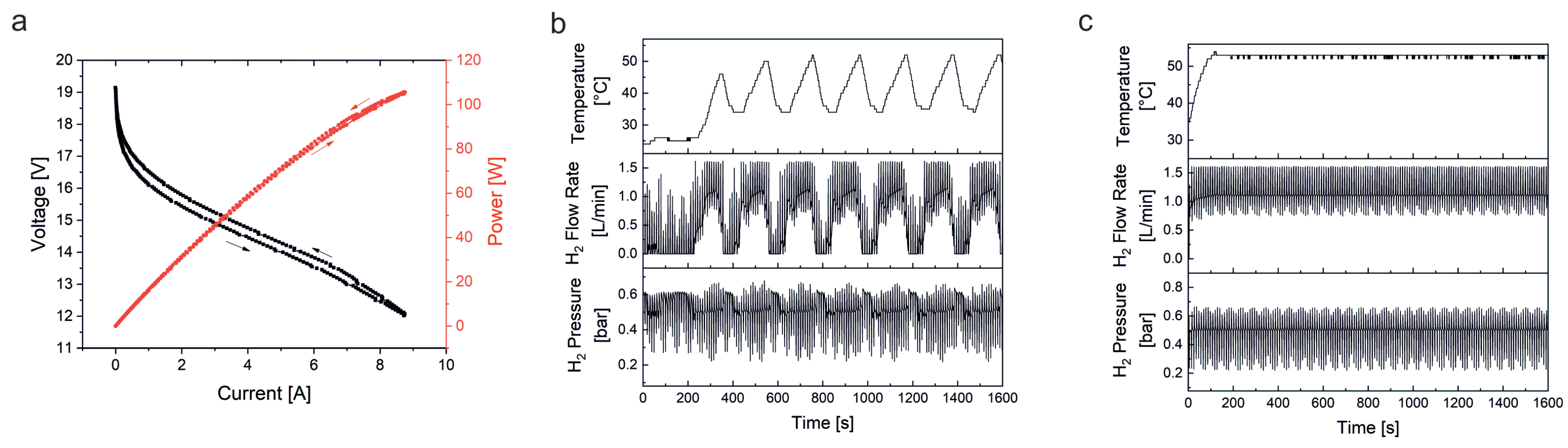
Continuous high load and dynamic load fuel cell tests were performed on a home-built fuel cell test station. The setup consisted of a commercial air-cooled, self-humidified, open-cathode 100 W fuel cell stack (type H-100, Horizon Fuel Cell Technologies, Singapore) consisting of 20 individual fuel cells linearly connected by bipolar plates. Each cell was characterized by an anode catalyst loading of 0.08 mg/cm Pt/C, a cathode catalyst loading of 0.35 mg/cm Pt/C, and the 18 μm thick Gore 18 polymer electrolyte membrane. The active area was about 20 cm/cell. The stack was operated with H (5.0 purity grade, Westfalen, Münster, Germany) and ambient air. A digital pressure controller (Vögtlin Instruments, Muttenz, Switzerland) was used to hold a hydrogen overpressure of 0.5 bar at the inlet of the fuel cell stack and to assure a controlled hydrogen supply for every fuel cell load. A power supply (Voltcraft/Conrad Electronic, Hirschau, Germany) was used to run the air blower of the fuel cell. The power potentiostat PP211 and the electrochemical workstation Zennium Pro (both from ZAHNER-Elektrik, Kronach, Germany) were used to precisely program and potentiostatically control the respective load cycle applied to the fuel cell stack. A cold trap (glass flask in an ice bath) was connected with the exhaust gas outlet of the fuel cell to condense and collect the product water for platinum analysis. The fuel cell stack gas outlet was connected to a gas valve, which was automatically opened every 10 seconds to release the exhaust gas into the cold trap. Residual hydrogen in the exhaust gas was monitored by a hydrogen sensor (neo hydrogen sensors, Neuss, Germany).
2.2. Reagents
All chemicals used were of analytical grade purity (pro analysi) unless otherwise stated. 1000 mg/L bismuth and platinum stock solutions were purchased from CPI international (Santa Rosa, USA) and Merck (Darmstadt, Germany), respectively, and diluted as required. For the preparation of the Pt standard solutions, the appropriate amount of Pt stock solution was dissolved in 0.1 M hydrochloric acid (32%, Bernd Kraft, Duisburg, Germany). To prepare the coating solution, 100 mg/L bismuth solution was added to a 0.2 M 1:1 sodium acetate (>99%, Merck, Darmstadt, Germany)/acetic acid (>99%, Carl Roth, Karlsruhe, Germany) buffer. Ammonia (25%, Carl Roth, Karlsruhe, Germany)/ammonium chloride (99.5%, reinst Ph. Eur., Grüssing, Filsum, Germany) buffer containing 5 × 10 mol/L dimethylglyoxime (DMG; >99%, Fluka, Seelze, Germany) served as electrolyte for the BiFE measurement. A 0.1 M DMG stock solution was prepared by dissolving the appropriate amount of DMG in pure ethanol.
For HMDE measurements, a solution of 0.72 mol/L sulfuric acid (95–97%, Bernd Kraft, Duisburg, Germany), 3 mmol/L hydrazine sulfate (>99%, Fluka, Seelze, Germany) and 6.71 mmol/L formaldehyde (37%, AppliChem, Darmstadt, Germany) served as electrolyte. The electrolyte solution had to be prepared every day due to its instability.
All solutions were prepared from Milli-Q water.
2.3. BiFE and BiSPE Preparation
The following procedure was used for coating the solid state electrode and the screen-printed electrode. The coating solution consisted of a 0.2 M sodium acetate/acetic acid buffer containing 100 mg/L bismuth. The working electrode was cleaned with Milli-Q water and then immersed in the coating solution. Before coating, 50 cleaning cycles were performed on the solid state electrode in the range of −0.6 to −1.1 V. On the screen-printed electrode no cleaning steps were applied since this option is missing for the used 910 PSTAT mini setup. For both electrodes, a cleaning potential of −0.15 V was applied for 2 s.
For the accumulation mode, a potential of −1.0 V was applied for 300 s. During coating, the solution was stirred at 200 rpm. After a rest time of 5 s, a voltammogram was recorded in the range of −0.6 and −1.1 V with a sweep rate of 0.04 V/s, a pulse amplitude of 0.05 V, and a pulse time of 0.04 s. After coating, the electrode was carefully rinsed with Milli-Q water.
2.4. Procedure for Pt Determination at the BiFE and BiSPE
Except for the cleaning and purging step, the same procedure was used for both the solid state and screen-printed electrode. On the screen-printed electrode there was no possibility for a purging and cleaning step before the measurement with the used 910 PSTAT mini setup. For the determination of platinum, 9 mL of electrolyte consisting of a 0.01 M ammonia/ammonium chloride buffer (pH = 9.25) containing 5 × 10 mol/L DMG was used. 1 mL of sample was added to the electrolyte. The platinum solutions used consisted of the appropriate amount of a 1 g/L platinum standard solution dissolved in 0.1 M hydrochloric acid. Electrolyte as well as platinum solutions had to be prepared daily due to their instability. Prior to each measurement, the measurement solution was purged with N (4.8, Westfalen, Münster, Germany) for 300 s to eliminate O in the solution of the solid state electrode. In addition, cleaning cycles were performed before analysis at this electrode. For this, a potential between −0.4 and −1.4 V was cycled for 50 times. At both electrodes an accumulation potential of −0.7 V was applied for 60 s while the solution was stirred at 200 rpm. After 10 s of equilibration time, the voltammogram was recorded from −0.4 to −1.4 V with a sweep rate of 0.04 V/s in differential pulse mode. The pulse amplitude was set to 0.05 V and the pulse time was 0.04 s. For all measurements, the measuring cell was cooled in an ice bath.
2.5. Procedure for Pt Determination at the HMDE
Pt determination at the HMDE was carried out in 1.5 mL electrolyte consisting of 0.72 mol/L sulfuric acid, 3 mmol/L hydrazine sulfate, and 6.71 mmol/L formaldehyde. The electrolyte was added to 10 mL of the sample. Before the measurement, the measuring cell was purged with N (4.8, Westfalen, Münster, Germany) for 300 s to remove O. An accumulation potential of −0.6 V was then applied for 120 s while the solution was stirred at 2000 rpm. After an equilibration time of 10 s, a scan from −0.6 to −1.1 V was performed in differential pulse mode. The sweep rate was set to 0.02 V/s and the pulse amplitude was 0.05 V.
2.6. Sample Preparation
The condensed fuel cell product water was stored in polypropylene sample vessels at −18 C after collection. To ensure stability, 0.01 mL concentrated hydrochloric acid was added per 10 mL sample. The sample vessels were cleaned with 10% HNO (65%, Bernd Kraft, Duisburg, Germany) and Milli-Q water before use.
Prior to each analysis, samples were treated with UV irradiation in a 909 UV Digester (Metrohm, Herisau, Switzerland). To each 10 mL sample, 0.01 mL concentrated hydrochloric acid and 0.05 mL hydrogen peroxide solution (30%, Carl Roth, Karlsruhe, Germany) were added. The UV treatment of the prepared samples was run for 90 min at 90 C.
2.7. Procedure for Continous High Load and Dynamic Load Fuel Cell Tests
Two different load profiles were used for the fuel cell test studies. The continuous high load cycle was run at constantly high power at approximately 100 W by potentiostatically holding the fuel cell stack voltage at 13 V. The dynamic cycle was adapted from the worldwide harmonized light duty vehicle test procedure (WLTP) [
36]. To generate as much product water as possible, only the most power intensive sections “high” and “extra high” of the WLTP were used. To create the program code, speed values of the WLTP were first linearly converted into percentages, i.e., 100% corresponded to maximum speed. The percentages obtained were then transferred to the set power of the fuel cell with 100% corresponding to maximum power. For this purpose, a polarization curve was recorded for the fuel cell stack on the test station to obtain the corresponding voltage for each power setting. With the obtained voltages a dynamic test cycle was programmed and applied to the fuel cell stack (potentiostatic control with potentiostat/electrochemical workstation).
4. Discussion
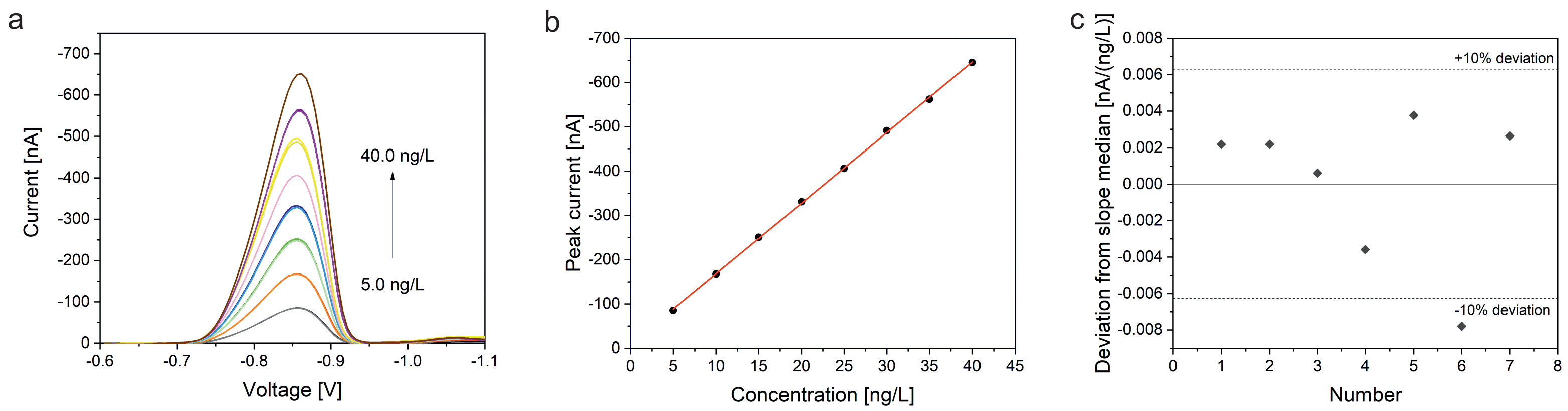
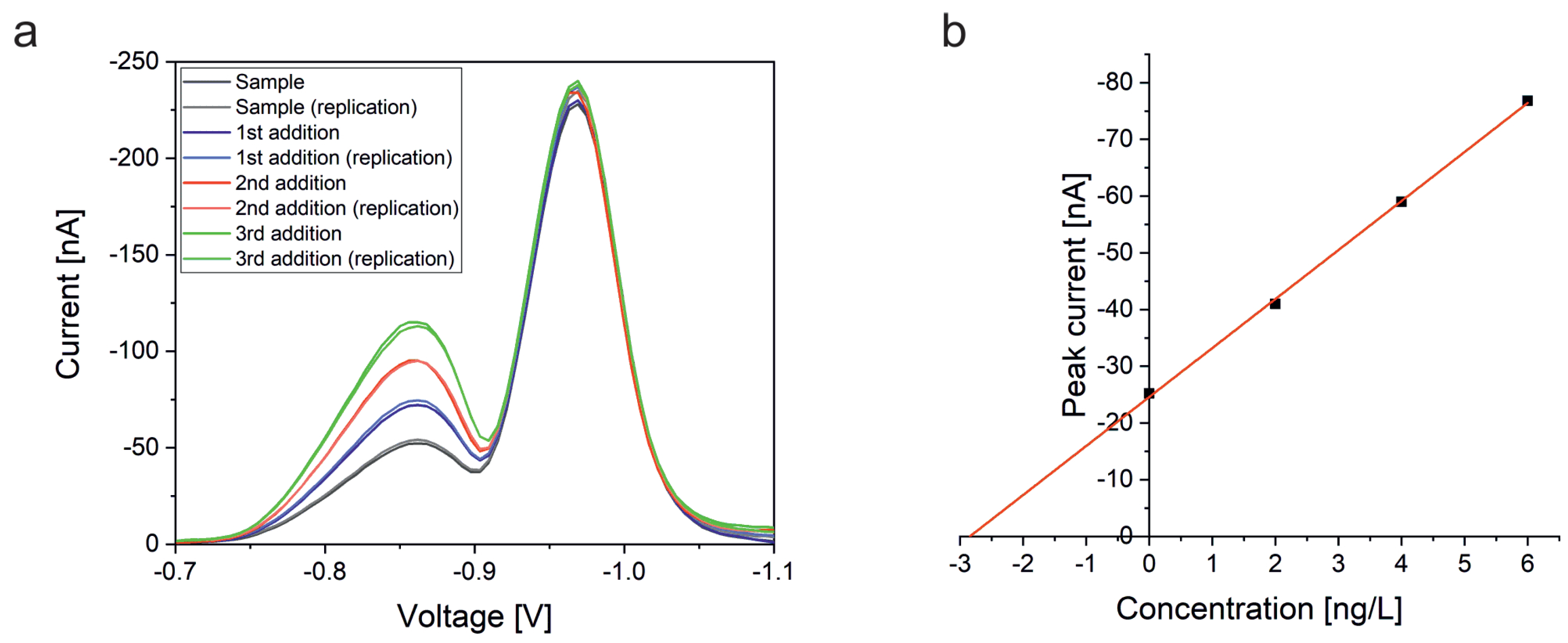
Our presented work focuses on methods for the determination of platinum using the HMDE and bismuth film electrodes deposited on glassy carbon. Platinum determination by adsorptive stripping voltammetry with an HMDE is a standard method and the method could be successfully validated for the quantitation of platinum in the product water of a 100 W fuel cell stack. As for the bismuth film on glassy carbon solid state electrode, various conditions were optimized. After a successful development of the method, it was also successfully validated for the determination of platinum by adSV. During the validation it turned out that both methods produce reproducible results with high accuracy. In addition, linear working ranges could be defined. Detection and determination limits were determined using the calibration line method. LOD and LOQ values of the method based on the HMDE are in a concentration range more than three orders of magnitude lower than those obtained with the bismuth film electrode.
In the validation of the bismuth film on a screen-printed glassy carbon electrode, no clear linear relationship between concentration and current was found. A much better fit was obtained by using a second-order calibration function. One factor that could affect linearity is the nature of the bismuth film. The bismuth film could not have formed ideally or may have become unstable, resulting in a detachment from the carbon electrode as a consequence of repeated adsorption and desorption of the analyte. Another reason could be the change in the morphology of the bismuth film during the measurements as reported by Baldrianova et al. [
39]. It should be noted that no conditioning cycles were carried out prior to the measurement in order to clean the electrode. So there could be impurities on the electrode surface that occupy the adsorption sites. Furthermore, no conventional Ag/AgCl electrode was used as the reference electrode, but a screen-printed silver electrode. Unlike the Ag/AgCl electrode, this electrode is in direct contact with the measurement solution. It is therefore possible that the reference electrode potential changes during the measurement and could influence the voltage setting. Moreover, no nitrogen purging was performed prior to the measurement. Hence, the dissolved oxygen or its reduction products such as hydrogen peroxide could also negatively effect the stability of the film or the adsorption reaction of the platinum complex. Van der Linden et al. showed that the presence of oxygen in the solution prevents the formation of monolayers on mercury films [
40]. A similar behavior is also thinkable for bismuth films. In general, all effects that result in passivation of the electrode could be a reason for the non-linear behavior. In this case, fewer and fewer active centers would be available for adsorption of the analyte complex, which leads to ever smaller signals. However, the validation with the quadratic calibration function gave a very good precision and recovery rate proving the principle applicability of this method for the determination of platinum. Overall, the accuracy of all three methods is with average recovery rates of 106.8% (HMDE), 113.6% (BiFE), and 103.8% (BiSPE) very good. In addition, a high precision could be proven for all methods as well, as supported by average RSD values of 4.2% (HMDE; without outlier), 5.9% (BiFE), and 2.8% (BiSPE), respectively, obtained by analyzing six standard samples measured at two different days.
Due to the rather high LOD and LOQ values of the bismuth film electrodes, only the HMDE could be used to measure the platinum dissolution in fuel cells. In the product water of the measured 100 W fuel cell stack, a signal at −0.88 V showed up that can be unambiguously assigned to platinum. As a result, platinum dissolution rates of 8.3 × 10
g/(cm
s) for the dynamic cycle and 3.3 × 10
g/(cm
s) for the continuous high load cycle were measured. Obviously, the effect of a constantly high current density (and low voltage) results in an approximately four times higher platinum dissolution rate than observed in the dynamic cycle. Usually, stronger platinum oxidation is observed at higher potentials (close to the open circuit potential, OCP) and the membrane is mechanically more stressed under dynamic potential cycling conditions. However, since with the higher current in the constant high load cycle more product water is generated per time, it might cause a stronger leaching out of soluble platinum. The dissolution rates found in our study are still orders of magnitude lower than the ones reported by Wang et al. (1.4–1.7 × 10
g/(cm
s)) [
16]. In contrast to our measurements, they measured the platinum dissolution of carbon supported platinum nanoparticles at a potential of 0.9 V, which is close to the OCP. At this potential, platinum is much more likely to be oxidized which might explain the higher platinum dissolution. Xie et al. do not give a concrete dissolution rate, but report on a platinum concentration of 40–160 ppt in the cathodic product water of a fuel cell aged in a cyclovoltammetry measurement under high-humidity conditions for up to 2000 h [
27]. These values are only slightly higher than the concentrations observed in our experiment.
In addition to the platinum peak, a second peak at −0.98 V appeared in the voltammogram of the fuel cell product water. The peaks are sufficiently separated, hence the platinum analysis was fortunately not disturbed. A literature search revealed that with formazone also rhodium can be analyzed [
14]. The peak potential of rhodium is expected at approximately −1.1 V. This, however, does not agree very well with the peak found at −0.98 V in our case. Palladium is another platin group element which is often found in conjunction with platinum and rhodium in catalytic applications. However, the expected palladium signal at −0.7 V [
14] does not match well with the signal in question. However, it has been reported that zinc can interfere with platinum when using formazone as complexing agent. A peak at approximately −1 V was associated with such zinc species [
29,
41]. For this reason it seems plausible that the additional peak might be caused by zinc. For an accurate and reliable identification of the second peak further experiments are essential, but were not the focus of this study.