Abstract
Naturally occurring phytonutrients/phyto-components are likely to have therapeutic values. These phyto-derived naturally occurring components, such as polyphenols, phenolics, flavonoids and phenolic acids have a hydrocarbon background with a polyphenolic ring, an ester bond with a polyphenolic ring, etc. Their structures play a critical role in determining the chemical and physical attributes that define their activity/functions and roles. Owing to their chemical structure, most of them are electroactive. Thus, these phytochemicals can be used in the preparation of electrochemical sensors. Gaining an understanding of functional genotypical units using electrochemistry is a unique study. The feasibility of incorporating an array of biosensors into a fully-automated micro-electrochemical system is further explored. This review is intended to provide in-depth knowledge of biosensors’ applications based on/for Plantae kingdom and varieties. The discussion focuses primarily on the fields associated with the fully-automated micro-electrochemical system and appropriate methods for its advancement. The intended approach is to provide a selective outlook including the setbacks/shortcomings and usefulness of opting for the concerned technique.
1. Introduction
Herbal formulations made from phytochemicals with therapeutic values are currently an extensive topic of research. They represent quite a vast pool of biologically active, extensive sets of macro- and micro-elements. Surveys executed on the subject of the usage of medicinal plants have shown a rise of approximately 10% over the last 2 years. The appropriate mix of health and food has come into the mainstream due to the health emergency created by the emergence of novel/new viruses in our lives. The phytomedicines have re-emerged in advertisements, social media and retail outlets with the arrival of multinational pharmaceuticals attracted to these domains [1]. The past decade has witnessed the succession of nutraceuticals wherein phyto-constituents ensure additional health benefits for the long term [2]. The major distinguishing factor between nutraceuticals and phytomedicine is that, nutraceuticals have a nutritional role that arises from using them for a longer time frame (e.g., chemoprevention) [1]. Thus, this field has now encompassed pharmacognosy, phytochemistry and nutraceuticals. Plant cytochrome P450s are an essential part of the plant with the ability to catalyze many unfavorable chemical reactions and hence called “nature’s blow torches”, ensuring high specificity and stereochemistry. Thus, the cytochromes have a pivotal role in the synthesis of a plethora of metabolic compounds that are involved in the functioning and survival of sessile organisms under stressful conditions. Bavishi et al. reported the mediator-free electrochemical investigation of plant cytochrome P450s and their NADPH P450 oxidoreductase [3]. Later, Udit et al. studied the direct electrochemistry for cytochrome P450 BM3 heme protein using graphite electrodes [4]. The free radicals are an essential part of both phytomedicine and nutraceuticals.
Reactive oxygen intermediates, superoxide anions, alkoxy radicals, etc., are all examples of free radicals. They are special species containing more than one unpaired electron, and thus are chemically very reactive or unstable. Their high reactivity is termed “ ‘stress’ or the ‘unbalance between the oxidants and antioxidants causing damage to the individual’ ” [5,6]. Free radicals are essential for oxidizing nucleic acids and lipids, initiating degenerative disease generation, etc. Epidemiological evidence indicates the necessary association of a cardiovascular diet with fruits and vegetables [7]. Vitamin E and Vitamin C have the intrinsic ability to scavenge free radicals, thus protecting the body from being damaged. These antioxidants are preventive agents in relation to various diseases, such as cancer, mutagens, risk of cardiovascular diseases, etc., as they modulate the enzyme activities thereby reducing chances of carcinogenicity or oxidative stress-inducing agents [8]. Apart from their constructive nature, they can also be highly destructive with the presence of metal ions leading to cell disorders [9,10]. The reactive oxygen species (ROS) lead to cell injuries by disrupting the oxidative balance and contribute to the development of many diseases over time such as cataractogenesis, cancer, rheumatoid arthritis and ageing [11]. Hence, the widespread empirical demand for wild plant groups needs proper information based on their phytochemical and antioxidant abilities. Thus, many techniques are working together to complete their analysis. Many plant-based systems have to be engineered into various sensing platforms, after the incorporation of micro-reservoirs in the thread along with detection dots, to become a wearable sensor.
Various sensing platforms have been introduced for the detection of potential chemicals, moieties (proteins, molecules) and byproducts of any chemical reaction. The principle of detection is based on physical, chemical or biological changes. However, the major drawbacks observed in these sensors are the pre-sampling, the requirement for a skilled technician and the large amount of sample required with sophisticated instrumental set-ups, hence uneconomical. This has paved the way for electrochemical sensing which involves nano-quantities of analytes, without any pre-sampling and sensitive data output. Surachet et al. reported the detection of reactive oxygen species using fluorescence spectroscopy. He quoted that the “sensitivity and selectivity of both fluorescence and electrochemical techniques are similar. The simplicity of electrochemical methods has gained the attention to many researchers.” He also added that, “the electrochemical techniques have potential for real time detection using micro or nano-scale electrodes, readily available for real time sensing irrespective of the type or location” [12].
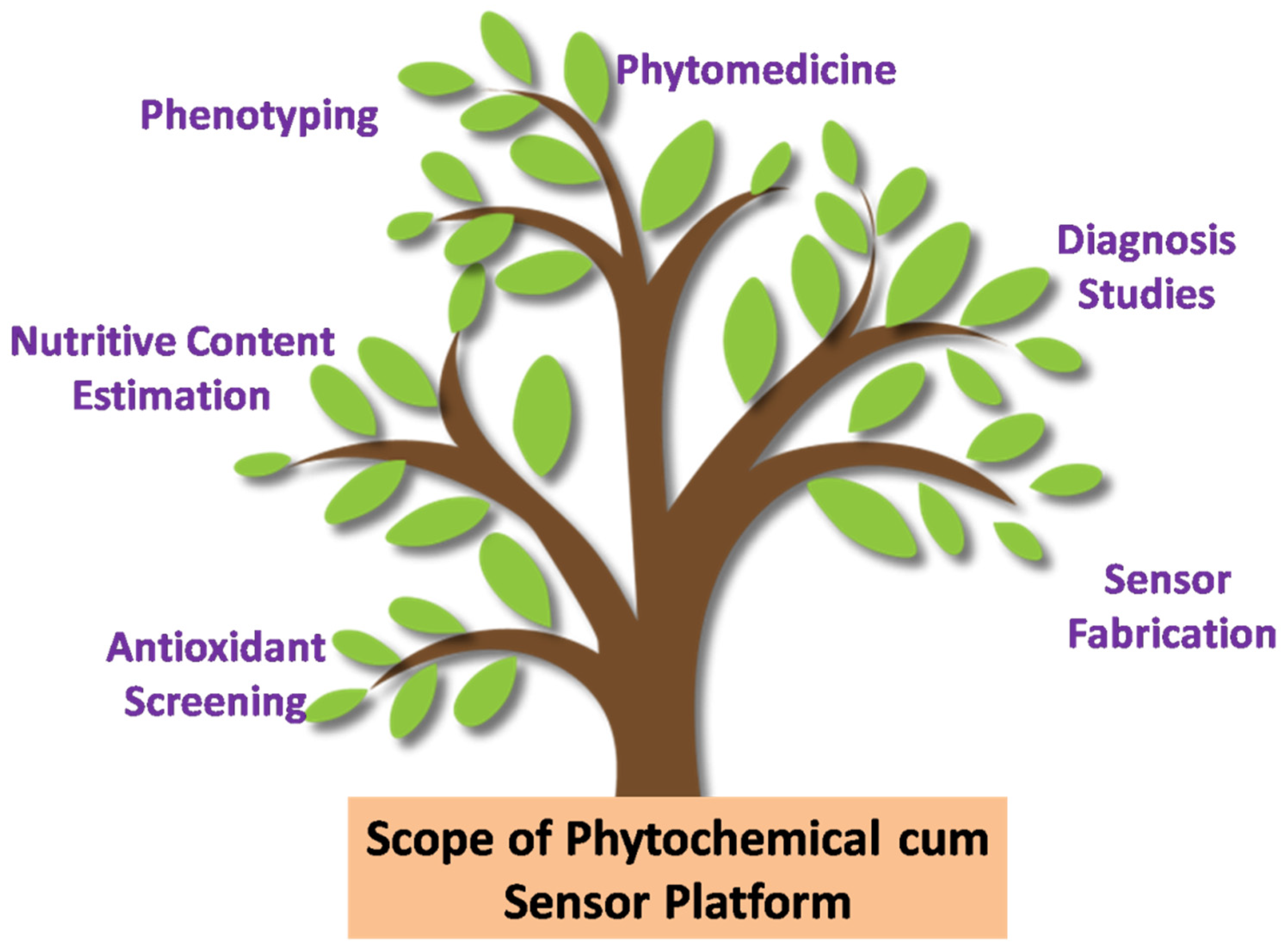
The electroanalysis field has brought in a new revolution to combat the activities of free radicals via the identification of the molecules responsible. An ideal electrochemical sensor should have key attributes such as accuracy, repeatability, sensitivity, least toxicity, biostatistical support, reliability, and easy sample preparation protocols, showing high specificity [13]. Figure 1 accounts an illustrative image portraying the various scopes that can be explored regarding the phyto based electrochemical sensor platforms.The antioxidants help in counteracting the free radicals by preventive chain reactions and disrupting the oxygen activation thereby preventing the damage caused by free radicals [11]. Antioxidants are present in fresh fruits and green leafy vegetables [14]. The reason behind all the parents and doctors asking for a higher intake of green vegetables and fruits may be to diminish/stop the incidence of chronic and degenerative diseases. The consumption of carrots, sweet potatoes and oranges are protective against cancer [15]. In addition, including onions, honey and grapes, i.e., sources of polyphenols, into the diet can help in preventing cardio-vascular and anti-viral activity in the body [16,17]. Many classical methods such as high-pressure liquid chromatography (HPLC), spectrophotometry, gas chromatography (GC), potentiometry, micellar capillary electrophoresis, etc., have been reported for the qualitative estimation of antioxidants [2,14,15]. The protocols are ORAC (Folin Ciocalteu assays, oxygen radical absorbance capacity), FRAP (the ferric reducing ability of plasma), DPPH (2,2-diphenyl 1-picrylhydrazyl) assays, lipid peroxide inhibition, DCHF-DA (dichlorofluorescein-diacetate), etc., for assessing their total antioxidant activity [18].
Figure 1.
Illustrative image portraying the various scopes that can be explored regarding phytochemical and/or sensor platforms. Rights and permission, copyright [13].
The detection of disease biomarkers, sequencing of the genome, and quantitative recognition of specific metabolites are quite intellectually challenging parameters to be studied and equally relevant in the medical and healthcare sector. Due to the evolution of all organisms with time, micro-organisms have mutated as a “survival of fittest” concept to sustain in varied environmental conditions via a broad distribution of protein stabilities, variable denaturation temperature and subsequent growth temperatures, lack of evolutionary trade-off, obtaining a protective coat, etc. [19,20]. Balol et al. have studied in depth the various unusual sources of genetic variation, and provided tabulated data for the diversity value of various distinct plant viruses [20]. Due to these variations, genetic alterations have come into existence which have led to a huge loss in economic terms basically due to the acceleration in development of pests and pathogens witnessed by the agricultural sector. As of now, not much research has been processed to detect the existence of plant diseases using in-field instrumentation. Meanwhile, the off-field pathogens, volatile organic compounds (VOCs), etc., released by plants during unfavorable conditions (biotic stress) have been assessed.
Synergistic interactions among different antioxidant species present in plants make it a tough task for accurate antioxidant assays. The goal of this paper is to understand the correlations between electrochemical profiles that can reveal possible interactions based on the biological activity of plants [21]. This is highlighted via the presence of various characteristics (phytochemicals) of herbs and spices available in nature (different parts of plants are used to treat different diseases and conditions) as explained in Section 2. Following this, the understanding of phytochemicals (chemical class and its compounds) with their mechanism of action is highlighted. Furthermore, the role of these plant-based system have been amalgamated with electrochemical approaches viz-a-viz their electrochemical quantitative and qualitative estimation of compounds; phenotyping; establishment of an electrochemical platform having phytochemical properties for sensor development, and many more possible opportunities explained in Section 3.
2. Therapeutic Value
Herbs and spices have an old-fashioned reputation for their usage with sturdy roots within cultural territory and heritage. Moreover, they have an incomparable association with nourishment and its links. Validating their benefits by scientific means has always remained a challenging task, particularly when compared to the standards applied for gauging pharmaceutical agents. Food can be eaten in various combinations, and in relatively large and unmeasured quantities. Herein, the real challenge is not proving whether foods have health benefits but rather developing scientific methods to expose them qualitatively and quantitatively. Table 1 provides a listing of such phytochemical defense foods with the part of the plant consumed, advantages, and disadvantages.

Table 1.
Accounts the name of various phytochemical defense compounds/foods used in our daily routines with tremendous benefits and few side effects.
Seasonings can be any part of the plant vis à vis the stigma of flowers (saffron), bark (cinnamon), roots (ginger), aromatic seeds (cumin), berries (peppercorns) and buds (cloves). Another interesting example is coriander which can be employed as an herb using its leaves while its dried seeds are used as a spice. Plant-based systems (herbs and spices) have an extended past of both culinary use and of providing health benefits, as well as acting as preservatives [37]. In 1555 BCE, ancient Egyptian papyri recorded the use of coriander, fennel, juniper, cumin, garlic, and thyme [38]. In 5000 BCE, accounts were found of the Sumerians using thyme for its health attributes, followed by the farmers of Mesopotamian in 3000 BCE growing garlic [39]. The global trade in spices goes back to 4500–1900 BCE, chiefly in Ethiopia [40]. The early Egyptians worshipped garlic and its cloves, which were found in the tomb of King Tutankhamen. The records of Egyptians having wooden cloves of garlic in their tombs to preserve the future meals of the afterlife is an astonishing detail. In addition, Hippocrates (460–377 BCE) had a catalogue consisting of 300 medications that included garlic, cinnamon, rosemary, etc. They seemingly included garlic to cure uterine cancer, whilst mint was treasured for its positive effects on the intestinal system, and licorice was initially used as a sweet but can be utilized as an herb too, based on its anti-inflammatory actions for asthma, chest problems, and mouth ulcers. The herb, rosemary, was used to improve and strengthen memory. The Greeks published a book that encompassed more than 500 herb varieties unfolding the variability of choice while a store in Rome has a strong inspiration for herbal remedies that consists of complicated mixtures, consisting of more than a hundred ingredients from 162 CE. Another significant example of such herbs is cinnamon for treating colds and flu, galangal used to treat abdominal pain, and nutmeg for a cure for diarrhea.
Moreover, traditional Ayurveda evolved more than 5000 years ago in the Himalayas, with knowledge being transmitted orally until it was first jotted down in Sanskrit poetry in 1500 BCE as “The Vedas”. Ayurveda prospered in the 7th century and focused on disease demotion and health promotion, highlighting dietary habits. Ayurveda includes the protection of various organs and functions: basil → heart, cinnamon → stimulate circulation, turmeric → jaundice, mace → stomach inflection, and ginger → universal medicine, essentially to cure indigestion and relieve nausea. With the decline of the Roman Empire and built upon the knowledge of Galen (476 CE), the development of Arabic medicine (500–1300 CE) preserved some of the awareness of the surrounding health benefits [41].
The topic of functional foods includes scientific endeavors, technological advancements, advertising, and standard guidelines. Based on a scientific standpoint, functional foods have been defined as “foods that provide advantages beyond the basic nutritional needs” [42]. However, the food modules of today are not just limited to concepts of preventing clinical deficits and sustaining homeostasis but account for a budding recognition of how food constituents actively interact within the body to support health and check abnormalities and overt infections. Herbs and spices fit into this picture in numerous ways. In particular, with supplements, the emphasis may be on their role in a dietary regime rather than their usage as medicines. They provide a path for the future investigation and development of an appreciation of the potential assistance of herbs and spices to health and well-being. They incorporate a significant character in dietary flavonoid intake. Chamomile, rosemary, licorice, onions, thyme, rosemary, and sage have enriched flavonoid content [43]. Herbs have anti-carcinogenic effects from different stages. An example is diallyl sulfide (found in garlic) which is an effective inhibitor of cytochrome P450 (CYP)3 IIE1, i.e., a Phase I enzyme and providing considerable surges of a variety of Phase II enzymes, such as glutathione S-transferase, quinone reductase, and uridine diphosphate-glucuronosyltransferase, which are implicated in the detoxification of carcinogens [44,45]. Even animal models of cancers have achieved some success with basil, mint, lemongrass, rosemary, and turmeric. Herbs involve protection against oxidative stress and inflammation, both of which can lead to the risk of commencement and advancement of cancer. Parsley, a culinary herb, consists of myristicin, an inducer of phase II enzyme glutathione S-transferase as tested on albino mice [46]. Even sedatives may be replaced by Passiflora incarnate, as suggested by the German Commission E in 1987. Another sedative is Valeriana officinalis which interacts with GABA systems (systems affected by amino butyric acid) in the brain [47].
The major question that arises is why are these plant-based systems (examples as listed in Table 1) so advantageous. The plant-derived super foods consist of tannins, catechins, alkaloids, steroids, and polyphenolic acids. These play a crucial role in plant defense mechanisms against invasion by foreign agents [15,39,42]. Substances such as terpenoids are responsible for odour (quinones and tannins) plus the pigment of the plant. Many of these substances account for the plants’ flavor (e.g., terpenoid capsaicin from chili peppers). Alkaloids include heterocyclic nitrogen compounds, an example being morphine isolated in 1805 from Papaver somniferum (opium poppy) [48]. The name morphine comes from the Greek word Morpheus, which means “God of dreams”. Codeine and heroin are both derivatives of morphine. Flavones are phenolic structures containing one carbonyl group. They are hydroxylated phenolic substances that are linked to an aromatic ring. Dimethoxyflavone and bonducellin were isolated. Essential oils and terpenoids are the anti-microbial properties of aromatic volatility oils derived from medicinal, or other edible, plants that have a high content of phenolic derivatives such as carvacrol and thymol. Some traditional medicinal plants, their parts, and the name of compounds are enlisted in Table 2.

Table 2.
Accounts of various traditional medicinal plants and their inhibitory activities.
Peptides are inhibitory to micro-organisms as first reported in 1942. Peptides called “cathelicidins” represent an important native component of innate host defense in mice and protect against necrotic skin infections caused by Streptococcus [54]. The broad spectrum activity displayed by anti-microbial peptides is considered as a “chemical condom” against HIV infection and the Herpes simplex virus [55]. Tannins are a group of phenolic substances capable of tanning leather or precipitating gelatin from solutions, by a property known as “astringency”. The growth of fungi, yeast, bacteria, and viruses was inhibited by tannins, whilst the ones with mixtures such as neem (Azadirachta indica), verasingam pattai (Zanthoxylum limonella), and Indian Babool (Acacia nilotica) stick are widely used as toothbrushes [56]. Strawberry extracts are strong inhibitors of Salmonella bacteria [54]. The dried flower-heads of Chrysanthemum morifolium are an oriental drug as well as a popular herbal tea in China, and also used for the treatment of eye diseases in Japan [57]. The scopes of/for phytochemical-based sensors are illustrated in Figure 2.
Figure 2.
Diagrammatic illustration of various scopes that can be explored regarding phytochemical and/or sensor platforms.
2.1. Phytomedicine
Due to their in-built abilities, traditional plants are recognized to be powerful ingredients that can substitute for anti-inflammatory, diuretic, and diaphoretic medicines [8]. They are well established with antioxidant and radical scavenging properties with minimal side effects compared to chemical therapeutics [58]. Thus, the use of traditional medicines for primary healthcare has steadily increased worldwide. Nearly 80% of the pharmaceutical-derived plants are used as anti-microbes for treating infectious diseases. These inhibit the germ and exhibit greater selectivity toxicity towards infecting germ and host cells. The beneficiary medical effects of plants are a result of their secondary metabolites [6]. The chemotaxonomic considerations and target-directed screening play a crucial role. These plant-based medicines have come as an end product in the market.
In comparison to synthetic pharmaceuticals based on a single constituent, phytomedicines exert a synergistic action of several chemical compounds acting at a single or multiple target site, thus eliminating the problematic/side effects associated with a physiological process. Williamson and his coworkers, in 1999, extensively documented how synergistic interactions imply underlying effectiveness [59].
2.2. Nutritive Content Estimation
A new interface with a class of sensors has been developed, such as a transistor-based sensor for sap fluid nutritive content analysis. However, the drawback observed was the invasive approach that affects plant activities [60]. Based on differential pulse voltammetry (DPV) data, we can determine the peak height which reflects the concentration of species following the peak potential for identification of species. The shape of the peaks helps in distinguishing molecules having the same potential characteristics. Gandhi et al. involved a GCE/CB electrode for the qualitative and quantitative estimation of nutritive content—sesamol in various natural samples (sesame seeds and oil varieties)—in neutral media without any pre sampling [61]. Another real-time approach accounts for using a pencil graphite electrode for testing various tea assays [62]. Square Wave Voltammetry is one such technique which distinguishes diverse samples based on peak current values. The most important characteristic is the elimination of capacitive current values. A few of their examples are tabulated in Table 3.

Table 3.
Tabulation of various sensors used for the nutritive estimation by various researchers.
2.3. Therapeutic Protein Expression Using Plants
The beginning of recombinant DNA technology has provided the gears for generating recombinant proteins that could be employed as therapeutic agents [66]. Recently, advances can be developed involving phytosystems as bioreactors to harvest therapeutic proteins directed against infection and diseases. The unceasing hazard of disease-causing micro-organisms is a serious apprehension/concern and has evolved a paradigm modification in the pharmaceutical and biotechnological enterprises, encouraging them to exploit the heterologous expression of amalgams in the living individuals. Plant-based biofactories promise rapid developments as biopharmaceutical agents and edible vaccines [67]. Table 4 shows the different recombinant types. These support the production of great yields, lower production cost and storage expenses, exclusion of pathogen contamination, few/limited processing steps, and the safe and secure distribution of oral vaccines that have boosted the practice of such systems in recent years. Nevertheless, bottlenecks lead to reduced expression, encouraging researchers to comprehensively investigate heterologous protein expression in phytosystems for the evolution of innovative approaches with a defined expression of biopharmaceutical peptides that encourage various immune responses. The advent of recombinant DNA manipulation in 1977 initiated the use of E. Coli, for therapeutic proteins, ground-breaking research, and manufacturing of recombinant DNA [68]. The US Food and Drug Administration (FDA)-approved Human Insulin was launched in 1982, followed by an E. coli-based insulin in 1979 [69]. However, very few plant-based expression systems excel in the manufacturing of vaccines, as suggested by Goeddel and his coworkers in 1979. Furthermore, they have tremendous growth potential as the basis of a modern discipline, providing immediate cures for infectious diseases. The expression of an alien gene in a host plant cell is dependent on cumulative effects for several essential components required for cell transformation (including the coding sequence, promoter region, transcript termination, etc.). In the plant cell pH, the efficiency and accuracy of the transcriptional and translational machinery, plant cell biochemistry, and the ease of amino acid are required by the plant for recombinant protein, including the interaction between the storage of expressed proteins in the cellular environment. An elevated safety concern due to transgene contamination strongly discourages the commercialization strategy for plant-derived pharmaceutical production.

Table 4.
Tabulation of distinct types of transformation in various host, their vector and the recombinant protein targeted.
3. Phytochemical Based Analysis and Sensor Systems
3.1. Phenotyping Tool/Phylogenic Classification
The valuation of basic plant traits including architecture, growth, development, defense, physiology, tolerance, resistance, etc., is the primary characteristic of measurement for an individual’s quantitative constraints that laid the foundation for complex trait evaluation.
An electrochemical platform can be employed to distinguish phylogenetic data based on the electrochemical approximation that can be used to build relationships in evolutionary patterns. The electrochemical combination is a rapid phenotyping tool accounting for the distinct combinations of mutants in plants, animals, and microbes. Tansil et al. gave a new outlook to DNA detection using a simplified electrocatalytic imidazole-naphthalene diimide-functionalized system as a threading intercalator [76]. The practicability of the proposed electrochemical platform incorporates a non-labelling procedure carried out at a neutral phosphate buffer. Carbo and his coworkers published an electrochemistry-based chemotaxonomy for different varieties of Plantae using microparticle methodology [77]. Even the infragenic identification based on petal tissue fingerprinting can be easily worked out using the electrochemical approach, as reported by Fu et al. [78].
The basic backbone of any nucleotide consists of phosphoric acids and base units that make the molecule heavily charged. This charge corresponds to the potential of the backbone unit which is generally negative.
A new approach related to an integrated chip-on-plant modified sensing-platform for the monitoring of gene expression has been explored by Pandey et al. [79]. Herein, the β-glucuronidase (GUS) expression bio-sensing is validated using chrono-amperometry in tomato and tobacco plants. The electrode microchip is fabricated to transduce the GUS enzyme expression based on genetic modifications.
3.2. Antioxidant Screening
The growing interest of the population in the functional foods and health sector has invoked a wave of antioxidant studies increasing the understanding of the role of free radicals in disease generation [11]. The vegetal kingdom and its associated products are the targets for the assays using various distinct methodological and protocols developed over time. Traditional approaches account for undefined reaction times with a separate setup required for each constituent with tedious protocols. The output of traditional approaches is quite specific for the concerned systems. Meanwhile, a universal criterion should be maintained for assay as it is not solely dependent on one specific reaction but many different mechanisms exist contributing to antioxidant ability. Thus, an electrochemical platform can be a potential approach to be applied for an overall antioxidant capacity determination as suggested by numerous reports using the solution-phase technique [80,81,82,83]. The lower the oxidation potential, the more intense the total electrochemical antioxidant power (TEAP). The non-essential compounds can be masked, reducing the chances of false or wrong information. The minor oxidation potential is recommended to understand the antioxidant concentration but sometimes that can cause trouble with wave broadening and shoulder-type signals. Quercetin is referred to as a standard antioxidant and has a peak potential of 0.16 V (oxidation) [84]. Table 5 is the culmination of such examples. The oxidation potential is a characteristic feature contributed by the free radical scavenging ability of plant extracts. Similarly, other phytochemicals can be accounted adequately based on various potentials with their subsequent current value; the higher the current “I”, the higher the antioxidant concentration. Arbelaez and his research group gave an elaborate review on an antioxidant capacity determination using Cyclic Voltammetry (CV), Chronoamperometry, differential pulse voltammetry (DPV), and square wave voltammetry (SWV) in various vegetable varieties including spinach, chicory, edible oils, cabbage, etc. [85,86]. Based on the CV data, peak separation, i.e., Ep, was obtained which helps us to determine the number of electrons transferred (n). Following the variation in potential is linear to the logarithmic activity of species. The peak correlates with the type of reductant, and the low oxidant potentials relate to greater strength for the electron-donating characteristics. Many reports also involve the estimation of oxidation peaks with that of ascorbic acid extendable to non-transparent samples [87]. Zegarac et al. have confirmed that the results compared between classical and CV techniques are reliable and comparable. The reason for the similarity is that, the mechanism is same incorporating one electron transfer. The electrochemical method is simple and two times faster than usual. They even stated a good correlation between the methods under the same conditions; however, the spectrophotometric technique was affected by color and turbidity constraints. Meanwhile, the electrochemical set-up was easier with no pre-requisites of color, stability, and pre-sampling steps.

Table 5.
Tabulation for the determination of Antioxidant Assay by researchers in the last two decades.
3.3. Sensor Platform Fabrication
Plant component- or phyto-nutrient-based sensing platforms can be a green initiative for the development of sustainable and eco-friendly sensors. Moreover, the involvement of a renewable source for the sensing element or the transducer (which converts one form of signal to another) can be an additional step to enhance the technological process which is significantly crucial for added advantages and eliminates the step for synthetic organic components. Table 6 shows various phytochemicals used for sensor development.

Table 6.
Account for various sensing platforms used with different phytonutrients.
Hong et al. initiated qualitative nucleic acid investigation using the amperometric technique [96]. A gold bi-layer electrode was prepared with oligonucleotide and mercaptahexadendoic acid on an Au electrode via a self-assembly approach. The fabricated biosensor involved 16S gene detection in a mixture of gene and PCR products at 0.15 V [96]. The presence of ascorbic acid was correlated proportionally to miRNA content [97].
3.4. Disease Diagnostic Functions/Disease Biomarker
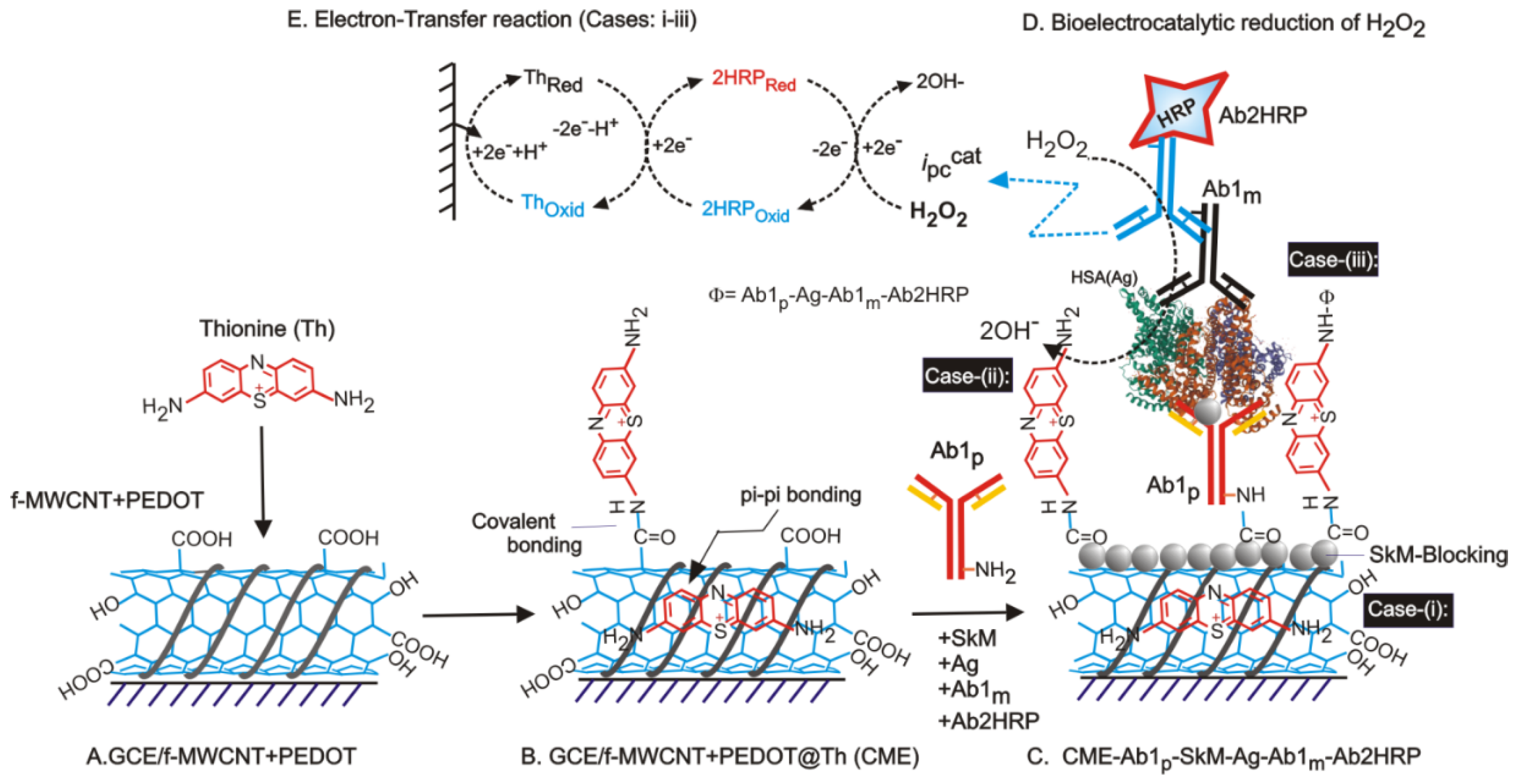
The discovery and sequencing of disease via various methodologies involve understanding the valuable insight as to how diseases can be selectively diagnosed at a nano-scale level within a few minutes. It contributes to the early-stage detection and monitoring of the situation in a better way, so that the least destruction in terms of mortality and economic aspects is achieved. Current detection methods are based on meticulous experimental requirements, expensive setups with skilled technicians, and complicated understanding. However, to overcome the issues, electrochemical techniques pave an easy way for early diagnosis and are quite economical. Electrochemistry-based sensors will become more prominent diagnostic tools for various clinical assays, widening the bandwidth for genetic-related disorders and diseases. Gandhi et al. introduced a simple and novel concept of the phytochemical-based sensor for immuno-sensing of White Spot Syndrome Virus [61]. Figure 3 illustrates the preparation of electrochemical biosensor for human serum albumin (HSA) present in urine samples using a sandwich ELISA involving CME-Ab1p-SkM-Ag(HSA)-Ab1m-Ab2HRP modified electrodes (A–C) and its (D) bio-electrocatalytic reduction of H2O2. The Ab1p-Polyclonal primary antibody, Ab1m-Monoclonal primary antibody, SkM-Skim milk power as a blocking agent, Ag = HSA, Ab2HRP = Horseradish peroxide enzyme tagged Ab1p. Cases-(i–iii) are possible routes for molecular orientations of surface-confined-Th and its interactions with biological systems. The figureshows the mechanistic pathway observed for the development of an electrochemical biosensor for human serum albumin (HSA) in this regard. Another such immunosensor is fabricated for the detection of urinary human serum albumin (HSA) [102]. Table 7 shows the disease detection process using the electrochemical platforms in various conditions. Furthermore, Khatar and his coworkers have reported, using a citrus Tristeza virus, the detection of destructive viruses in plants using screen--printed carbon electrodes modified with gold nanoparticles in 0.01 M Phosphate buffer involving electrochemical impedance spectroscopy [103]. Fang et al. reported a bi-enzyme sensor for the detection of methyl salicylate (allelochemical; a volatile organic compound released by plants during pathogenic infection and infestation) via a molecular tethering technique [104].
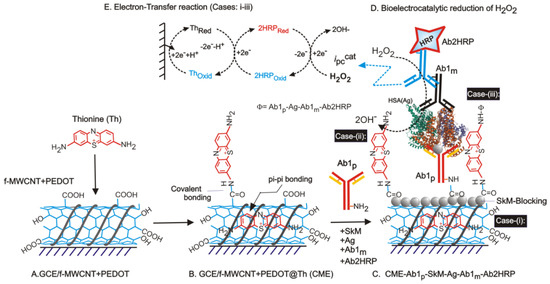
Figure 3.
Diagrammatic illustration for preparation of electrochemical biosensor for human serum albumin (HSA) present in urine samples using a sandwich ELISA involving CME-Ab1p-SkM-Ag(HSA)-Ab1m-Ab2HRP modified electrodes (A–C) and its (D) bio-electrocatalytic reduction of H2O2. The Ab1p =Polyclonal primary antibody, Ab1m =Monoclonal primary antibody, SkM =Skim milk power as a blocking agent, Ag = HSA, Ab2HRP = Horseradish peroxide enzyme tagged Ab1p. Cases-(i–iii) are possible routes for molecular orientations of surface-confined -Th and its interactions with biological systems. Rights and permissions, copyright [102].

Table 7.
Disease detection using the electrochemical platforms in various conditions.
4. Bottlenecks/Drawbacks
The insolubility of essential oils and non-polar extracts makes it very difficult for them to be used in aqueous media [110].
Many VOCs are generated by plants due to unfavorable events; these are termed Green Leaf Volatiles (GLVs); they are non-specific to pathogenic attacks. The release of such GLVs can interfere with VOCs detection.
The electrochemical investigation studies are hampered due to the background noise caused by the fluctuations in the signal. These fluctuations need to be suppressed and a proper determination of changes in electrical potential, impedance values, current output, and kinetics change should be monitored after performing background correction.
The performance of any electrochemistry-based sensing platform is particularly dependent on affinity confined/initiated by the probe with the target molecules which are based on the exploitation of structure, design, amplification modules, technique, and environmental conditions. However, with the proper adjusting of the parameters, we can enhance the response of sensors [111].
The presence of metals with plant extracts can lead to their uptake and the transport mechanism. Their presence can put restrictions on the binding capacity or binding competition and can further deteriorate their transportation activity. Pawel et al. studied the electrochemical fingerprinting of plants rich in flavonoids and even studied the interaction of electro-active metal species, especially of iron (Fe) and copper (Cu). There is an inverse output between the flavonoids’ detectability with increasing metal ions [112].
This instability is frequently encountered by a biosensor with miniature oligonucleotide detection probes (8–10 nucleotide long) that binds to the similar template of miRNA [96], thus paving a way toward chemical-electrochemical amplified assays.
The biggest challenge encountered is eliminating the non-specific redox process under a specific potential window, especially when the redox feature of a particular substrate is at the lowest potential which should be quite close to the thermodynamic potential of the substrate concerned [113].
The amperometric technique of electrochemistry is quite accurate, non-invasive, and selective. However, this Amp i-t accounts for the role of faradaic contribution only while the non-faradaic current can lead to a non-steady response of systems. Hence, to ensure a steady current response, constant potential amperometry (a technique called ”Chronoamperometry”) must be applied [104].
The use of intercalators is a substitute for labelling drawbacks but their usage leads to noise problems as the binding is only established between single strands and not the double-stranded DNA. An advancement in this field has been approached via designing new intercalators that offer better discrimination between DNA strands and segments with achieving a better signal/noise ratio [75].
Frequently, voltammograms show a similar response of several molecules that restricts their usage and even makes it a complication to differentiate between substances [87].
In enzyme-oriented assays for DNA detection, the background current is observed in the range of nanoamperes due to non-specific adsorption and electrostatic abrupt changes. These excess current/background current can be minimized with a bi-layer configured sensor.
5. Advantages
The same equipment, i.e., galvanostat/potentiostat and a few add-ons such as an electrode, stirrer, etc., is sufficient to gather diverse information with a choice of techniques inbuilt into the operating system.
Much enhanced sensitivity and accuracy were achieved using a biosensor and reducing the effort of running multiple replicates [114,115].
The electrochemical approach has in-built techniques to gather information based on various spheres as large quantities of data can be obtained within the same set-up (a voltammetric charge, electrode potential, diffusion current, electron transfer characteristics, half wave potential, etc.).
Usually, the DNA-based sensors have high Gibbs free energy (1 to 10 kcal/mol) based on the thermodynamics. Hence, the energy barrier had to be overcome vis à vis high temperature and ion concentration gradient in the bio-systems. The electrochemical sensors make this happen/realistic/easy as the electric field generated (one hundred millivolts) due to potentials, can overcome the energy barrier.
These nano-electrode systems are highly specific and extremely efficient concerning power consumption, recognition interval time, smaller electrical potentials, and have a low throughput process compared to traditional approaches.
This technique avoids the use of labelling, which makes the investigation complex and cumbersome with an alternative to using redox-active intercalators [75].
EC sensors are based on the simple manipulation of the molecules and their properties within the sample due to the electronic charge variation by the potentials and current parameter optimization. This enhances the homogenous mixing of the sample and their ability to achieve a precise output [61].
Low noise potentiostats/bi-potentiostats can monitor microampere currents which are far more economical compared to sensitive non-electrochemical instrumentation [111].
Amperometric detection at lesser negative or positive potentials squeezes the interferences and background variables, yielding a lower signal/noise ratio with an improved detection limit [62].
This provides a simple strategic concept that can enable portable, handheld electrochemical apparatus for in-field testing of samples [102].
6. Conclusions and Future Outlook
In this review, after introducing and classifying various phyto-based nutrient systems, we highlighted how they have been employed in subsequent fields, and described their potential appealing values that could be utilized in future. Because of the inherent and unique characteristics of the Plantae kingdom, interesting bioactive or nature-based compounds are used in a myriad of fields. They consist of a plethora of fascinating therapeutic properties which are utilized in the planned system and could be a boon for the living. Thus, a simple, low throughput process providing a novel set-up for phenotyping the plant models is set to become a new avenue for scientists. As explained in the review part of the article, the phytochemical defense compounds within plants can be an avenue to solve major health issues for mankind, as illustrated in Section 1 and Section 2. This led us to their role as phytomedicine and their therapeutic usage in day-to-day lives. They can be further extended for therapeutic protein expression using plants. Various phytochemical-based analysis and sensor systems have been developed for phenotyping and classification along with antioxidant screening, which could be a boon to the healthcare sector. Apart from these, various systems have been extended as transducer elements for the development of biosensing systems for point-of-care setups. This plant physiological approach has a vital role to play in the burgeoning field. However, research is ongoing for bottlenecks related to sample handling and fluidic processing with detection on/using a well-defined platform. Thus, further research in this pattern should be allowed on a highly advanced platform for tailoring according to their structural properties and for expanding their potential applications.
Author Contributions
M.G.—Conceptualization, Methodology, Writing, Project administration, Validation, Investigation and Writing; K.A.—Validation, Investigation, Supervision; B.K.T.—Validation, Investigation, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Mansi Gandhi thanks the Indian Council of Medical Research (ICMR) for the award of her SRF (Id: 2019-4952) during her PhD studies (year: 2016–2021). Khairunnisa Amreen acknowledges St. Ann’s College for women, Mehdipatnam, Hyderabad.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487. [Google Scholar] [PubMed]
- Bavishi, K.; Laursen, T.; Martinez, K.L.; Møller, B.L.; Della Pia, E.A. Application of nanodisc technology for direct electrochemical investigation of plant cytochrome P450s and their NADPH P450 oxidoreductase. Sci. Rep. 2016, 6, 29459. [Google Scholar] [CrossRef] [PubMed]
- Udit, A.K.; Hill, M.G.; Gray, H.B. Electrochemistry of cytochrome P450 BM3 in sodium dodecyl sulfate films. Langmuir 2006, 22, 10854–10857. [Google Scholar] [CrossRef] [PubMed]
- Mcquaid, K.E.; Keenan, A.K. Physiological Society Symposium: Impaired Endothelial and Smooth Muscle Cell Function in Oxidative Stress Endothelial Barrier Dysfunction and Oxidative Stress: Roles for Nitric Oxide? Exp. Physiol. 1997, 82, 369–376. [Google Scholar] [CrossRef]
- Nath, S.; Tamuli, K.J.; Gogoi, B.; Bordoloi, M.; Das, A.; Barua, C.C.; Barua, I.C. Antioxidant properties, phenolic and mineral profiling, assessment of angiotensin I converting enzyme (ACE) inhibitory potential of Elsholtzia communis (Collett & Hemsl.) Diels from North East India. Eur. J. Integr. Med. 2020, 40, 101247. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Barros, L.; Cabrita, L.; Boas, M.V.; Carvalho, A.M.; Ferreira, I.C.F.R. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011, 127, 1600–1608. [Google Scholar] [CrossRef]
- Chen, C.Y.; Stemberger, R.S.; Klaue, B.; Blum, J.D.; Pickhardt, P.C.; Folt, C.L. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol. Oceanogr. 2000, 45, 1525–1536. [Google Scholar] [CrossRef]
- Dales, J.P.; Desplat-Jégo, S. Metal imbalance in neurodegenerative diseases with a specific concern to the brain of multiple sclerosis patients. Int. J. Mol. Sci. 2020, 21, 9105. [Google Scholar] [CrossRef]
- He, Z.; Xu, Q.; Newland, B.; Foley, R.; Lara-Sáez, I.; Curtin, J.F.; Wang, W. Reactive oxygen species (ROS): Utilizing injectable antioxidative hydrogels and ROS-producing therapies to manage the double-edged sword. J. Mater. Chem. B 2021, 9, 6326–6346. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Dong, Y.; Gong, J.; Li, Z.; Zhang, J. Core-shell structured gold nanorods on thread-embroidered fabric-based microfluidic device for Ex Situ detection of glucose and lactate in sweat. Sens. Actuators B Chem. 2021, 353, 131154. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Amaral, G.; Dantas Dos Santos, H.; Oliveira Da Conceição, A.; Faustino De Oliveira, F.; Aparecida De Oliveira, R. Evaluation of triterpenes isolated from stems of Pouteria macahensis TD. Trends Phytochem. Res. 2019, 3, 181–188. [Google Scholar]
- Gandhi, M.; Amreen, K. Electrochemical Profiling of Plants. Electrochem 2022, 3, 434–450. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef]
- Chen, P.; Shakhnovich, E.I. Thermal adaptation of viruses and bacteria. Biophys. J. 2010, 98, 1109–1118. [Google Scholar] [CrossRef]
- Domingo, E. Rna Virus Mutations. Annu. Rev. Microbiol. 1997, 51, 151–178. [Google Scholar] [CrossRef]
- Balol, G.B.; Divya, B.L.; Basavaraj, S.; Sundaresha, S.; Mahesh, Y.S.; Erayya HS, D. Sources of genetic variation in plant virus populations. J. Pure Appl. Microbiol. 2010, 4, 803–808. [Google Scholar]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future (review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid.-Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Nassar, R.; Eid, S.; Chahine, R.; Chabi, B.; Bonnieu, A.; El Sabban, M.; Najjar, F.; Hamade, A. Antioxidant effects of lebanese Crocus sativus L. and its main components, crocin and safranal, on human skeletal muscle cells. Eur. J. Integr. Med. 2020, 40, 101250. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Ring, D.J.; Dalrymple, S.N. Treatment of the common cold. Am. Fam. Physician 2019, 100, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Awang, D.V.C. Prescribing therapeutic feverfew (Tanacetum parthenium (L.) Schultz bip., syn. Chrysanthemum parthenium (L.) Bernh.). Integr. Med. 1998, 1, 11–13. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Mahomoodally, M.; Aumeeruddy, M.; Rengasamy, K.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2021, 69, 140–149. [Google Scholar] [CrossRef]
- Rojas, P.; Montes, P.; Rojas, C.; Serrano-García, N.; Rojas-Castañeda, J.C. Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson’s disease: Therapeutic perspectives. Nutrition 2012, 28, 1081–1088. [Google Scholar] [CrossRef]
- Choi, M.K.; Song, I.S. Interactions of ginseng with therapeutic drugs. Arch. Pharmacal Res. 2019, 42, 862–878. [Google Scholar] [CrossRef]
- Gurley, B.J.; Swain, A.; Hubbard, M.A.; Hartsfield, F.; Thaden, J.; Williams, D.K.; Gentry, W.B.; Tong, Y. Supplementation with goldenseal (Hydrastis canadensis), but not kava kava (Piper methysticum), inhibits human CYP3A activity in vivo. Clin. Pharmacol. Ther. 2008, 83, 61–69. [Google Scholar] [CrossRef]
- Singh, Y.N.; Singh, N.N. Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs 2002, 16, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, B.O.; Omolabi, F.K.; Olajide, P.A. Pharmacological, Biochemical and Therapeutic Potential of Milk Thistle (Silymarin): A Review. World News Nat. Sci. 2021, 37, 75–91. [Google Scholar]
- Bloch, M.H.; Mulqueen, J. Nutritional supplements for the treatment of ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 883–897. [Google Scholar] [CrossRef]
- Presley, C.L.; Kolodziejczyk, T.C.; Pulsipher, K.J.; Maghfour, J.; Militello, M.; Rietcheck, H.R.; Fonseca, A.; Olayinka, T.J.; Rundle, C.W.; Waller, J.D.; et al. A Scoping Review of Pharmacotherapy, Complementary, and Alternative Medicine (CAM), and Surgical Therapies for Androgenic Alopecia. Curr. Dermatol. Rep. 2021, 10, 48–54. [Google Scholar] [CrossRef]
- Tammadon, M.R.; Nobahar, M.; Hydarinia-Naieni, Z.; Ebrahimian, A.; Ghorbani, R.; Vafaei, A.A. The Effects of Valerian on Sleep Quality, Depression, and State Anxiety in Hemodialysis Patients: A Randomized, Double-blind, Crossover Clinical Trial. Oman Med. J. 2021, 36, e255. [Google Scholar] [CrossRef]
- Lone, R.; Shuab, R.; Kamili, A.N. Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Shoja, M.M.; Tubbs, R.S.; Bosmia, A.N.; Fakhree, M.A.A.; Jouyban, A.; Balch, M.W.; Loukas, M.; Khodadoust, K.; Khalili, M.; Eknoyan, G. Herbal diuretics in medieval Persian and Arabic medicine. J. Altern. Complement. Med. 2015, 21, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Shimelis, T. Spices production and marketing in Ethiopia: A review. Cogent. Food Agric. 2021, 7, 1915558. [Google Scholar] [CrossRef]
- Masic, I.; Skrbo, A.; Naser, N.; Tandir, S.; Zunic, L.; Medjedovic, S.; Sukalo, A. Contribution of Arabic Medicine and Pharmacy to the Development of Health Care Protection in Bosnia and Herzegovina—The First Part. Med. Arch. 2017, 71, 364–372. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Eldridge, A.L.; Bhagwat, S.; Gebhardt, S.E.; Holden, J.M.; Beecher, G.R.; Peterson, J.; Dwyer, J. Flavonoid Content of Vegetables; Agricultural Research Service: Washington, DC, USA, 2002.
- Pinto, J.T.; Rivlin, R.S. Recent Advances on the Nutritional Effects Associated with the Use of Garlic. J. Nutr. 2001, 131, 1058–1060. [Google Scholar] [CrossRef]
- Lii, C.K.; Tsai, C.W.; Wu, C.C. Garlic allyl sulfides display differential modulation of rat cytochrome P450 2B1 and the placental form glutathione S-transferase in various organs. J. Agric. Food Chem. 2006, 54, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Tijerina, M.T.; Tobola, A.S. Preferential overexpression of a class MU glutathione S-transferase subunit in mouse liver by myristicin. Biochem. Biophys. Res. Commun. 1997, 236, 825–828. [Google Scholar] [CrossRef]
- Awad, R.; Levac, D.; Cybulska, P.; Merali, Z.; Trudeau, V.L.; Arnason, J.T. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007, 85, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, F. Fate of Food-Relevant Toxic Plant Alkaloids during Food Processing or Storing and Analytical Strategies to Unveil Potential Transformation Products. J. Agric. Food Chem. 2022, 70, 5975–5981. [Google Scholar] [CrossRef]
- Hadad, M.; Gattuso, S.; Gattuso, M.; Feresin, G.; Tapia, A. Anatomical studies of Baccharis grisebachii Hieron. (Asteraceae). Used in folk medicine of San Juan province, Argentina. Dominguezia 2013, 29, 41–47. [Google Scholar]
- Akomolafe, R.O.; Adeoshun, I.O.; Ayoka, A.O.; Elujoba, A.A.; Iwalewa, E.O. An in vitro study of the effects of Cassia podocarpa fruit on the intestinal motility of rats. Phytomedicine 2004, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.M.; Singh, R.; Chandra, R. Therapeutic uses of curcuma longa (Turmeric). Indian J. Clin. Biochem. 2001, 16, 153–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandal, S.K.; Maji, A.K.; Mishra, S.K.; Ishfaq, P.M.; Devkota, H.P.; Silva, A.S.; Das, N. Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues. Pharmacol. Res. 2020, 160, 105085. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Perez Gil, A.L.; Barbosa Navarro, L.; Patipo Vera, M.; Petricevich, V.L. Anti-inflammatory and antinociceptive activities of the ethanolic extract of Bougainvillea xbuttiana. J. Ethnopharmacol. 2012, 144, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2020, 86, 153170. [Google Scholar] [CrossRef]
- Hani, K.; Zairi, A.; Tangy, F.; Bouassida, K. Dermaseptins and magainins: Antimicrobial peptides from frogs’ skin-new sources for a promising spermicides microbicides-a mini review. J. Biomed. Biotechnol. 2009, 2009, 452567. [Google Scholar] [CrossRef]
- Sajankumar, R.P.; Hegde, V.; Shetty, P. Antimicrobial effectiveness of Neem (Azadirachta indica) and Babool (Acacia nilotica) on Streptococcus mutans: An in vitro study. J. Indian Assoc. Public Health Dent. 2015, 13, 517. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Gopalakrishnakone, P. Therapeutic potential of plants as anti-microbials for drug discovery. Evid.-Based Complement. Altern. Med. 2010, 7, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Assessing Antioxidant Activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Coppedè, N.; Janni, M.; Bettelli, M.; Maida, C.L.; Gentile, F.; Villani, M.; Ruotolo, R.; Iannotta, S.; Marmiroli, N.; Marmiroli, M.; et al. An in vivo biosensing, biomimetic electrochemical transistor with applications in plant science and precision farming. Sci. Rep. 2017, 7, 16195. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Rajagopal, D.; Parthasarathy, S.; Raja, S.; Huang, S.; Kumar, A.S. In Situ Immobilized Sesamol-Quinone/Carbon Nanoblack-Based Electrochemical Redox Platform for E fficient Bioelectrocatalytic and Immunosensor Applications. ACS Omega 2018, 3, 10823–10835. [Google Scholar] [CrossRef]
- Vishnu, N.; Gandhi, M.; Badhulika, S.; Kumar, A.S. Tea quality testing using 6B pencil lead as an electrochemical sensor. Anal. Methods 2018, 10, 2327–2336. [Google Scholar] [CrossRef]
- Vishnu, N.; Gandhi, M.; Rajagopal, D.; Kumar, A.S. Pencil graphite as an elegant electrochemical sensor for separation-free and simultaneous sensing of hypoxanthine, xanthine and uric acid in fish samples. Anal Methods 2017, 9, 2265–2274. [Google Scholar] [CrossRef]
- Araújo, D.A.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A lab-made screen-printed electrode as a platform to study the effect of the size and functionalization of carbon nanotubes on the voltammetric determination of caffeic acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Lobsey, C.R.; Rossel, R.A.V.; Mcbratney, A.B.; Minasny, B. Proximal Soil Sensing; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Sahoo, S.; Kariya, T.; Ishikawa, K. Targeted delivery of therapeutic agents to the heart. Nat. Rev. Cardiol. 2021, 18, 389–399. [Google Scholar] [CrossRef]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.S.; Ahmed, M.M.; Liao, Y.C.; Waheed, M.T.; Sameeullah, M.; Hussain, S.; Saud, S.; Hassan, S.; et al. Recent developments in therapeutic protein expression technologies in plants. Biotechnol. Lett. 2015, 37, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.M.; Thomas, J. Edible Vaccines: Promises and Challenges. Mol. Biotechnol. 2020, 62, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Sempere, R.N.; Gómez, P.; Truniger, V.; Aranda, M.A. Development of expression vectors based on pepino mosaic virus. Plant Methods 2011, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, F.; Wang, Y.; Stevanato, P.; Arcioni, S.; Bellucci, M. Genetic transformation of the sugar beet plastome. Transgenic Res. 2009, 18, 17–30. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Soria-Guerra, R.E.; Olivera-Flores, M.T.D.J.; López-Revilla, R.; Argüello-Astorga, G.R.; Jiménez-Bremont, J.F.; la Cruz, R.F.G.-D.; Loyola-Rodríguez, J.P.; Alpuche-Solís, G. Expression of Escherichia coli heat-labile enterotoxin b subunit (LTB) in carrot (Daucus carota L.). Plant Cell Rep. 2007, 26, 969–976. [Google Scholar] [CrossRef]
- Peyret, H.; Lomonossoff, G.P. When plant virology met Agrobacterium: The rise of the deconstructed clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef]
- Hennegan, K.; Yang, D.; Nguyen, D.; Wu, L.; Goding, J.; Huang, J.; Guo, F.; Huang, N.; Watkins, S. Improvement of human lysozyme expression in transgenic rice grain by combining wheat (Triticum aestivum) puroindoline b and rice (Oryza sativa) Gt1 promoters and signal peptides. Transgenic Res. 2005, 14, 583–592. [Google Scholar] [CrossRef]
- Buhrow, L.M.; Clark, S.M.; Loewen, M.C. Identification of an attenuated barley stripe mosaic virus for the virus-induced gene silencing of pathogenesis-related wheat genes. Plant Methods 2016, 12, 12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tansil, N.C.; Xie, H.; Xie, F.; Gao, Z. Direct detection of DNA with an electrocatalytic threading intercalator. Anal. Chem. 2005, 77, 126–134. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Ibars, A.M.; Prieto-Mossi, J.; Estrelles, E.; Scholz, F.; Cebrián-Torrejón, G.; Martini, M. Electrochemistry-based chemotaxonomy in plants using the voltammetry of microparticles methodology. New J. Chem. 2015, 39, 7421–7428. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Wu, M.; Zhang, H.; Wang, A.; Su, W.; Chen, F.; Yu, J.; et al. An electrochemical method for plant species determination and classification based on fingerprinting petal tissue. Bioelectrochemistry 2019, 129, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Teig-Sussholz, O.; Schuster, S.; Avni, A.; Shacham-Diamand, Y. Integrated electrochemical Chip-on-Plant functional sensor for monitoring gene expression under stress. Biosens. Bioelectron. 2018, 117, 493–500. [Google Scholar] [CrossRef]
- Yang, B.; Kotani, A.; Arai, K.; Kusu, F. Estimation of the Antioxidant Activities of Flavonoids from Their Oxidation Potentials. Anal. Sci. 2001, 17, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Machado De Carvalho, L.; Martini, M.; Valencia, D.P.; Cebrián-Torrejón, G. Electrochemical monitoring of the pharmacological activity of natural products. In Studies in Natural Products Chemistry; Elsevier: Berlin/Heidelberg, Germany, 2015; Volume 45, pp. 59–84. [Google Scholar] [CrossRef]
- Teixeira, J.; Oliveira, C.; Amorim, R.; Cagide, F.; Garrido, J.; Ribei-ro, J.A.; Pereira, C.M.; Silva, A.F.; Andrade, P.B.; Oliveira, P.J.; et al. Development of hydroxybenzoic-based plat-forms as a solution to deliver dietary antioxi-dants to mitochondria. Sci. Rep. 2017, 7, 6842. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Polyquercetin/MWNT-modified Electrode for the Determination of Natural Phenolic Antioxidants. Electroanalysis 2017, 29, 2610–2619. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Jara, M.J.; José, P.; Hernández, M.; Francisco, H.; Aneta, J.H. Phenolic compounds and antioxidant activity of twelve grape cultivars measured by chemical and electrochemical methods. Eur. Food Res. Technol. 2018, 244, 1933–1943. [Google Scholar] [CrossRef]
- Lanfer-marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Cosio, M.S.; Buratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Ligaj, M.; Kobus-Cisowska, J.; Maciejewska, P.; Tichoniuk, M.; Szulc, P. Application for novel electrochemical screening of antioxidant potential and phytochemicals in Cornus mas extracts. CYTA J. Food 2019, 17, 781–789. [Google Scholar] [CrossRef]
- Juárez-Gómez, J.; Ramírez-Silva, M.T.; Guzmán-Hernández, D.S.; Romero-Romo, M.; Palomar-Pardavé, M. Novel electrochemical method to evaluate the antioxidant capacity of infusions and beverages, based on in situ formation of free superoxide radicals. Food Chem. 2020, 332, 127409. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Jiang, X.; Liu, F.; Liu, Q.; Liu, X. Construction of silver-cotton carbon fiber sensing interface and study on the protective effect of antioxidants on hypoxia-induced cell damage. Microchem. J. 2020, 159, 105345. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Messina, G.A.; Bertolino, F.A.; Pereira, S.V.; Raba, J.; Nazareno, M.A. Comparative study of different methodologies for the determination the antioxidant activity of Venezuelan propolis. Microchem. J. 2020, 158, 105244. [Google Scholar] [CrossRef]
- Tomac, I.; Šeruga, M.; Labuda, J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Zhang, H.; Gai, P.; Zhang, X.; Chen, J. Electrochemical evaluation of total antioxidant capacities in fruit juice based on the guanine/graphene nanoribbon/glassy carbon electrode. Talanta 2013, 106, 206–211. [Google Scholar] [CrossRef]
- Rodrí, E. Electrochemical Quantification of the Antioxidant Capacity of Medicinal Plants Using Biosensors. Sensors 2014, 14, 14423–14439. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, C.; Gao, Z. Amperometric Detection of Nucleic Acid at Femtomolar Levels with a Nucleic Acid/Electrochemical Activator Bilayer on Gold Electrode. Anal. Chem. 2004, 76, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Lillehoj, P.B.; Ho, C.M. DNA diagnostics: Nanotechnology-enhanced electrochemical detection of nucleic acids. Pediatr. Res. 2010, 67, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Bueno-Díaz, C.; Montiel, V.R.-V.; Povedano, E.; Reviejo, A.; Villalba, M.; Campuzano, S.; Pingarrón, J. First electrochemical immunosensor for the rapid detection of mustard seeds in plant food extracts. Talanta 2020, 219, 121247. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Park, J.S.; Jo, D.G.; Cho, M.; Lee, Y. Curcumin-based electrochemical sensor of amyloid-Β oligomer for the early detection of Alzheimer’s disease. Sens. Actuators B Chem. 2018, 273, 1593–1599. [Google Scholar] [CrossRef]
- Amreen, K.; Shukla, V.K.; Shukla, S.; Rajagopal, D.; Kumar, A.S. Redox behaviour and surface-confinement of electro active species of ginger extract on graphitized mesoporous carbon surface and its copper complex for H2O2 sensing. Nano-Struct. Nano-Objects 2017, 11, 56–64. [Google Scholar] [CrossRef]
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Electrochemical determination of capsaicin in pepper samples using sustainable paper-based screen-printed bulk modified with carbon black. Electrochim. Acta 2020, 354, 136628. [Google Scholar] [CrossRef]
- Gandhi, M.; Indiramma, J.; Jayaprakash, N.S.; Kumar, A.S. An efficient electrochemical sandwich ELISA for urinary human serum albumin-biomarker based on highly redox-active thionine surface-confined MWCNT/PEDOT.PSS platform. J. Electroanal. Chem. 2022, 906, 116018. [Google Scholar] [CrossRef]
- Khater, M.; de la Escosura-Muñiz, A.; Quesada-González, D.; Merkoçi, A. Electrochemical detection of plant virus using gold nanoparticle-modified electrodes. Anal. Chim. Acta 2019, 1046, 123–131. [Google Scholar] [CrossRef]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. A novel bi-enzyme electrochemical biosensor for selective and sensitive determination of methyl salicylate. Biosens. Bioelectron. 2016, 81, 39–45. [Google Scholar] [CrossRef]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Ma, H.; Wang, H.; Wang, Y.; Fan, D.; Du, B.; Wei, Q.; Zhang, N. Ultrasensitive amyloid-β proteins detection based on curcumin conjugated ZnO nanoparticles quenching electrochemiluminescence behavior of luminol immobilized on Au@MoS2/Bi2S3 nanorods. Biosens. Bioelectron. 2019, 131, 136–142. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Chen, C. Impedimetric biosensor modified with hydrophilic material of tannic acid/polyethylene glycol and dopamine-assisted deposition for detection of breast cancer-related BRCA1 gene. J. Electroanal. Chem. 2017, 791, 204–210. [Google Scholar] [CrossRef]
- Alipour, E.; Shahabi, H.; Mahmoudi-Badiki, T. Introducing curcumin as an electrochemical DNA hybridization indicator and its application for detection of human interleukin-2 gene. J. Solid State Electrochem. 2016, 20, 1645–1653. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A. Using electrochemical oxidation of Rutin in modeling a novel and sensitive immunosensor based on Pt nanoparticle and graphene–ionic liquid–chitosan nanocomposite to detect human chorionic gonadotropin. Sens. Actuators B Chem. 2016, 222, 1103–1111. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, H.H.; Heller, A. Enzyme-amplified amperometric detection of 3000 copies of DNA in a 10-μL droplet at 0.5 fM concentration. Anal. Chem. 2003, 75, 3267–3269. [Google Scholar] [CrossRef]
- Konieczyński, P. Electrochemical fingerprint studies of selected medicinal plants rich in flavonoids. Acta Pol. Pharm. Drug Res. 2015, 72, 655–661. [Google Scholar]
- Gao, Z.; Yu, Y.H. A microRNA biosensor based on direct chemical ligation and electrochemically amplified detection. Sens. Actuators B Chem. 2007, 121, 552–559. [Google Scholar] [CrossRef]
- Naghdi, T.; Faham, S.; Mahmoudi, T.; Pourreza, N.; Ghavami, R.; Golmohammadi, H. Phytochemicals toward Green (Bio)sensing. ACS Sens. 2020, 5, 3770–3805. [Google Scholar] [CrossRef]
- Olvera, D.; Monaghan, M.G. Electroactive material-based biosensors for detection and drug delivery. Adv. Drug Deliv. Rev. 2021, 170, 396–424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).