A Novel Label-Free Electrochemical Immunosensor Based on a Self-Assembled Monolayer-Modified Electrode for Polychlorinated Biphenyl (PCB) in Environmental Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Equipment and Instruments
2.3. Cyclic Voltammetry for Pretreatment of Electrodes
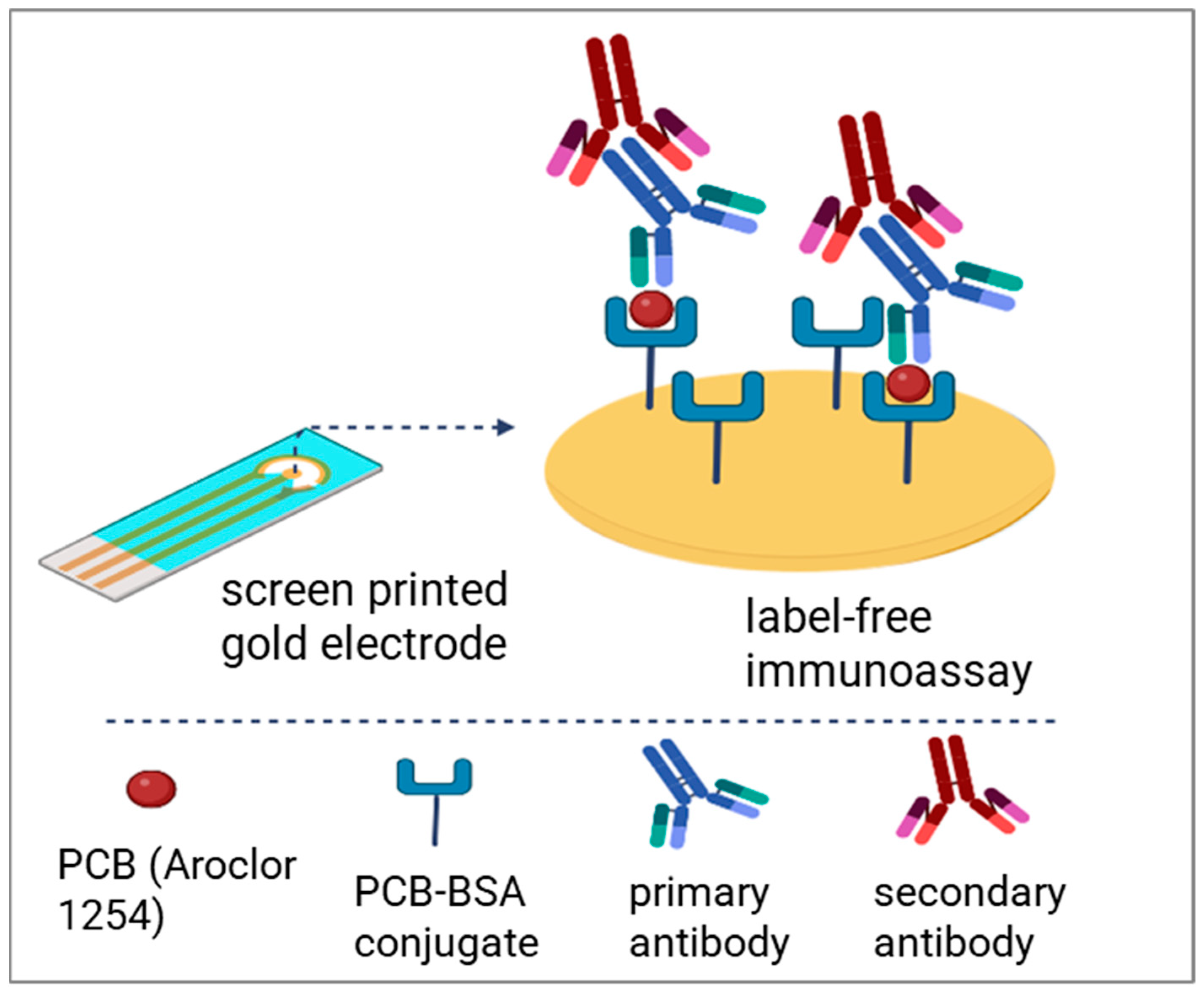
2.4. Modification of SPG Electrodes
2.5. Optimization of Label-Free Immunoassay
2.6. Real Sample Analysis
3. Results and Discussion
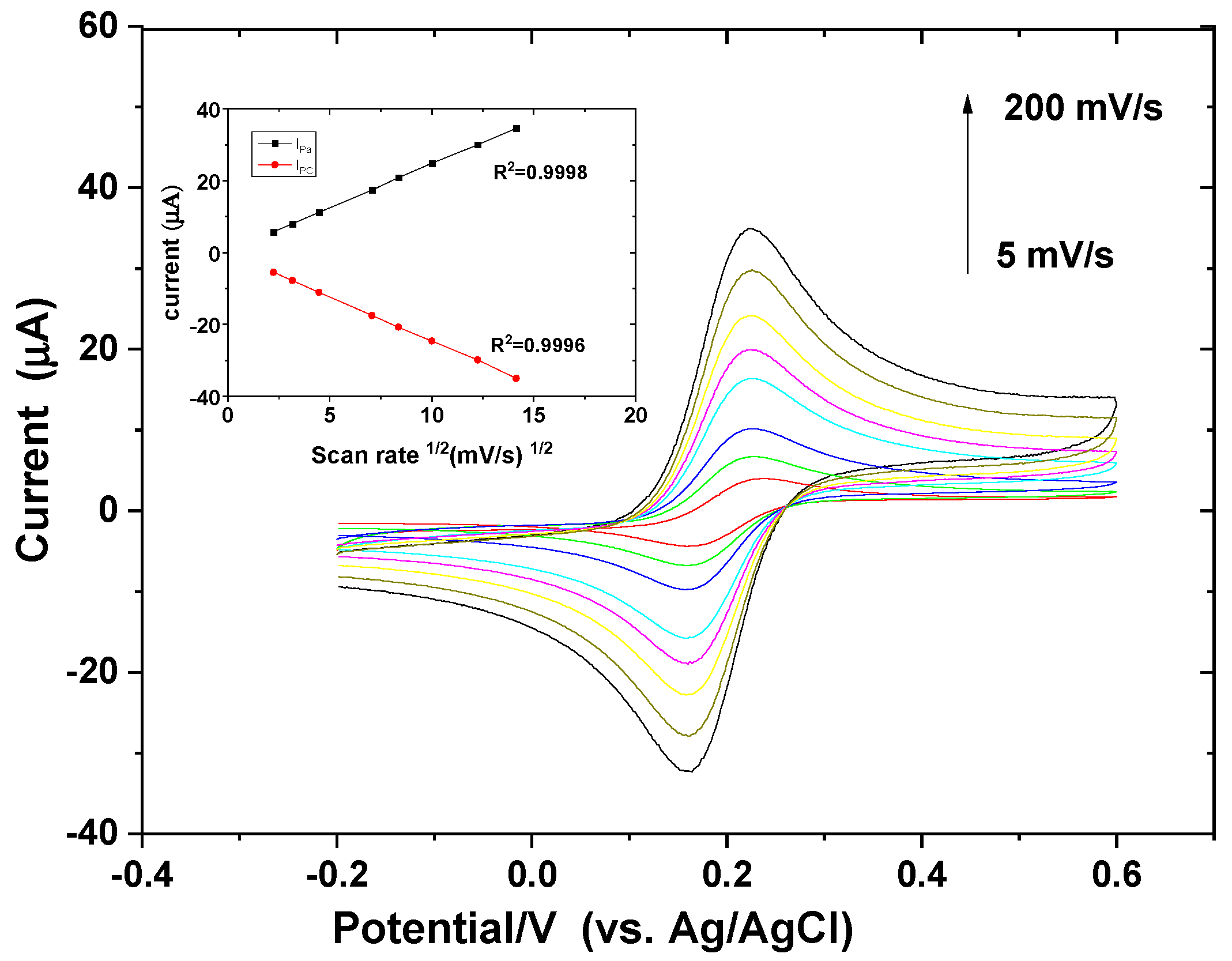
3.1. Electrochemical Characterization of the Electrode
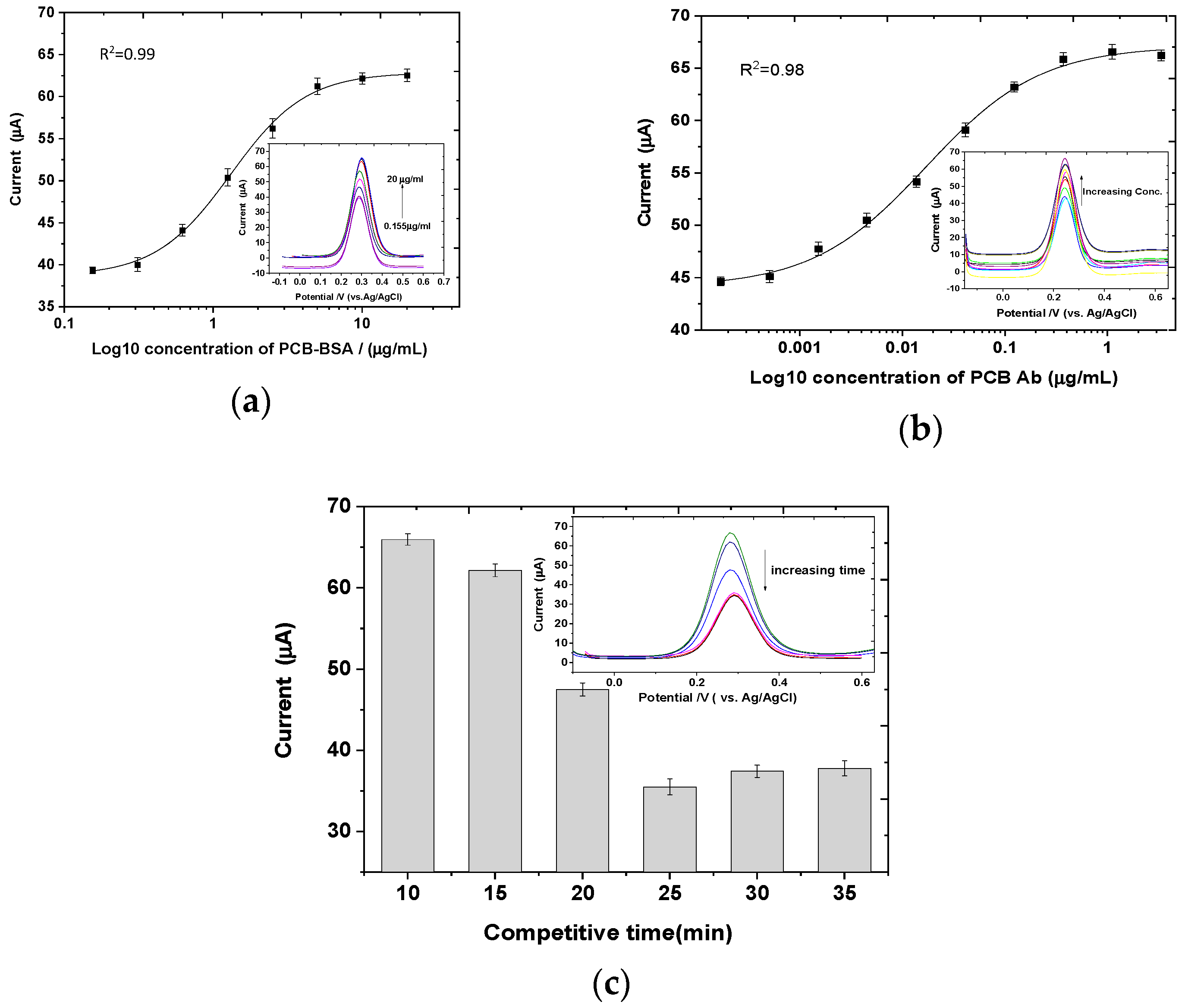
3.2. Optimization of the Assay Conditions
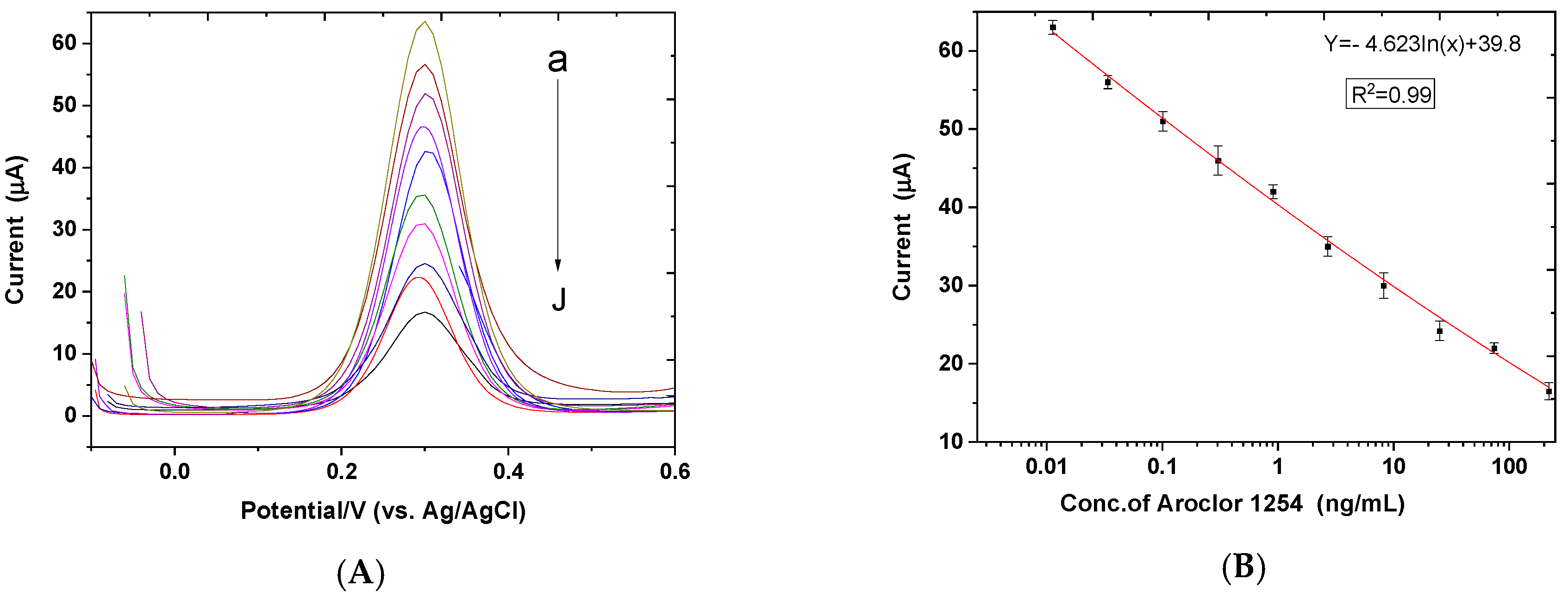
3.3. Sensitivity of the Immunosensor for PCB Aroclor 1254 Determination
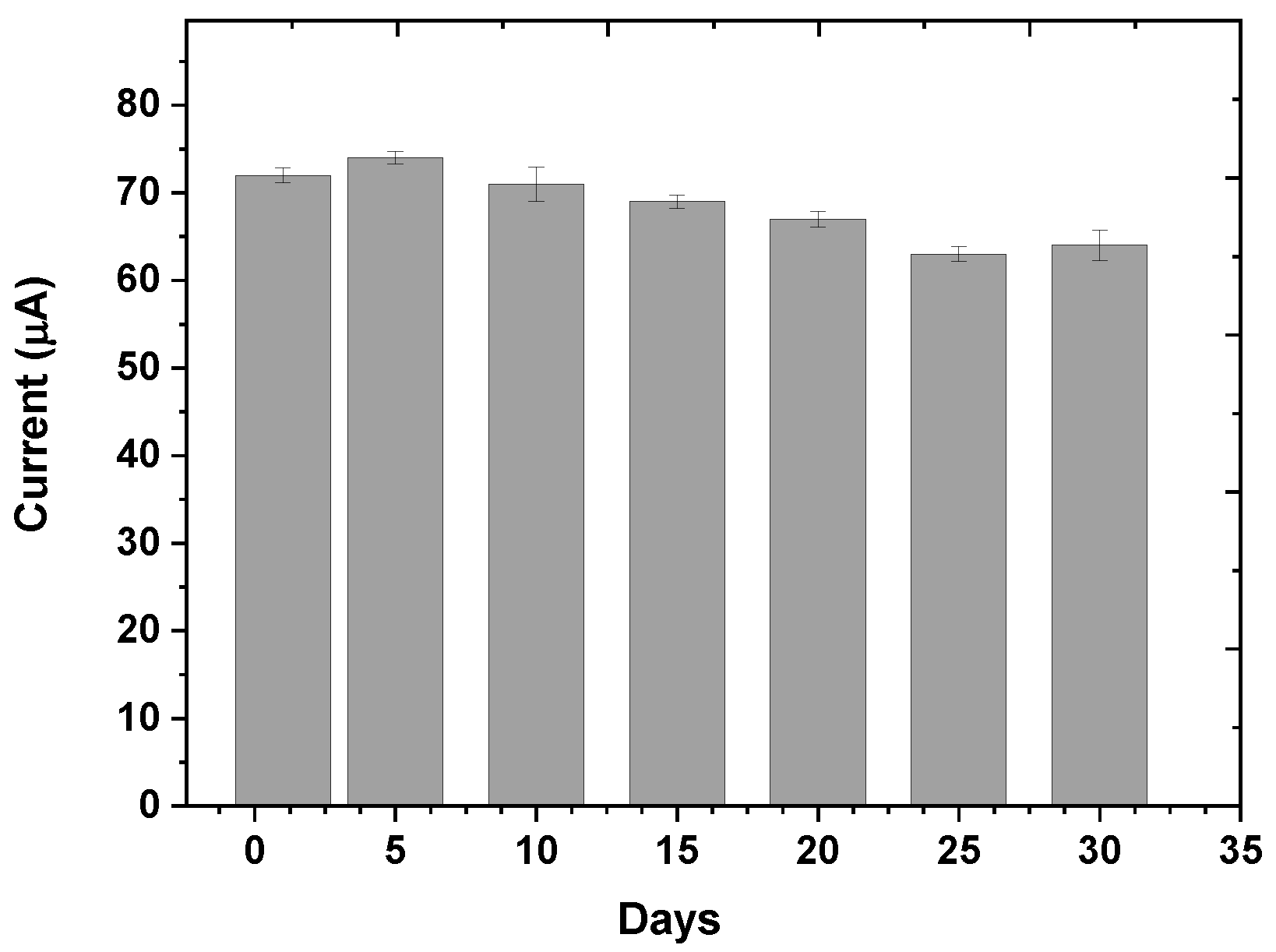
3.4. Reproducibility and Stability of the Designed Immunosensor
3.5. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ∆Ep | peak-to-peak separation |
| 11-MUA | 11-mercaptoundecanoic acid |
| Ag/AgCl | silver/silver chloride |
| Au | gold |
| BSA | bovine serum albumin |
| CV | cyclic voltammetry |
| EDC | 1-Ethyl-3-(3-(dimethylamino)-propyl) carbodiimide |
| ELISA | enzyme-linked immunosorbent assay |
| HPLC | high performance liquid chromatography |
| K3Fe(CN)6 | potassium ferricyanide |
| K4Fe(CN)6 | potassium ferrocyanide |
| KCI | potassium chloride |
| LOD | limit of detection |
| MeOH | methanol |
| NaCl | sodium chloride |
| NaCNBH4 | sodium cyanoborohydride |
| NaOH | sodium hydroxide |
| NHS | N-hydroxy succinimide |
| PAb | polyclonal antibodies |
| PBS | phosphate-buffered saline |
| PCB | polychlorinated biphenyls |
| Rct | charge transfer resistance |
| SAM | self-assembled monolayer |
| SPGE | screen-printed gold electrodes |
| TRIS | tris(hydroxylmethyl)aminomethane |
References
- Biziuk, M. Environmental fate and global distribution of polychlorinated biphenyls Related papers Environmental Fate and Global Distribution of Polychlorinated Biphenyls. Artic. Rev. Environ. Contam. Toxicol. 2009, 201, 137–158. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef] [Green Version]
- Alsefri, S.; Balbaied, T.; Moore, E. Electrochemical Development of an Immunosensor for Detection Polychlorinated biphenyls (PCBs) for Environmental Analysis. Chemosensors 2021, 9, 307. [Google Scholar] [CrossRef]
- Han, D.; Currell, M.J. Persistent organic pollutants in China’s surface water systems. Sci. Total Environ. 2017, 580, 602–625. [Google Scholar] [CrossRef]
- Fu, J.; Mai, B.; Sheng, G.; Zhang, G.; Wang, X.; Peng, P.; Xiao, X.; Ran, R.; Cheng, F.; Peng, X.; et al. Persistent organic pollutants in environment of the Pearl River Delta, China: An overview. Chemosphere 2003, 52, 1411–1422. [Google Scholar] [CrossRef]
- Laschi, S.; Fránek, M.; Mascini, M. Screen-Printed Electrochemical Immunosensors for PCB Detection. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2000, 12, 1293–1298. [Google Scholar] [CrossRef]
- Hong, S.; Kang, T.; Oh, S.; Moon, J.; Choi, I.; Choi, K.; Yi, J. Label-free sensitive optical detection of polychlorinated biphenyl (PCB) in an aqueous solution based on surface plasmon resonance measurements. Sens. Actuators B Chem. 2008, 134, 300–306. [Google Scholar] [CrossRef]
- Bender, S.; Sadik, O.A. Direct Electrochemical Immunosensor for Polychlorinated Biphenyls. Environ. Sci. Technol. 1998, 32, 788–797. [Google Scholar] [CrossRef]
- Helen, C.; Lemasle, M.; Laplanche, A.; Genin, E. Determination of polychlorodibenzo-p-dioxins and polychlorodibenzofurans by gas chromatography/tandem mass spectrometry and gas chromatography/triple mass spectrometry in a quadrupole ion trap. J. Mass Spectrom. 2001, 36, 546–554. [Google Scholar] [CrossRef]
- Ottonello, G.; Ferrari, A.; Magi, E. Determination of polychlorinated biphenyls in fish: Optimisation and validation of a method based on accelerated solvent extraction and gas chromatography–mass spectrometry. Food Chem. 2014, 142, 327–333. [Google Scholar] [CrossRef]
- Popp, P.; Keil, P.; Möder, M.; Paschke, A.; Thuss, U. Application of accelerated solvent extraction followed by gas chromatography, high-performance liquid chromatography and gas chromatography–mass spectrometry for the determination of polycyclic aromatic hydrocarbons, chlorinated pesticides and polychlorinated dibenzo-p-dioxins and dibenzofurans in solid wastes. J. Chromatogr. A 1997, 774, 203–211. [Google Scholar] [CrossRef]
- Zajicek, J.L.; Tillitt, D.E.; Schwartz, T.R.; Schmitt, C.J.; Harrison, R.O. Comparison of an enzyme-linked immunosorbent assay (ELISA) to gas chromatography (GC)—Measurement of polychlorinated biphenyls (PCBs) in selected US fish extracts. Chemosphere 2000, 40, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Lasrado, J.A.; Santerre, C.R.; Zajicek, J.L.; Stahl, J.R.; Tillitt, D.E.; Deardorff, D. Determination of PCBs in Fish Using Enzyme-linked Immunosorbent Assay (ELISA). J. Food Sci. 2003, 68, 133–136. [Google Scholar] [CrossRef]
- Fillmann, G.; Galloway, T.S.; Sanger, R.C.; Depledge, M.H.; Readman, J.W. Relative performance of immunochemical (enzyme-linked immunosorbent assay) and gas chromatography–electron-capture detection techniques to quantify polychlorinated biphenyls in mussel tissues. Anal. Chim. Acta 2002, 461, 75–84. [Google Scholar] [CrossRef]
- Endo, T.; Okuyama, A.; Matsubara, Y.; Nishi, K.; Kobayashi, M.; Yamamura, S.; Morita, Y.; Takamura, Y.; Mizukami, H.; Tamiya, E. Fluorescence-based assay with enzyme amplification on a micro-flow immunosensor chip for monitoring coplanar polychlorinated biphenyls. Anal. Chim. Acta 2005, 531, 7–13. [Google Scholar] [CrossRef]
- Inui, H.; Takeuchi, T.; Uesugi, A.; Doi, F.; Takai, M.; Nishi, K.; Miyake, S.; Ohkawa, H. Enzyme-Linked Immunosorbent Assay with Monoclonal and Single-Chain Variable Fragment Antibodies Selective to Coplanar Polychlorinated Biphenyls. J. Agric. Food Chem. 2012, 60, 1605–1612. [Google Scholar] [CrossRef]
- Syahir, A.; Usui, K.; Tomizaki, K.; Kajikawa, K.; Mihara, H. Label and Label-Free Detection Techniques for Protein Microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, S.; Wei, X.; Zhang, S.; Liu, J.; Dong, Y.; Florescu, M.; Jaffrezic-Renault, N. An Improved Label-Free Indirect Competitive SPR Immunosensor and Its Comparison with Conventional ELISA for Ractopamine Detection in Swine Urine. Sensors 2017, 17, 604. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef]
- Švorc, Ľ. Determination of Caffeine: A Comprehensive Review on Electrochemical Methods. Int. J. Electrochem. Sci. 2013, 8, 5755–5773. [Google Scholar]
- Zheng, X.; Li, H.; Xia, F.; Tian, D.; Hua, X.; Qiao, X.; Zhou, C. An Electrochemical Sensor for Ultrasensitive Determination the Polychlorinated Biphenyls. Electrochim. Acta 2016, 194, 413–421. [Google Scholar] [CrossRef]
- Date, Y.; Aota, A.; Sasaki, K.; Namiki, Y.; Matsumoto, N.; Watanabe, Y.; Ohmura, N.; Matsue, T. Label-Free Impedimetric Immunoassay for Trace Levels of Polychlorinated Biphenyls in Insulating Oil. Anal. Chem. 2014, 86, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Haji-Hashemi, H.; Norouzi, P.; Safarnejad, M.R.; Ganjali, M.R. Label-free electrochemical immunosensor for direct detection of Citrus tristeza virus using modified gold electrode. Sens. Actuators B Chem. 2017, 244, 211–216. [Google Scholar] [CrossRef]
- Liu, X.; Duckworth, P.A.; Wong, D.K.Y. Square wave voltammetry versus electrochemical impedance spectroscopy as a rapid detection technique at electrochemical immunosensors. Biosens. Bioelectron. 2010, 25, 1467–1473. [Google Scholar] [CrossRef]
- Frederix, F.; Bonroy, K.; Laureyn, W.; Reekmans, G.; Campitelli, A.; Dehaen, W.; Maes, G. Enhanced performance of an affinity biosensor interface based on mixed self-assembled monolayers of thiols on gold. Langmuir 2003, 19, 4351–4357. [Google Scholar] [CrossRef]
- Briand, E.; Salmain, M.; Herry, J.M.; Perrot, H.; Compère, C.; Pradier, C.M. Building of an immunosensor: How can the composition and structure of the thiol attachment layer affect the immunosensor efficiency? Biosens. Bioelectron. 2006, 22, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, J.K.; Tan, Y.H.; Pandey, B.; Fujikawa, K.; Demchenko, A.V.; Stine, K.J. Electrochemical impedance spectroscopy study of Concanavalin A binding to self-assembled monolayers of mannosides on gold wire electrodes. J. Electroanal. Chem. 2016, 780, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Fischer, L.M.; Tenje, M.; Heiskanen, A.R.; Masuda, N.; Castillo, J.; Bentien, A.; Émneus, J.; Jakobsen, M.H.; Boisen, A. Gold cleaning methods for electrochemical detection applications. Microelectron. Eng. 2009, 86, 1282–1285. [Google Scholar] [CrossRef]
- Lu, M.; Li, X.; Yu, B.; Li, H. Electrochemical Behavior of Au Colloidal Electrode through Layer-by-Layer Self-Assembly. J. Colloid Interface Sci. 2002, 248, 376–382. [Google Scholar] [CrossRef]
- Fan, L.; Wang, G.; Liang, W.; Yan, W.; Guo, Y.; Shuang, S.; Dong, C.; Bi, Y. Label-free and highly selective electrochemical aptasensor for detection of PCBs based on nickel hexacyanoferrate nanoparticles/reduced graphene oxides hybrids. Biosens. Bioelectron. 2019, 145, 111728. [Google Scholar] [CrossRef]
- Dutta, G.; Lillehoj, P.B. Wash-free, label-free immunoassay for rapid electrochemical detection of PfHRP2 in whole blood samples. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Mehta, S.; Kumeria, T.; Shiddiky, M.J.A.; Popat, A.; Choudhury, S.; Bose, S.; Nayak, R. Rapid fabrication of homogeneously distributed hyper-branched gold nanostructured electrode based electrochemical immunosensor for detection of protein biomarkers. Sens. Actuators B Chem. 2021, 326, 128803. [Google Scholar] [CrossRef]
- Xiao, X.; Li, H.; Wang, M.; Zhang, K.; Si, P. Examining the effects of self-assembled monolayers on nanoporous gold based amperometric glucose biosensors. Analyst 2014, 139, 488–494. [Google Scholar] [CrossRef]
- Centi, S.; Laschi, S.; Fránek, M.; Mascini, M. A disposable immunomagnetic electrochemical sensor based on functionalised magnetic beads and carbon-based screen-printed electrodes (SPCEs) for the detection of polychlorinated biphenyls (PCBs). Anal. Chim. Acta 2005, 538, 205–212. [Google Scholar] [CrossRef]
- Deng, A.; Franek, M.; Kolar, V. Food and Agricultural Immunology Determination of Atrazine in Soil Samples by ELISA Using Polyclonal and Monoclonal Antibodies. Agric. Immunol. 2010, 11, 135–144. [Google Scholar] [CrossRef]
- Chen, D.; Li, J. Interfacial design and functionization on metal electrodes through self-assembled monolayers. Surf. Sci. Rep. 2006, 61, 445–463. [Google Scholar] [CrossRef]
- Laschi, S.; Mascini, M.; Scortichini, G.; Fra, M.; Nek, Ä.; Mascini, M. Polychlorinated Biphenyls (PCBs) Detection in Food Samples Using an Electrochemical Immunosensor. J. Agric. Food Chem. 2003, 51, 1816–1822. [Google Scholar] [CrossRef]
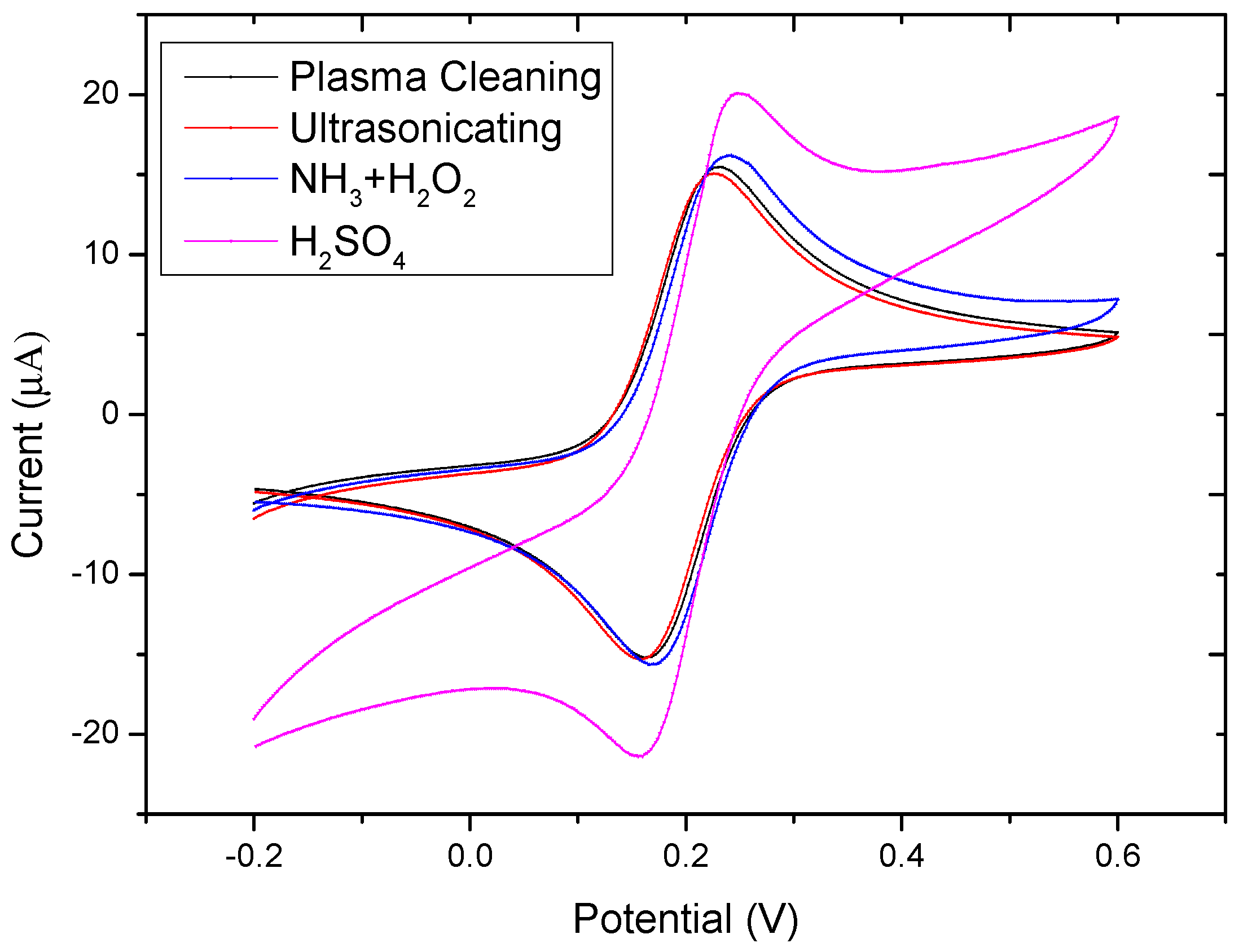
| Cleaning Protocols | Epa (V) | Epc (V) | ΔEP (mV) * |
|---|---|---|---|
| Plasma cleaning | 0.226 | 0.168 | 58 |
| Ultrasonicating | 0.226 | 0.160 | 66 |
| NH3 + H2O2 | 0.242 | 0.172 | 70 |
| H2SO4 | 0.246 | 0.160 | 86 |
| Scan Rate (mV/s) | Ipa (µA) c | ΔEp (mV) b | Emid vs. Ag/Ag CL (Mv) a |
|---|---|---|---|
| 200 | 34.59 | 58 | 193 |
| 150 | 30.01 | 64 | 194 |
| 100 | 24.80 | 58 | 193 |
| 70 | 20.83 | 62 | 191 |
| 50 | 17.38 | 64 | 192 |
| 20 | 11.18 | 68 | 192 |
| 10 | 7.98 | 68 | 194 |
| 5 | 5.71 | 70 | 198 |
| Method | Detection Mode | Linear Range | LOD | Amplification | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| DPV | - | 1–100 | 0.22 ng/L | None | Water | [30] |
| EIS | Noncompetitive | 0.01–10 μg/L | 0.001 μg/L | None | Oil | [22] |
| DPV | Competitive | 0.01–50 ppm | 5.2 ppm | AP (ALP) | Food | [37] |
| LSV | Competitive | 0.1–220 | 0.09 ng/mL−1 | AP (ALP) | Water | [3] |
| DPV | Competitive | 0.011–220 | 0.11 ng/mL−1 | None | Soil | This work |
| Techniques | Sample | Added (ng/mL−1) | Found (ng/mL−1) | Recovery % | RSD % (n = 3). |
|---|---|---|---|---|---|
| ELISA | Soil | 8.2 | 10.54 | 128.53 | 4.4% |
| ELISA | Soil | 25 | 26.41 | 105.64 | 3.6% |
| Electrochemical | Soil | 8.2 | 7.89 | 96.21 | 1.7% |
| Electrochemical | Soil | 25 | 22.74 | 90 | 2.5 % |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsefri, S.; Balbaied, T.; Albalawi, I.; Alatawi, H.; Moore, E. A Novel Label-Free Electrochemical Immunosensor Based on a Self-Assembled Monolayer-Modified Electrode for Polychlorinated Biphenyl (PCB) in Environmental Analysis. Electrochem 2022, 3, 451-462. https://doi.org/10.3390/electrochem3030031
Alsefri S, Balbaied T, Albalawi I, Alatawi H, Moore E. A Novel Label-Free Electrochemical Immunosensor Based on a Self-Assembled Monolayer-Modified Electrode for Polychlorinated Biphenyl (PCB) in Environmental Analysis. Electrochem. 2022; 3(3):451-462. https://doi.org/10.3390/electrochem3030031
Chicago/Turabian StyleAlsefri, Samia, Thanih Balbaied, Ibtihaj Albalawi, Hanan Alatawi, and Eric Moore. 2022. "A Novel Label-Free Electrochemical Immunosensor Based on a Self-Assembled Monolayer-Modified Electrode for Polychlorinated Biphenyl (PCB) in Environmental Analysis" Electrochem 3, no. 3: 451-462. https://doi.org/10.3390/electrochem3030031
APA StyleAlsefri, S., Balbaied, T., Albalawi, I., Alatawi, H., & Moore, E. (2022). A Novel Label-Free Electrochemical Immunosensor Based on a Self-Assembled Monolayer-Modified Electrode for Polychlorinated Biphenyl (PCB) in Environmental Analysis. Electrochem, 3(3), 451-462. https://doi.org/10.3390/electrochem3030031







