Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application
Abstract
:1. Introduction
2. Preparation Method of Transition Metal Anchored on Nitrogen-Doped Porous Carbon Catalysts
2.1. Post-Loading Method
2.1.1. Impregnation Method
2.1.2. Deposition-Precipitation Method
2.2. Simultaneous Introduction of Metals and Nitrogen into Pre-Synthesized Carbon Support
2.3. In-Situ Pyrolysis Method of Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts
3. Catalytic Application
3.1. Hydrogenation Reactions
3.2. Dehydrogenation Reaction
3.3. Oxidation Reactions
3.4. Hydroformylation Reactions
3.5. Electrocatalytic Reactions
3.5.1. Oxygen Reduction Reaction (ORR)
3.5.2. Hydrogen evolution reaction (HER)
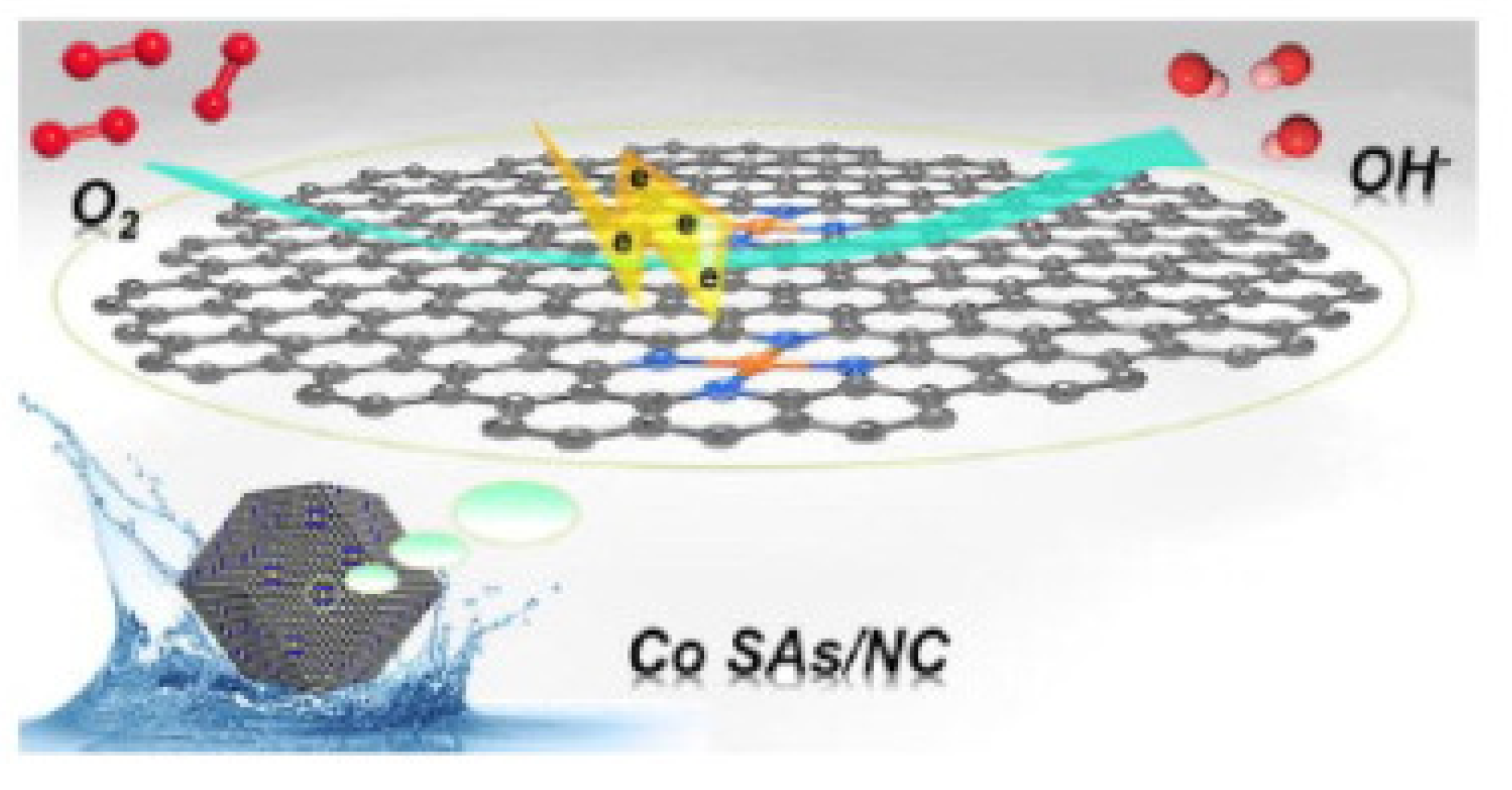
3.5.3. Oxygen Evolution Reaction (OER)
4. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-Based Single-Atom Catalysts for Advanced Applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Huang, Y.; Zhang, T.; Liu, B. Supported Noble-Metal Single Atoms for Heterogeneous Catalysis. Adv. Mater. 2019, 31, e1902031. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/Porous Carbon Composites for Heterogeneous Catalysis: Old Catalysts with Improved Performance Promoted by N-Doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Luna, P.D.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, eaav3506. [Google Scholar] [CrossRef]
- Wang, S.; Wu, T.; Lin, J.; Ji, Y.; Yan, S.; Pei, Y.; Xie, S.; Zong, B.; Qiao, M. Iron–Potassium on Single-Walled Carbon Nanotubes as Efficient Catalyst for CO2 Hydrogenation to Heavy Olefins. ACS Catal. 2020, 10, 6389–6401. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, C.; Liu, X.; Wang, X.; Zhou, F.; Wang, J.; Hu, Y.; Zhao, Y.; Yao, T.; Yang, L.-M.; et al. Single Atomic Cerium Sites with a High Coordination Number for Efficient Oxygen Reduction in Proton-Exchange Membrane Fuel Cells. ACS Catal. 2021, 11, 3923–3929. [Google Scholar] [CrossRef]
- Rangraz, Y.; Heravi, M.M.; Elhampour, A. Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations. Chem. Rec. 2021, 21, 1985–2073. [Google Scholar]
- Baroliya, P.K.; Chopra, J.; Pal, T.; Maiti, S.; Al-Thabaiti, S.A.; Mokhtar, M.; Maiti, D. Supported Metal Nanoparticles Assisted Catalysis: A Broad Concept in Functionalization of Ubiquitous C–H Bonds. ChemCatChem 2021, 13, 4655–4678. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Y.; Ma, D.; Foucher, A.C.; Xiong, L.; Zhang, J.; Stach, E.A.; Yue, Q.; Kang, Y. Atomic Fe Dispersed Hierarchical Mesoporous Fe–N–C Nanostructures for an Efficient Oxygen Reduction Reaction. ACS Catal. 2020, 11, 74–81. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar]
- Fan, Q.; Hou, P.; Choi, C.; Wu, T.S.; Hong, S.; Li, F.; Soo, Y.L.; Kang, P.; Jung, Y.; Sun, Z. Activation of Ni Particles into Single Ni–N Atoms for Efficient Electrochemical Reduction of CO2. Adv. Energy Mater. 2019, 10, 1903068. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Z.; Xu, R.; Zhang, W.; Chen, W.; Zheng, L.; Zhang, J.; Luo, J.; Wu, K.; Zhu, Y.; et al. Ordered Porous Nitrogen-Doped Carbon Matrix with Atomically Dispersed Cobalt Sites as an Efficient Catalyst for Dehydrogenation and Transfer Hydrogenation of N-Heterocycles. Angew. Chem. Int. Ed. 2018, 57, 11262–11266. [Google Scholar]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N–Pd Interaction in Nitrogen-Doped Carbon Nanotube Catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar]
- Xiong, Y.; Sun, W.; Xin, P.; Chen, W.; Zheng, X.; Yan, W.; Zheng, L.; Dong, J.; Zhang, J.; Wang, D.; et al. Gram-Scale Synthesis of High-Loading Single-Atomic-Site Fe Catalysts for Effective Epoxidation of Styrene. Adv. Mater. 2020, 32, e2000896. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar]
- Dong, C.; Li, Y.; Cheng, D.; Zhang, M.; Liu, J.; Wang, Y.-G.; Xiao, D.; Ma, D. Supported Metal Clusters: Fabrication and Application in Heterogeneous Catalysis. ACS Catal. 2020, 10, 11011–11045. [Google Scholar]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar]
- Dilpazir, S.; He, H.; Li, Z.; Wang, M.; Lu, P.; Liu, R.; Xie, Z.; Gao, D.; Zhang, G. Cobalt Single Atoms Immobilized N-Doped Carbon Nanotubes for Enhanced Bifunctional Catalysis toward Oxygen Reduction and Oxygen Evolution Reactions. ACS Appl. Energy Mater. 2018, 1, 3283–3291. [Google Scholar] [CrossRef]
- Alarawi, A.; Ramalingam, V.; He, J.-H. Recent advances in emerging single atom confined two-dimensional materials for water splitting applications. Mater. Today Energy 2019, 11, 1–23. [Google Scholar]
- Li, M.; Chen, S.; Jiang, Q.; Chen, Q.; Wang, X.; Yan, Y.; Liu, J.; Lv, C.; Ding, W.; Guo, X. Origin of the Activity of Co–N–C Catalysts for Chemoselective Hydrogenation of Nitroarenes. ACS Catal. 2021, 11, 3026–3039. [Google Scholar]
- Zhang, Q.; Li, T.; Kameyama, H.; Wu, Q.; Ma, X.; Wu, Y. Pt structured catalysts prepared using a novel competitive impregnation method for the catalytic combustion of propionic acid. Catal. Commun. 2014, 56, 27–31. [Google Scholar]
- Mao, F.; Qi, Z.; Fan, H.; Sui, D.; Chen, R.; Huang, J. Heterogeneous cobalt catalysts for selective oxygenation of alcohols to aldehydes, esters and nitriles. RSC Adv. 2017, 7, 1498–1503. [Google Scholar]
- Chan-Thaw, C.E.; Campisi, S.; Wang, D.; Prati, L.; Villa, A. Selective Oxidation of Raw Glycerol Using Supported AuPd Nanoparticles. Catalysts 2015, 5, 131–144. [Google Scholar]
- Chen, P.; Chew, L.M.; Xia, W. The influence of the residual growth catalyst in functionalized carbon nanotubes on supported Pt nanoparticles applied in selective olefin hydrogenation. J. Catal. 2013, 307, 84–93. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar]
- Sun, W.; Guo, K.; Fan, J.; Min, Y.; Xu, Q. Confined Selenium in N-Doped Mesoporous Carbon Nanospheres for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 16558–16566. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Li, Z. Fe-Based Metal–Organic Frameworks for Highly Selective Photocatalytic Benzene Hydroxylation to Phenol. ACS Catal. 2015, 5, 6852–6857. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Ghorbel, M.C.B.; Praetz, S.; Meiling, D.; Schlesiger, C.; Schomäcker, R.; Thomas, A. Confinement of Cobalt Species in Mesoporous N-Doped Carbons and the Impact on Nitroarene Hydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 11171–11182. [Google Scholar]
- Li, B.; Zhao, H.; Fang, J.; Li, J.; Gao, W.; Ma, K.; Liu, C.; Yang, H.; Ren, X.; Dong, Z. Ru nanoparticles anchored on porous N-doped carbon nanospheres for efficient catalytic hydrogenation of Levulinic acid to gamma-valerolactone under solvent-free conditions. J. Colloid. Interface Sci. 2022, 623, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, R.; Li, J.; Zhao, H.; Ma, J.; Dong, Z. Atomically dispersed Co-N4 sites anchored on N-doped carbon for aqueous phase transfer hydrogenation between nitroarenes and saturated N-heterocycles. Appl. Catal. B Envrion. 2021, 299, 120681. [Google Scholar]
- Cui, X.; Li, W.; Junge, K.; Fei, Z.; Beller, M.; Dyson, P.J. Selective Acceptorless Dehydrogenation of Primary Amines to Imines by Core–Shell Cobalt Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 7501–7507. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Han, J.-T.; Feng, W.-J.; Yu, Q.-Y.; Li, X.-H.; Antonietti, M.; Chen, J.-S. Tuning the Adsorption Energy of Methanol Molecules Along Ni-N-Doped Carbon Phase Boundaries by the Mott–Schottky Effect for Gas-Phase Methanol Dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 2697–2701. [Google Scholar]
- Li, B.; Fang, J.; Xu, D.; Zhao, H.; Zhu, H.; Zhang, F.; Dong, Z. Atomically Dispersed Co Clusters Anchored on N-doped Carbon Nanotubes for Efficient Dehydrogenation of Alcohols and Subsequent Conversion to Carboxylic Acids. Chem. Sus. Chem. 2021, 14, 4536–4545. [Google Scholar]
- Yan, Y.; Tong, X.; Wang, K.; Bai, X. Highly efficient and selective aerobic oxidation of alcohols in aqueous media by TEMPO-containing catalytic systems. Catal. Commun. 2014, 43, 112–115. [Google Scholar] [CrossRef]
- Yu, J.; Luan, Y.; Qi, Y.; Hou, J.; Dong, W.; Yang, M.; Wang, G. Hierarchical PS/PANI nanostructure supported Cu(ii) complexes: Facile synthesis and study of catalytic applications in aerobic oxidation. RSC Adv. 2014, 4, 55028–55035. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Guo, Y.; Liu, X.; Zhang, Y.; Wang, Y. N-doped carbon supported Pt catalyst for base-free oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Appl. Catal. A Gen. 2016, 526, 1–8. [Google Scholar]
- Sun, K.; Shan, H.; Neumann, H.; Lu, G.P.; Beller, M. Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles. Nat. Commun. 2022, 13, 1848. [Google Scholar]
- Wei, Q.; Wang, J.; Shen, W. Atomically dispersed Feδ+ anchored on nitrogen-rich carbon for enhancing benzyl alcohol oxidation through Mott-Schottky effect. Appl. Catal. B Envrion. 2021, 292, 120195. [Google Scholar]
- Escobar-Bedia, F.J.; Lopez-Haro, M.; Calvino, J.J.; Martin-Diaconescu, V.; Simonelli, L.; Perez-Dieste, V.; Sabater, M.J.; Concepción, P.; Corma, A. Active and Regioselective Ru Single-Site Heterogeneous Catalysts for Alpha-Olefin Hydroformylation. ACS Catal. 2022, 12, 4182–4193. [Google Scholar]
- Chandrasekaran, S.; Zhang, C.; Shu, Y.; Wang, H.; Chen, S.; Edison, T.N.J.I.; Liu, Y.; Karthik, N.; Misra, R.D.K.; Deng, L.; et al. Advanced opportunities and insights on the influence of nitrogen incorporation on the physico-/electro-chemical properties of robust electrocatalysts for electrocatalytic energy conversion. Coord. Chem. Rev. 2021, 449, 214209. [Google Scholar]
- Cheng, X.; Shen, Z.; Jiao, L.; Yang, L.; Wang, X.; Wu, Q.; Hu, Z. Tuning metal catalysts via nitrogen-doped nanocarbons for energy chemistry: From metal nanoparticles to single metal sites. Energy Chem. 2021, 3, 100066. [Google Scholar]
- Li, Z.; Leng, L.; Ji, S.; Zhang, M.; Liu, H.; Gao, J.; Zhang, J.; Horton, J.H.; Xu, Q.; Zhu, J. Engineering the morphology and electronic structure of atomic cobalt-nitrogen-carbon catalyst with highly accessible active sites for enhanced oxygen reduction. J. Energy Chem. 2022, 73, 469–477. [Google Scholar]
- Wang, D.; Yang, P.; Liu, L.; Wang, W.; Chen, Z. Atomically dispersed metal-nitrogen-carbon electrocatalysts for oxygen reduction reaction: From synthesis strategies to activity engineering. Mater. Today Energy 2022, 26, 101017. [Google Scholar]
- Chen, Y.-N.; Zhang, X.; Zhou, Z. Carbon-Based Substrates for Highly Dispersed Nanoparticle and Even Single-Atom Electrocatalysts. Small Methods 2019, 3, 1900050. [Google Scholar]
- He, D.; Jiang, Y.; Lv, H.; Pan, M.; Mu, S. Nitrogen-doped reduced graphene oxide supports for noble metal catalysts with greatly enhanced activity and stability. Appl. Catal. B Envrion. 2013, 132, 379–388. [Google Scholar]
- Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-Nitrogen-Doped Carbon Materials as Highly Efficient Catalysts: Progress and Rational Design. Adv. Sci. 2020, 7, 2001069. [Google Scholar]
- Ge, R.; Li, W.; Huo, J.; Liao, T.; Cheng, N.; Du, Y.; Zhu, M.; Li, Y.; Zhang, J. Metal-ion bridged high conductive RGO-M-MoS2 (M = Fe3+, Co2+, Ni2+, Cu2+ and Zn2+) composite electrocatalysts for photo-assisted hydrogen evolution. Appl. Catal. B Envrion. 2019, 246, 129–139. [Google Scholar]
- Zhang, Z.; Chen, Y.; Zhou, L.; Chen, C.; Han, Z.; Zhang, B.; Wu, Q.; Yang, L.; Du, L.; Bu, Y.; et al. The simplest construction of single-site catalysts by the synergism of micropore trapping and nitrogen anchoring. Nat. Commun. 2019, 10, 1657. [Google Scholar]
- Liu, X.; Deng, Y.; Zheng, L.; Kesama, M.R.; Tang, C.; Zhu, Y. Engineering Low-Coordination Single-Atom Cobalt on Graphitic Carbon Nitride Catalyst for Hydrogen Evolution. ACS Catal. 2022, 12, 5517–5526. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv. Mater. 2017, 29, 1604942. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.-J.; Du, P.; Hu, K.; Gao, J.; Li, H.; Liu, P.; Ina, T.; Ohara, K.; Ito, Y.; Chen, M. Metal and Nonmetal Codoped 3D Nanoporous Graphene for Efficient Bifunctional Electrocatalysis and Rechargeable Zn–Air Batteries. Adv. Mater. 2019, 31, 1900843. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H.; et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar]


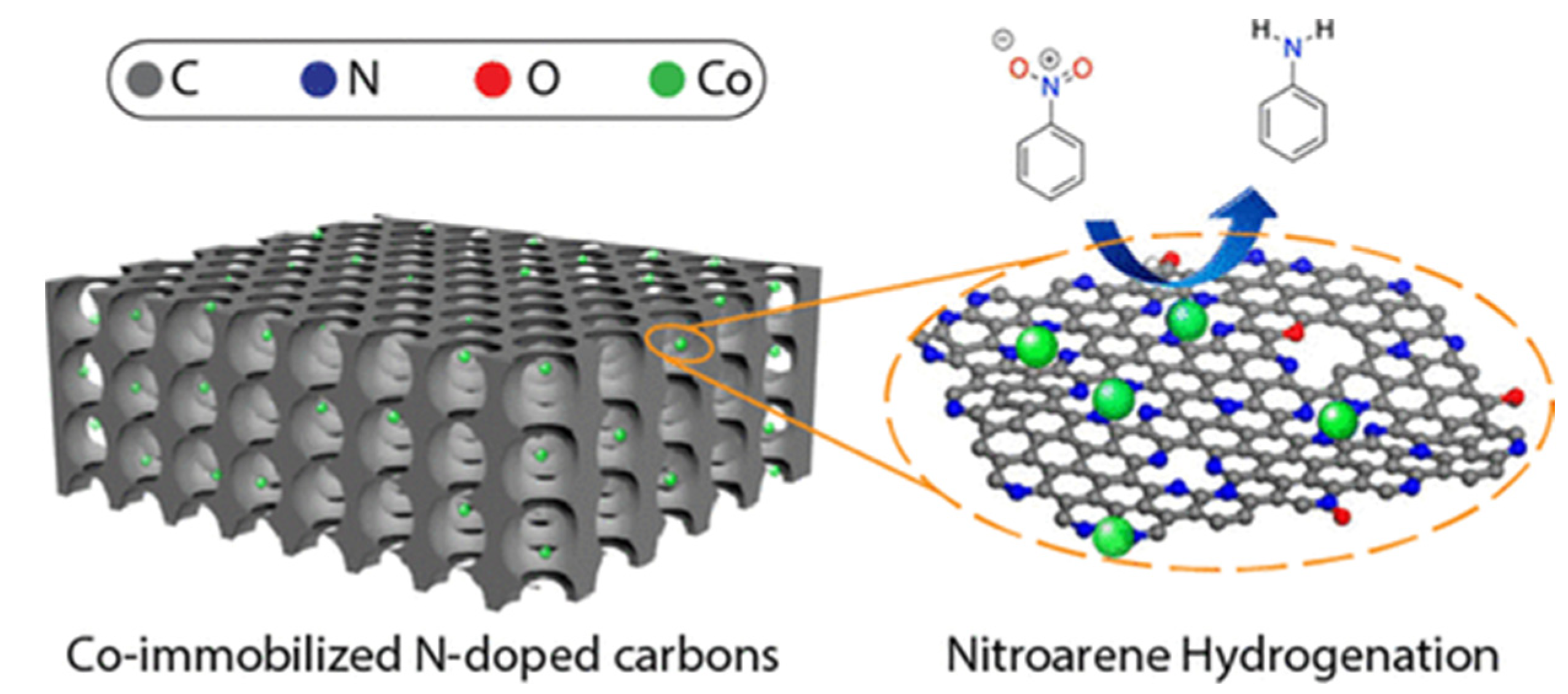
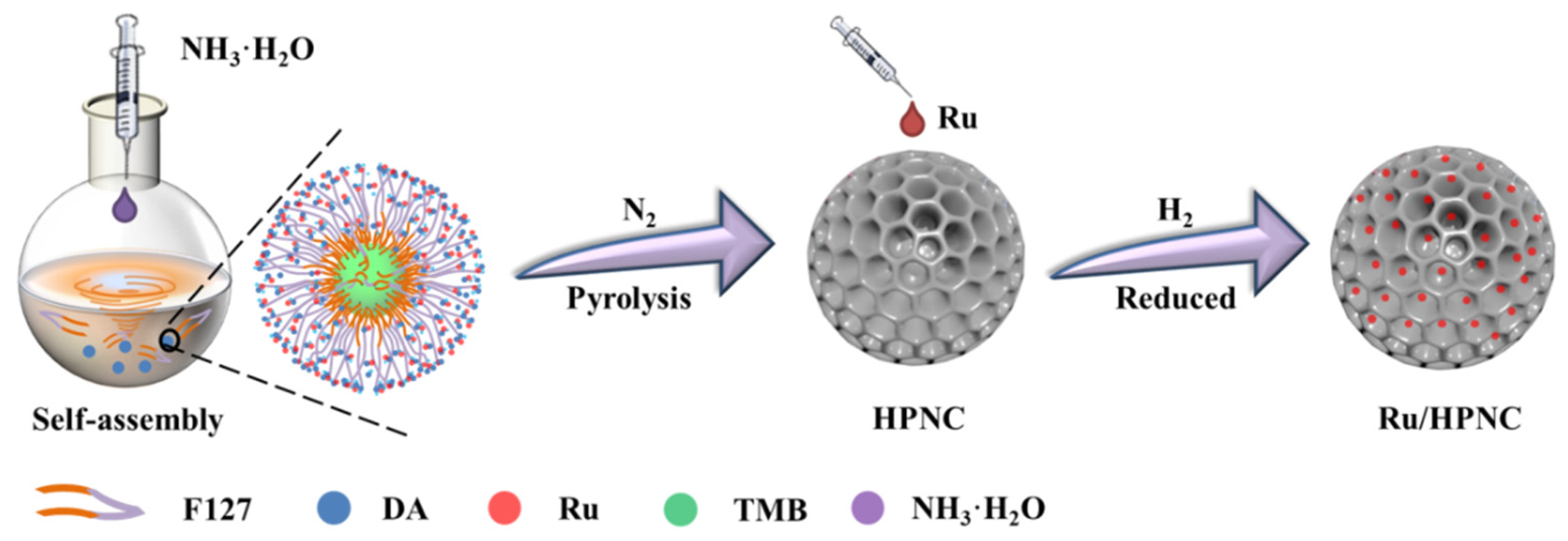

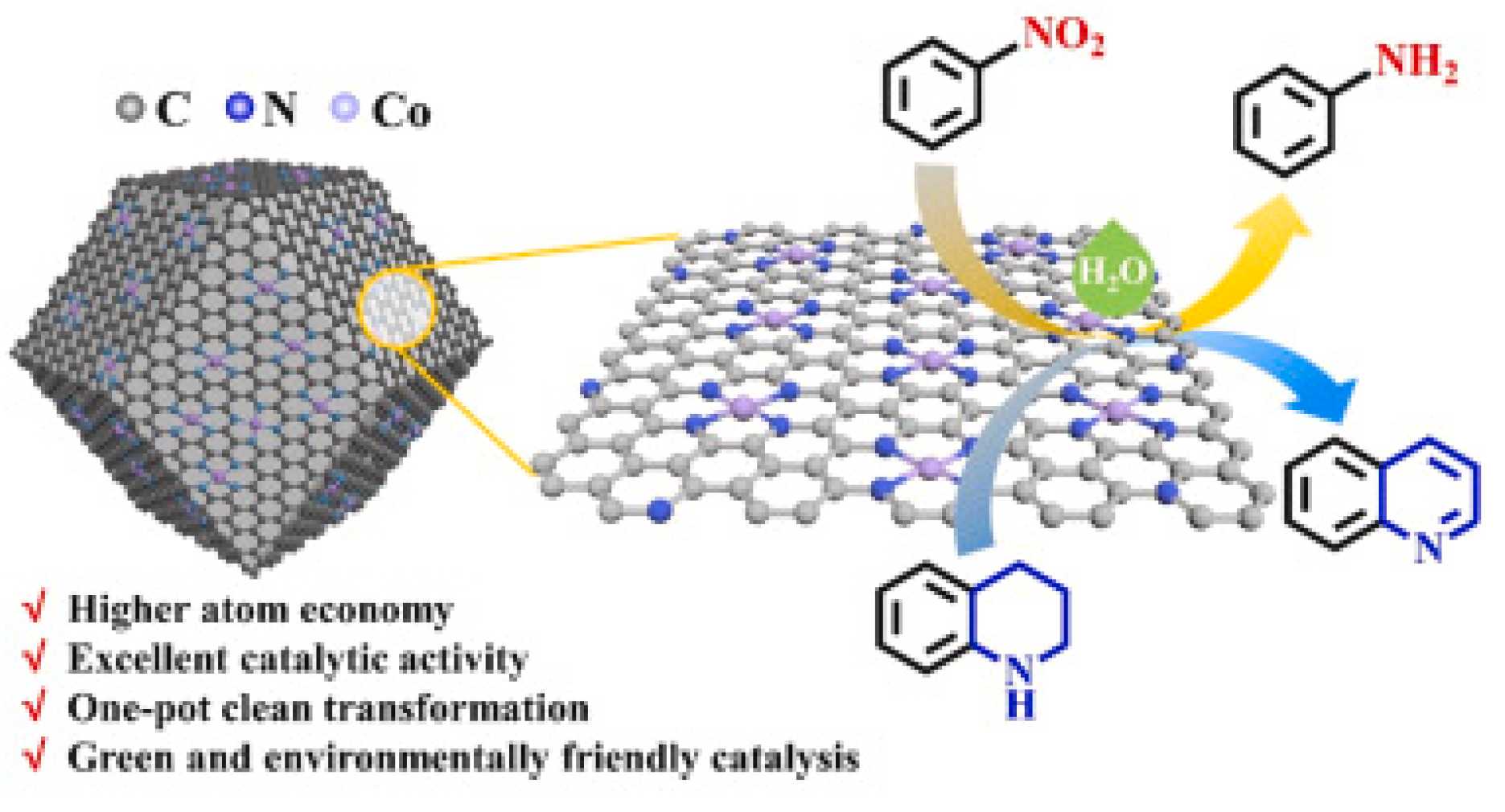



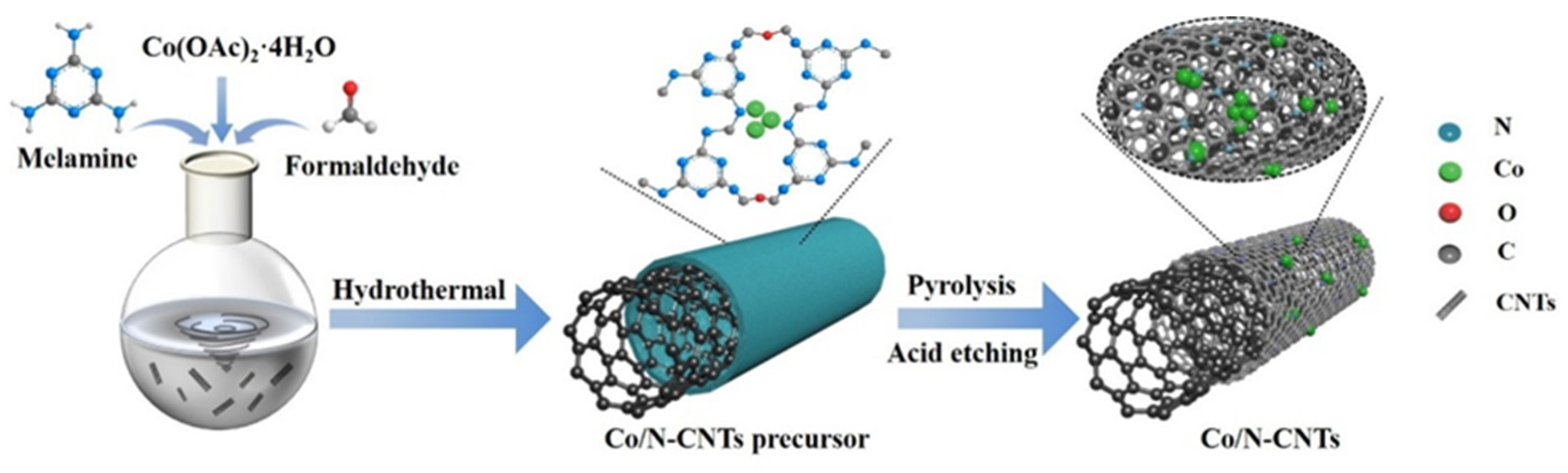



| Entry | Preparation Methods | Advantage | Disadvantage |
|---|---|---|---|
| 1 | Post-loading | Controllable structure and morphology Uniform particle size High utilization of active sites | Complex synthesis processes Lower metal loading rate |
| 2 | Simultaneous introduction of metals and nitrogen into pre-synthesized carbon support | Adjustable nitrogen content High-dispersed metal sites | Uncontrollable active size |
| 3 | In-situ pyrolysis | Adjustable nitrogen content High-dispersed metal sites Simplest synthesis steps and time-saving operation | Uncontrollable active size Active sites are easily confined by N-doped carbon framework Precious metals easily aggregate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, L.; Zhang, J.; Su, Y. Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application. Electrochem 2022, 3, 520-537. https://doi.org/10.3390/electrochem3030036
Li B, Zhang L, Zhang J, Su Y. Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application. Electrochem. 2022; 3(3):520-537. https://doi.org/10.3390/electrochem3030036
Chicago/Turabian StyleLi, Boyang, Lihua Zhang, Jianrui Zhang, and Yaqiong Su. 2022. "Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application" Electrochem 3, no. 3: 520-537. https://doi.org/10.3390/electrochem3030036
APA StyleLi, B., Zhang, L., Zhang, J., & Su, Y. (2022). Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application. Electrochem, 3(3), 520-537. https://doi.org/10.3390/electrochem3030036







