Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia vulgaris and Solanum tuberosum in Mild Steel Samples in 1 M Sulphuric Acid
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of Specimens
2.2. Extraction of Alkaloids
2.3. Test for Alkaloids
2.4. Physicochemical Characterization
2.5. Preparation of Media
2.6. Weight Loss Measurement
2.7. Potentiodynamic Polarization
2.8. Surface Morphology Study
3. Results and Discussion
3.1. Test for Alkaloids
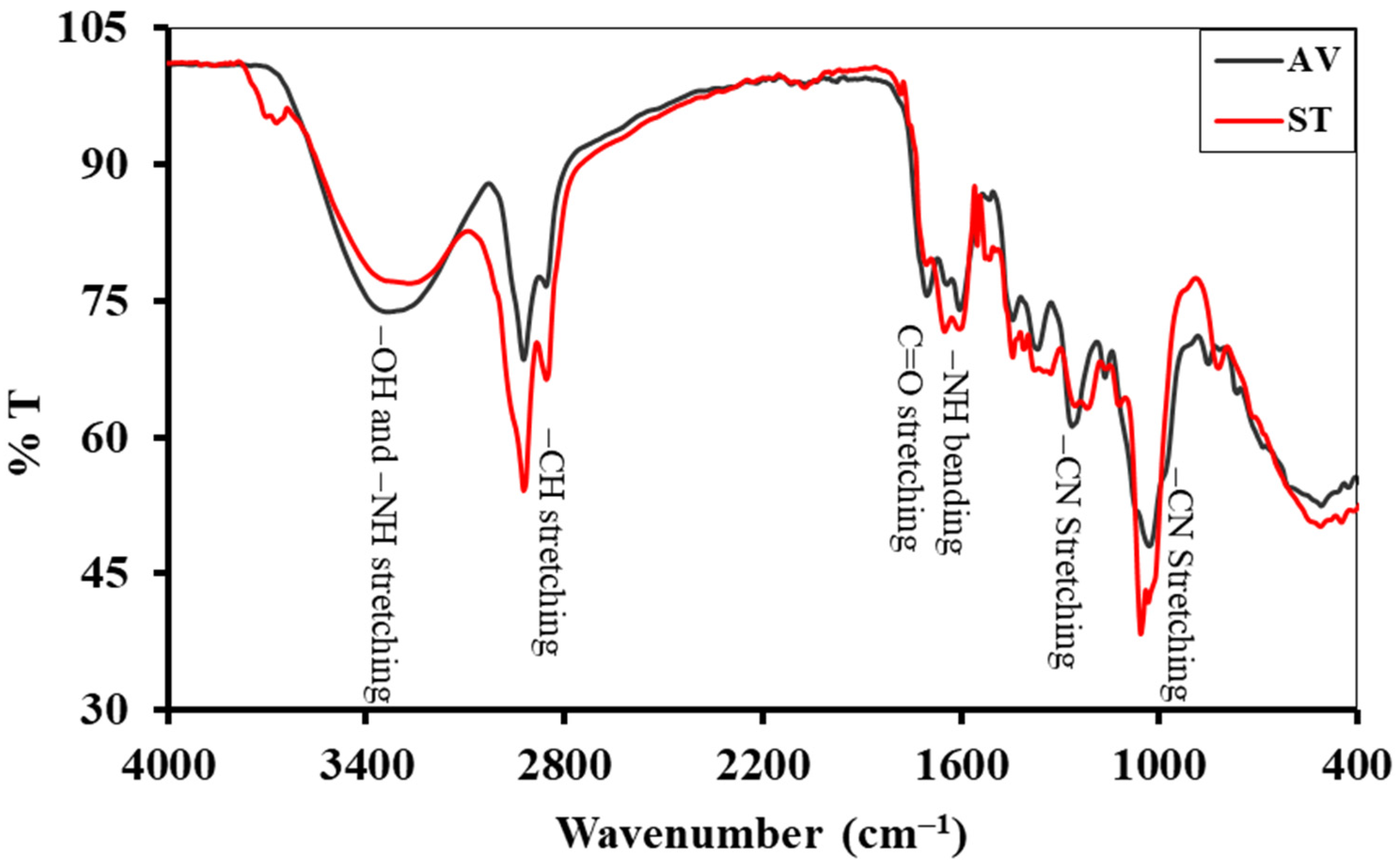
3.2. Fourier Transform Infra-Red (FTIR) Analysis
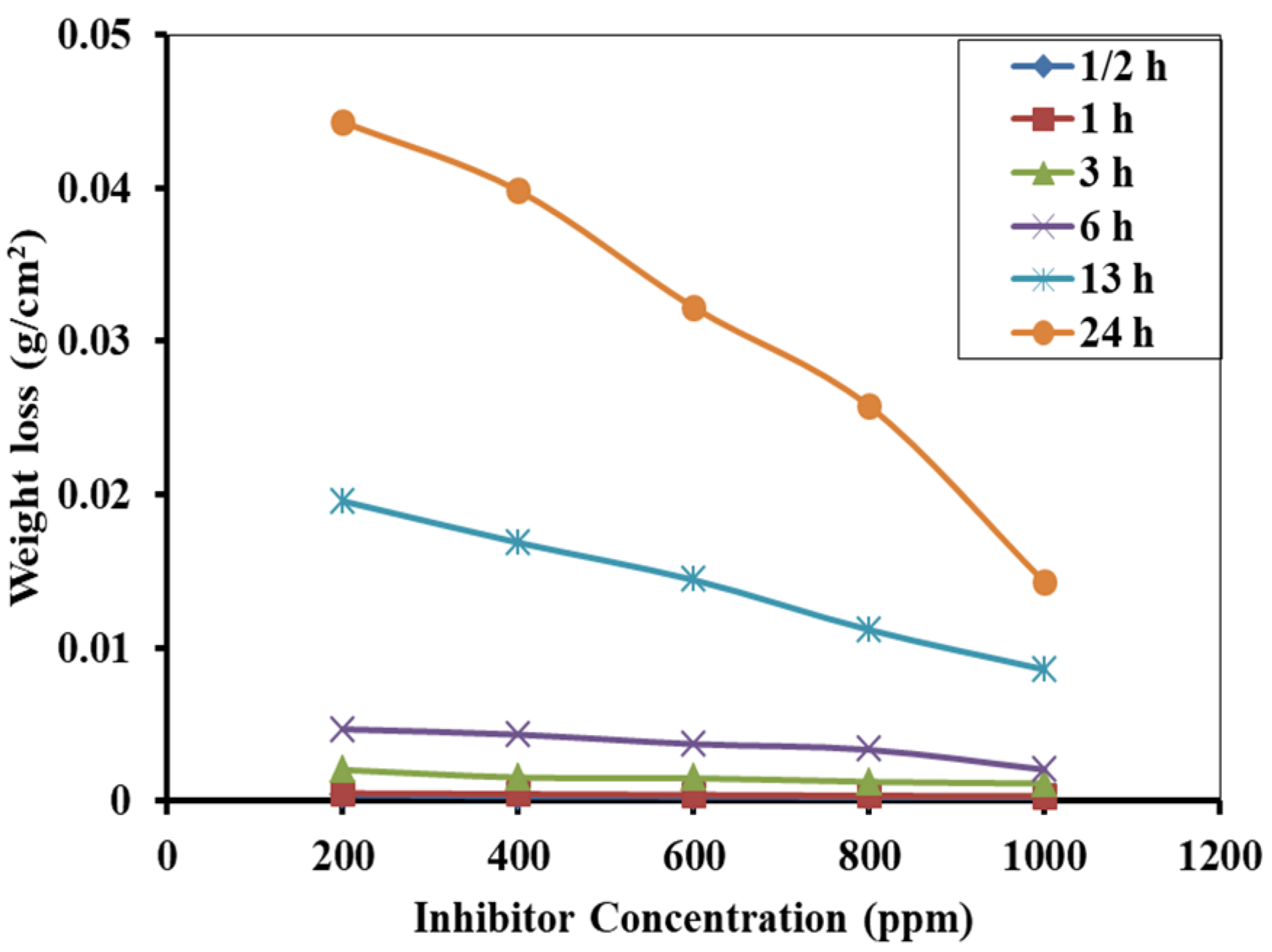
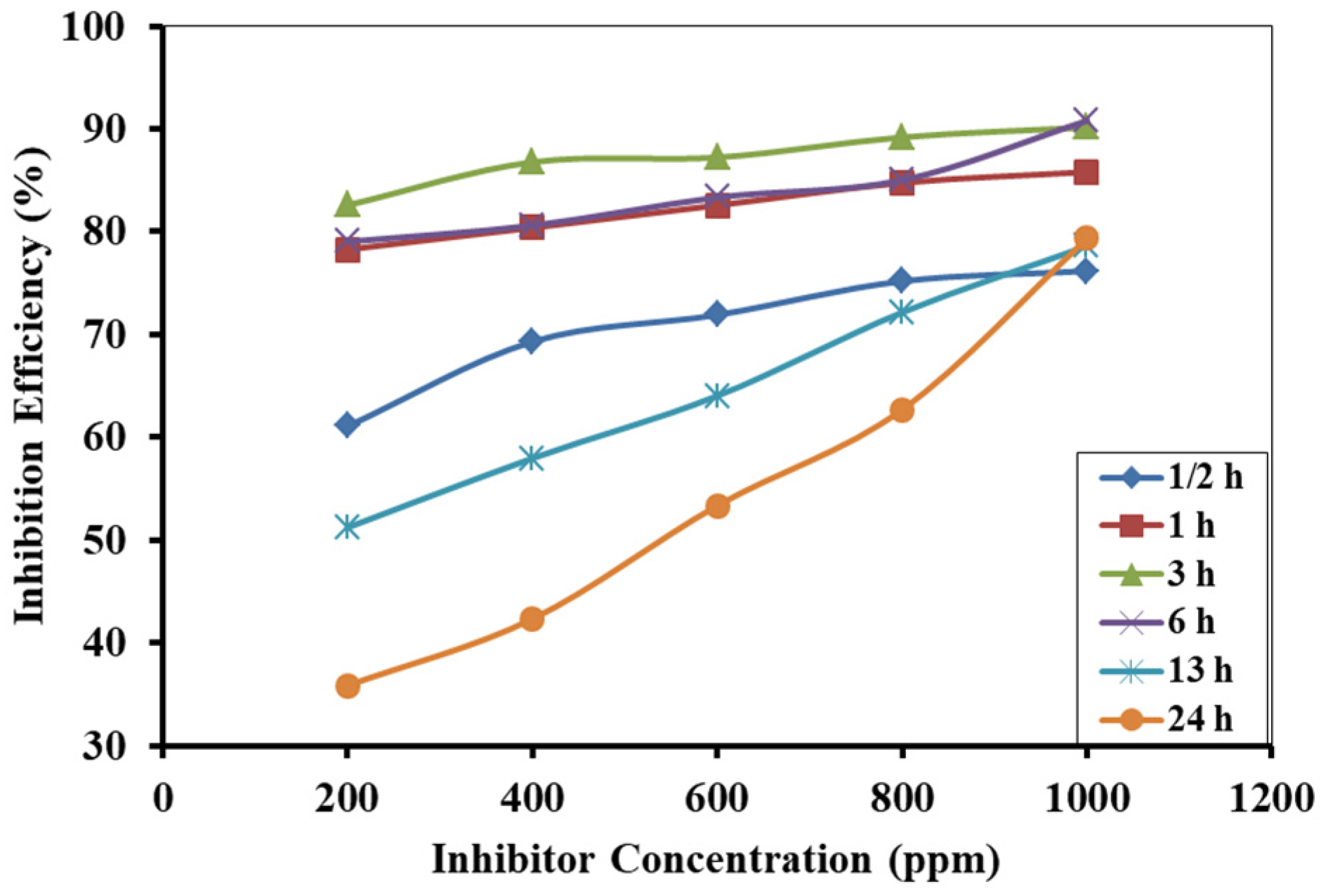
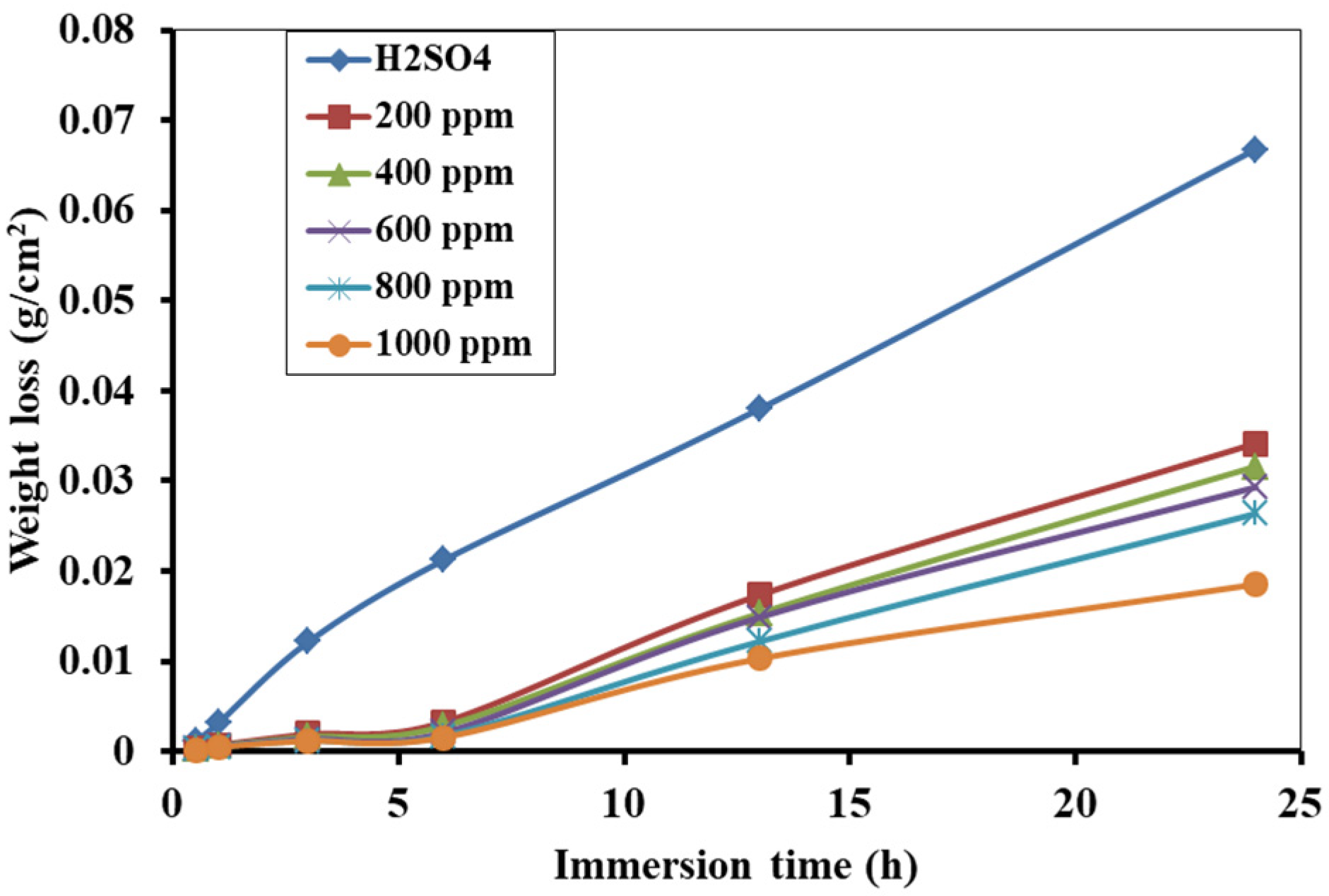
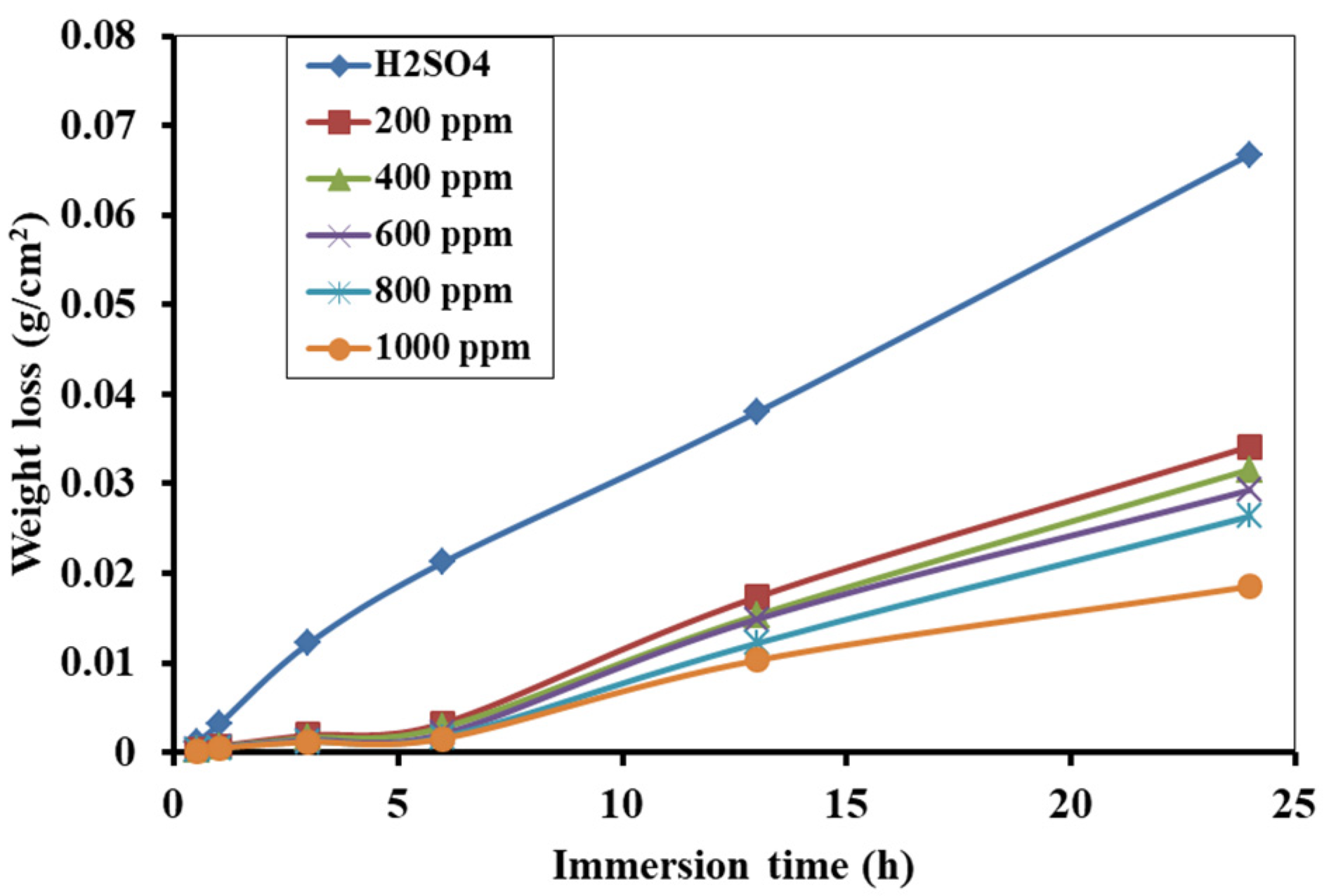
3.3. Weight Loss Measurement and Corrosion Inhibition Efficiency
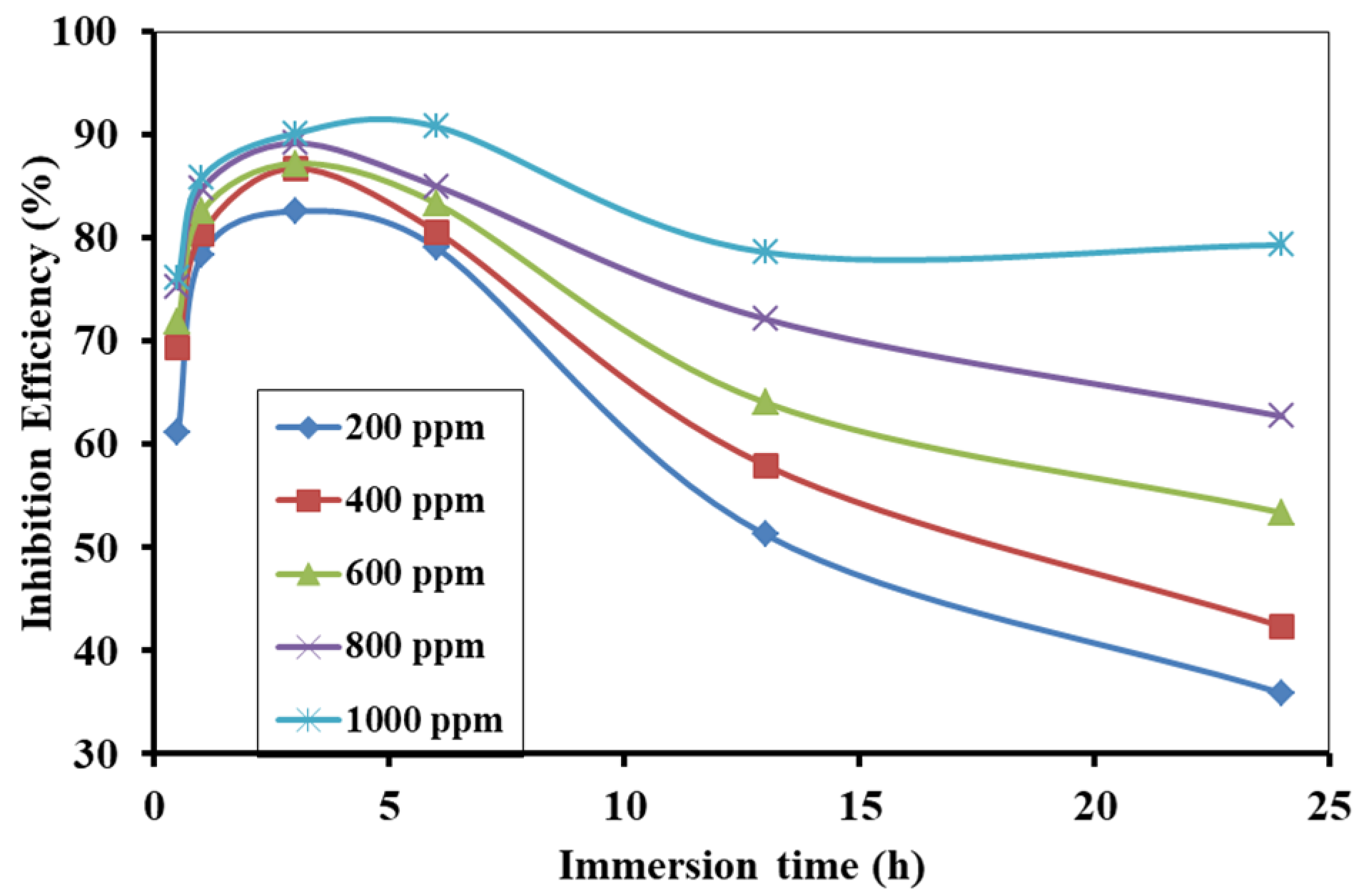
3.4. Effect of Inhibitor Concentration
3.5. Effect of Immersion Time
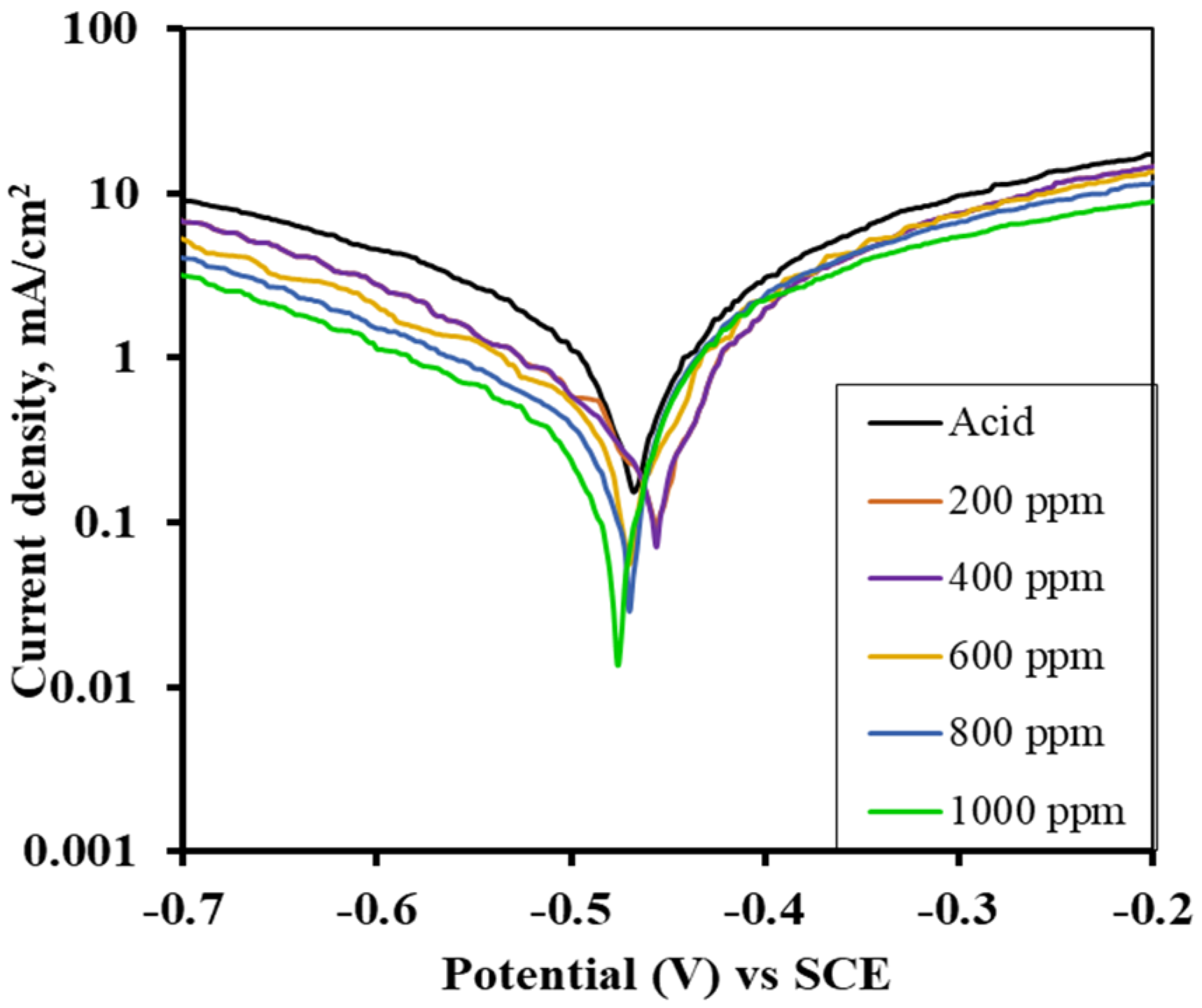
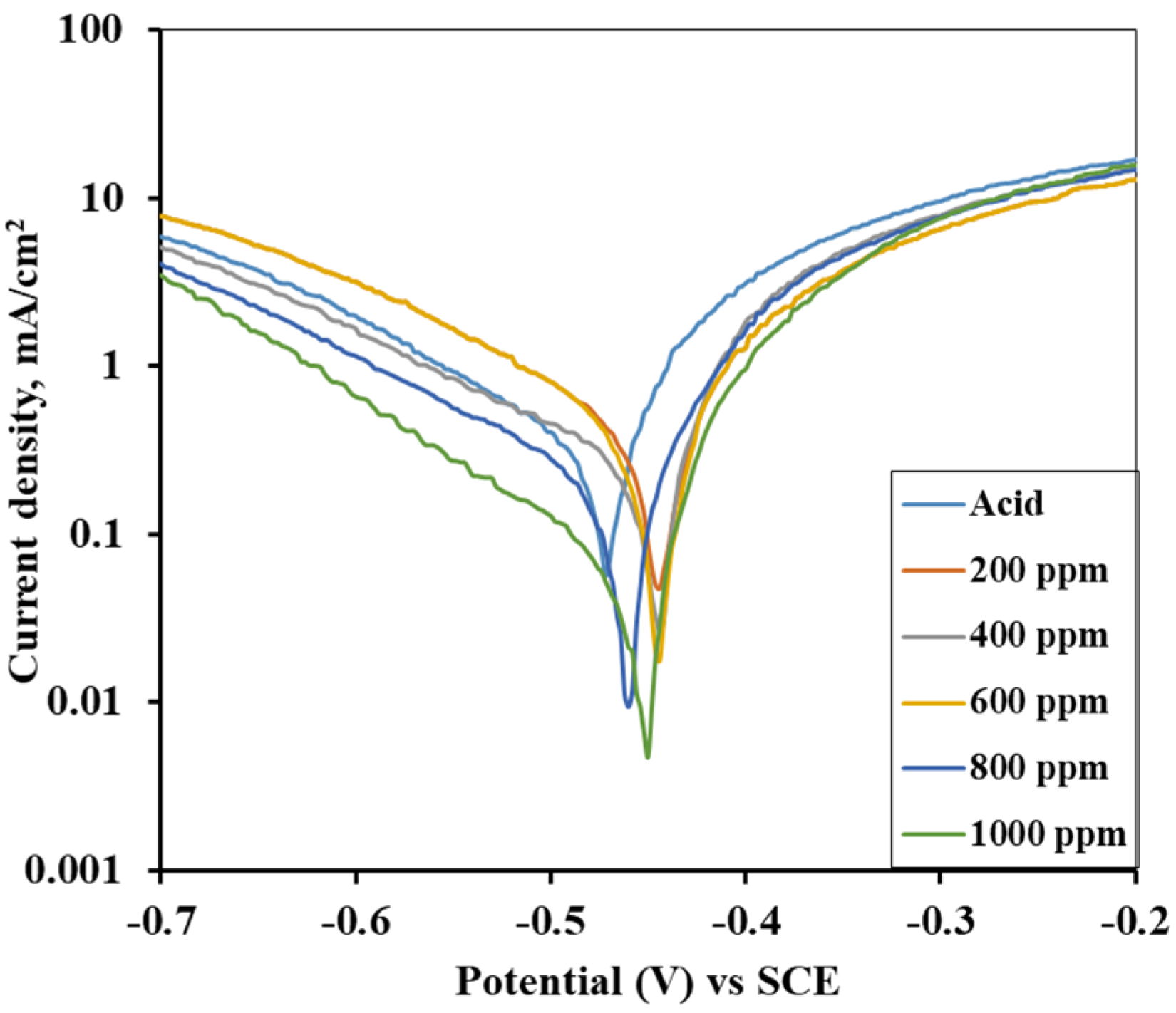
3.6. Potentiodynamic Polarization (as-Immersed and 1 h Immersed MS Sample)
3.7. Inhibition Efficiency of Alkaloid for Immersed and as-Immersed Condition
3.8. Comparative Study of Corrosion Inhibition of AV and ST
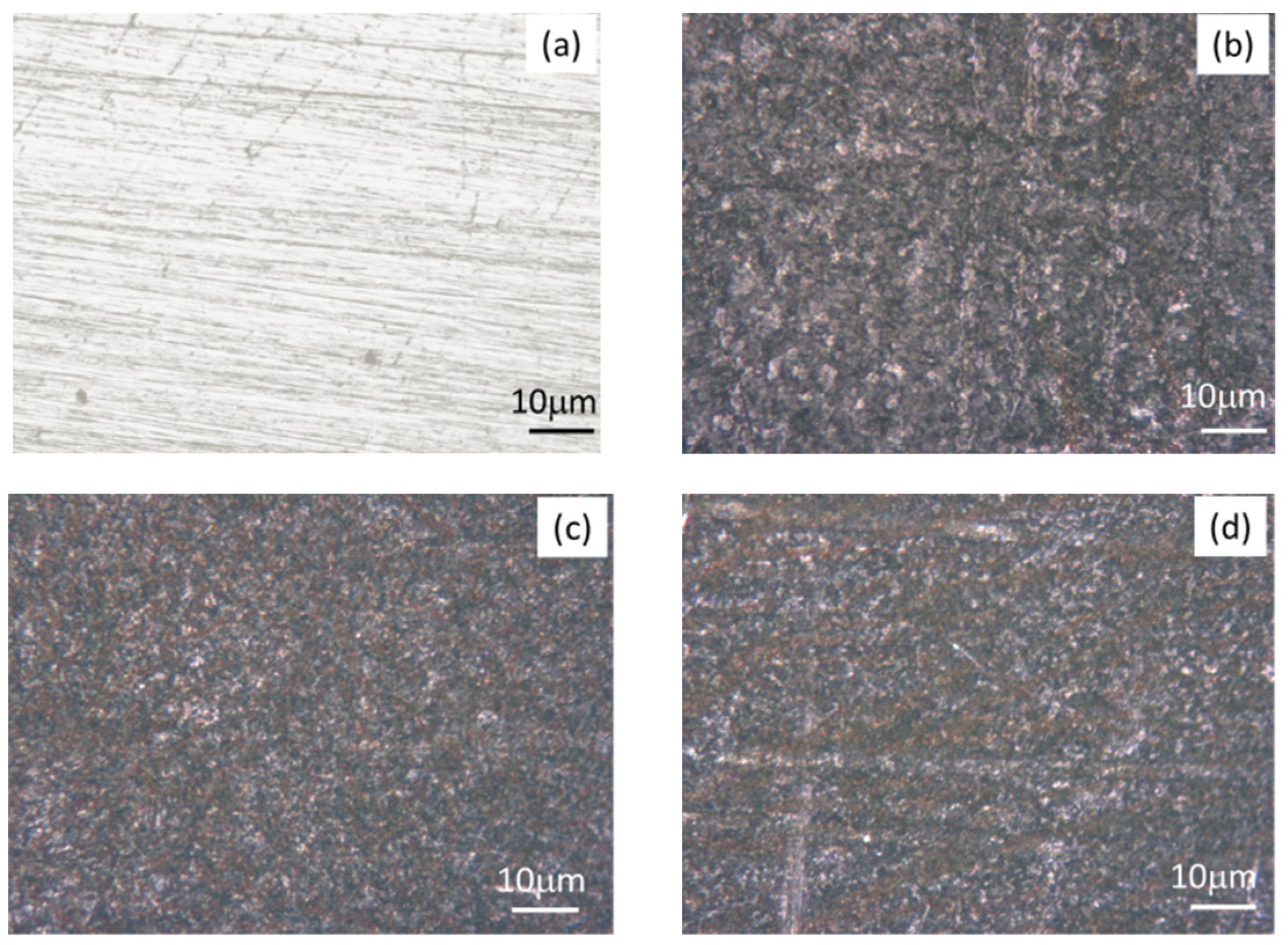
3.9. Surface Morphological Study
3.10. Mechanism of Green Corrosion Inhibitor
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarras, P.; Stenger-Smith, J. Corrosion processes and strategies for prevention: An introduction. In Handbook of Smart Coatings for Materials Protection; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–28. [Google Scholar]
- Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bhattarai, J. Frontiers of Corrosion Science, 1st ed.; Kshitiz Publication: Kathmandu, Nepal, 2010. [Google Scholar]
- Heusler, K.; Landolt, D.; Trasatti, S. Electrochemical corrosion nomenclature (Recommendations 1988). Pure Appl. Chem. 1989, 61, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Sastri, V.S. Challenges in Corrosion: Costs, Causes, Consequences, and Control; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Thapa, B.; Gupta, D.K.; Yadav, A.P. Corrosion inhibition of bark extract of Euphorbia royleana on mild steel in 1M HCl. J. Nepal Chem. Soc. 2019, 40, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International measures of prevention, application, and economics of corrosion technologies study. NACE Int. 2016, 216, 2–3. [Google Scholar]
- Faraj, L.; Khan, G.M. Application of Natural Product Extracts as Green Corrosion Inhibitors for Metals and Alloys in Acid Pickling Processes-A. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar]
- Rani, B.; Basu, B.B.J. Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012, 2012, 380217. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef] [Green Version]
- Karki, N.; Neupane, S.; Chaudhary, Y.; Gupta, D.K.; Yadav, A.P. Berberis aristata: A highly efficient and thermally stable green corrosion inhibitor for mild steel in acidic medium. Anal. Bioanal. Electrochem. 2020, 12, 970–988. [Google Scholar]
- Ahmed, M.H.O.; Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B.; Gaaz, T.S. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 8, 728–733. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 380217. [Google Scholar]
- Yahaya, L.; Aroyeun, S.; Ogunwolu, S.; Jayeola, C.; Igbinadolor, R. Green and Black Tea (Camellia sinensis) Extracts as Corrosion Inhibitor for Mild Steel in Acid Medium. World Appl. Sci. J. 2017, 35, 985–992. [Google Scholar]
- Bhardwaj, N.; Prasad, D.; Haldhar, R. Study of the Aegle marmelos as a green corrosion inhibitor for mild steel in acidic medium: Experimental and theoretical approach. J. Bio-Tribo-Corros. 2018, 4, 61. [Google Scholar] [CrossRef]
- Al-Mhyawi, S.R. Inhibition of mild steel corrosion using Juniperus plants as green inhibitior. Afr. J. Pure Appl. Chem. 2014, 8, 9–22. [Google Scholar]
- Fadare, O.; Okoronkwo, A.; Olasehinde, E. Assessment of anti-corrosion potentials of extract of Ficus asperifolia-Miq (Moraceae) on mild steel in acidic medium. Afr. J. Pure Appl. Chem. 2016, 10, 8–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Jiang, H.; Zhang, D.-x.; Hou, L.; Zhou, W.-j. Solanum lasiocarpum L. extract as green corrosion inhibitor for A3 steel in 1 M HCl solution. Int. J. Electrochem. Sci. 2019, 14, 1178–1196. [Google Scholar] [CrossRef]
- Shrestha, P.R.; Oli, H.B.; Thapa, B.; Chaudhary, Y.; Gupta, D.K.; Das, A.K.; Nakarmi, K.B.; Singh, S.; Karki, N.; Yadav, A.P. Bark extract of Lantana camara in 1M HCl as green corrosion inhibitor for mild steel. Eng. J. 2019, 23, 205–211. [Google Scholar] [CrossRef]
- Abeng, F.; Idim, V.; Nna, P. Kinetics and thermodynamic studies of corrosion inhibition of mild steel using Methanolic extract of erigeron floribundus (Kunth) in 2 M HCl solution. World News Nat. Sci. 2017, 10, 26–38. [Google Scholar]
- Nnanna, L.A.; Uchendu, K.O.; Nwosu, F.O.; Ihekoronye, U.; Eti, P. Gmelina Arborea Bark Extracts as a Corrosion Inhibitor for mild steel in an acidic environment. Int. J. Mater. Chem. 2014, 4, 34–39. [Google Scholar]
- Muthukrishnan, P.; Jeyaprabha, B.; Prakash, P. Mild steel corrosion inhibition by aqueous extract of Hyptis Suaveolens leaves. Int. J. Ind. Chem. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mathur, N.; Chhipa, R. Study of Corrosion Inhibitors (Pennisetum glaucum extracts) on Mild Steel used in Building Construction. Inter. J. Eng. Sci. Res. Technol. 2014, 3, 845. [Google Scholar]
- Okafor, P.C.; Ebenso, E.E.; Ekpe, U.J. Azadirachta indica extracts as corrosion inhibitor for mild steel in acid medium. Int. J. Electrochem. Sci. 2010, 5, 978–993. [Google Scholar]
- Rocha, J.C.d.; Gomes, J.A.d.C.P.; D’Elia, E. Aqueous extracts of mango and orange peel as green inhibitors for carbon steel in hydrochloric acid solution. Mater. Res. 2014, 17, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Njoku, V.; Oguzie, E.; Obi, C.; Ayuk, A. Baphia nitida leaves extract as a green corrosion inhibitor for the corrosion of mild steel in acidic media. Spectroscopy 2014, 24, 26–28. [Google Scholar] [CrossRef] [Green Version]
- Pandian, B.R.; Sethuraman, M.G. Solanum tuberosum as an inhibitor of mild steel corrosion in acid media. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2009, 28, 77–84. [Google Scholar]
- Fidrusli, A.; Mahmood, M. Ginger extract as green corrosion inhibitor of mild steel in hydrochloric acid solution. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Philadelphia, PA, USA, 2018; p. 012087. [Google Scholar]
- Okewale, A.; Olaitan, A. The use of rubber leaf extract as a corrosion inhibitor for mild steel in acidic solution. Int. J. Mater. Chem. 2017, 7, 5–13. [Google Scholar]
- Idouhli, R.; Koumya, Y.; Khadiri, M.; Aityoub, A.; Abouelfida, A.; Benyaich, A. Inhibitory effect of Senecio anteuphorbium as green corrosion inhibitor for S300 steel. Int. J. Ind. Chem. 2019, 10, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Ogunleye, O.; Arinkoola, A.; Eletta, O.; Agbede, O.; Osho, Y.; Morakinyo, A.; Hamed, J. Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef] [Green Version]
- Karki, N.; Neupane, S.; Gupta, D.K.; Das, A.K.; Singh, S.; Koju, G.M.; Chaudhary, Y.; Yadav, A.P. Berberine isolated from Mahonia nepalensis as an eco-friendly and thermally stable corrosion inhibitor for mild steel in acid medium. Arab. J. Chem. 2021, 14, 103423. [Google Scholar] [CrossRef]
- Lebrini, M.; Robert, F.; Lecante, A.; Roos, C. Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros. Sci. 2011, 53, 687–695. [Google Scholar] [CrossRef]
- Raja, P.B.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Awang, K. Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1 M HCl media. Corros. Sci. 2013, 69, 292–301. [Google Scholar] [CrossRef]
- Raja, P.B.; Qureshi, A.K.; Rahim, A.A.; Awang, K.; Mukhtar, M.R.; Osman, H. Indole Alkaloids of Alstonia angustifolia var. latifolia as Green Inhibitor for Mild Steel Corrosion in 1M HCl Media. J. Mater. Eng. Perform. 2013, 22, 1072–1078. [Google Scholar] [CrossRef]
- Kamal, C.; Sethuraman, M.G. Caulerpin—A bis-Indole Alkaloid As a Green Inhibitor for the Corrosion of Mild Steel in 1 M HCl Solution from the Marine Alga Caulerpa racemosa. Ind. Eng. Chem. Res. 2012, 51, 10399–10407. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Judzentiene, A. Chemical composition of essential oils of Artemisia vulgaris L. (mugwort) from plants grown in North Lithuania. Chemija 2006, 17, 12–15. [Google Scholar]
- Ashok, P.; Kumud, U. Preliminary phytochemical screening and physico-chemical parameters of aerial parts of Artemisia vulgaris. Int. J. Res. Ayurveda Pharm. 2010, 1, 206–211. [Google Scholar]
- Pandey, B.P.; Thapa, R.; Upreti, A. Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgaris and Gaultheria fragrantissima collected from Nepal. Asian Pac. J. Trop. Med. 2017, 10, 952–959. [Google Scholar] [CrossRef]
- Rashid, M.U.; Alamzeb, M.; Ali, S.; Ullah, Z.; Shah, Z.A.; Naz, I.; Khan, M.R. The chemistry and pharmacology of alkaloids and allied nitrogen compounds from Artemisia species: A review. Phytother. Res. 2019, 33, 2661–2684. [Google Scholar] [CrossRef]
- Bajracharya, M.; Sapkota, M. Profitability and productivity of potato (Solanum tuberosum) in Baglung district, Nepal. Agric. Food Secur. 2017, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Gnanasekaran, C.G.; Basalingappa, K.M. Solanum tuberosum L.: Botanical, Phytochemical, pharmacological and Nutritional significance. Int. J. Phytomed. 2018, 10, 115–124. [Google Scholar]
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J. Chromatogr. A 2004, 1054, 143–155. [Google Scholar] [CrossRef]
- Ikeuba, A.; Okafor, P.; Ekpe, U.; Ebenso, E.E. Alkaloid and non-alkaloid ethanolic extracts from seeds of Garcinia kola as green corrosion inhibitors of mild steel in H2SO4 solution. Int. J. Electrochem. Sci. 2013, 8, 7455–7467. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Carmona-Téllez, S.; Alarcon-Flores, G.; Zaleta-Alejandre, E.; Rivera-Alvarez, Z.; Meza-Rocha, A.; Martínez-Martínez, R.; Murrieta, H.; Aguilar-Frutis, M.; Falcony, C. Luminescent polystyrene films, a novel way to reduce styrofoam residues. Rev. Mex. Física 2015, 61, 323–329. [Google Scholar]
- Karki, N.; Choudhary, Y.; Yadav, A.P. Thermodynamic, Adsorption and Corrosion Inhibition Studies of Mild Steel by Artemisia vulgaris Extract from Methanol as Green Corrosion Inhibitor in Acid Medium. J. Nepal Chem. Soc. 2018, 39, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Palou, R.M.; Olivares-Xomelt, O.; Likhanova, N.V. Environmentally friendly corrosion inhibitors. Dev. Corros. Prot. 2014, 19, 431–432. [Google Scholar]
- Oli, H.B.; Parajuli, D.L.; Sharma, S.; Chapagain, A.; Yadav, A.P. Adsorption Isotherm and Activation Energy of Inhibition of Alkaloids on Mild Steel Surface in Acidic Medium. Amrit Res. J. 2021, 2, 59–67. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–principles, mechanisms and applications. Dev. Corros. Prot. 2014, 16, 365–378. [Google Scholar]




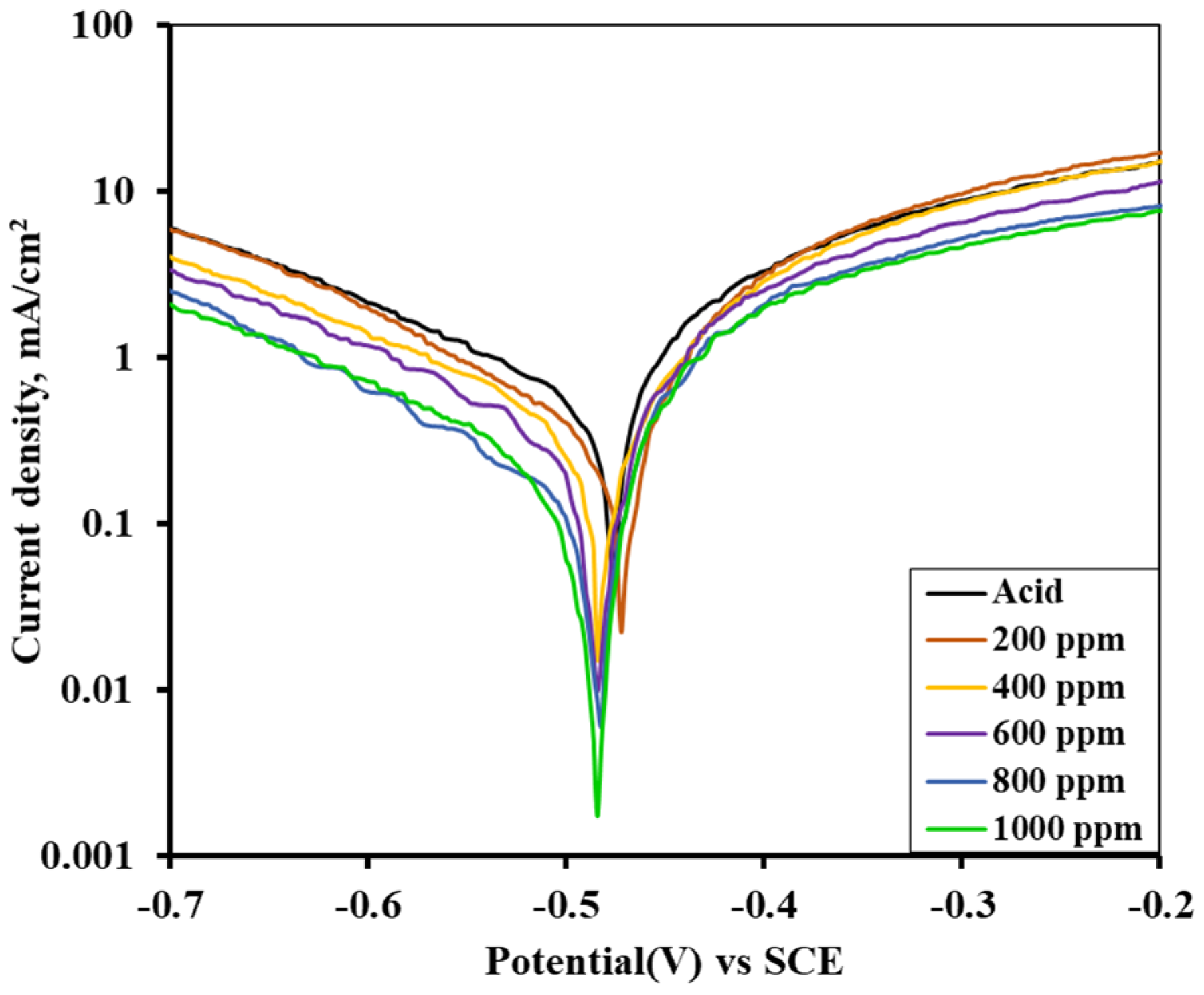
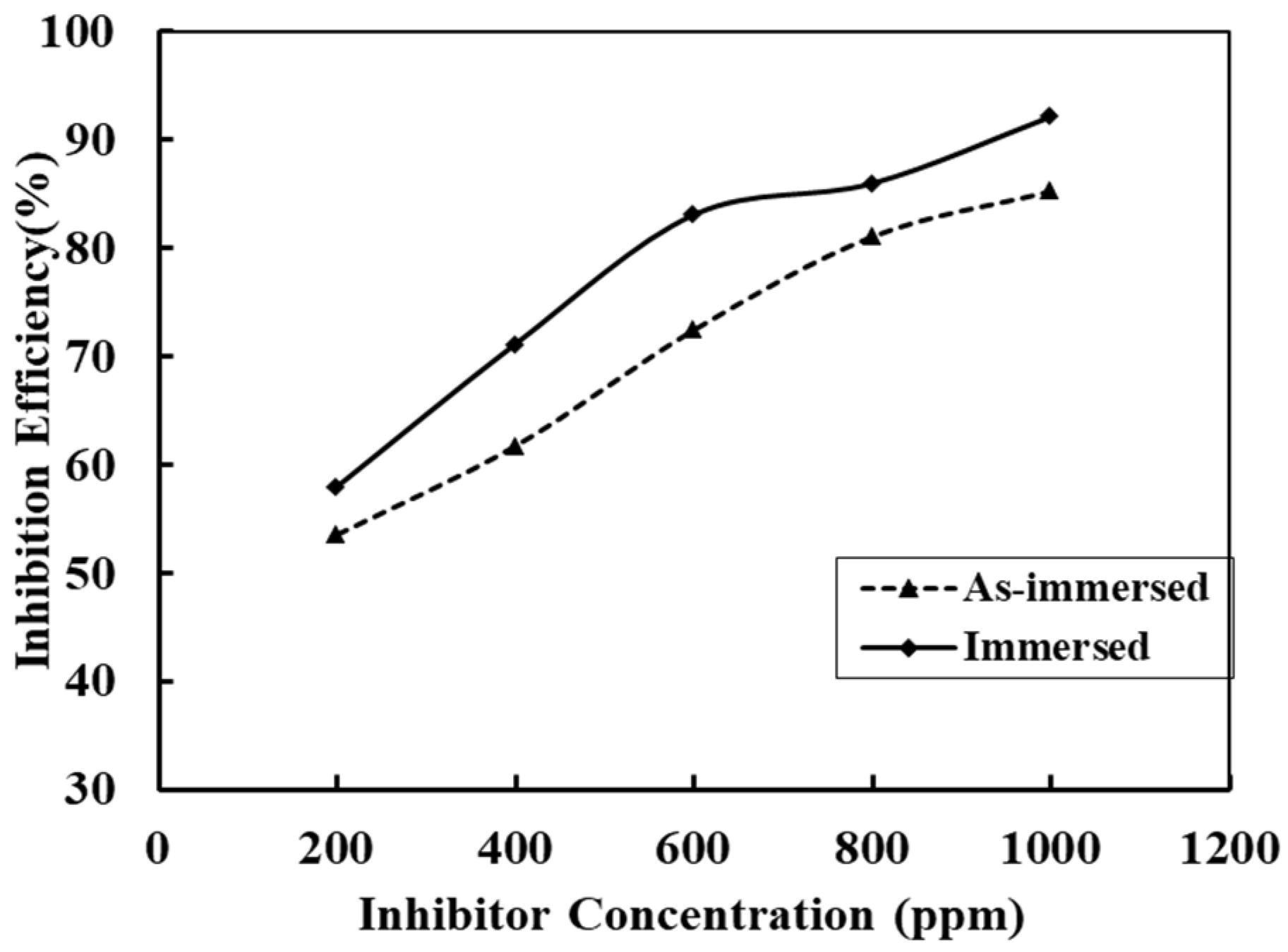


| Inhibitor Concentration (ppm) | Corrosion Current Density (icorr) in AV Alkaloid Inhibitor | Efficiency (%) of AV Alkaloids | Corrosion Current Density (icorr) in ST Alkaloid Inhibitor | Efficiency (%) of ST Alkaloids |
|---|---|---|---|---|
| 1 M H2SO4 | 0.476 | - | 0.502 | - |
| 200 | 0.221 | 53.57 | 0.226 | 54.98 |
| 400 | 0.182 | 61.77 | 0.208 | 58.57 |
| 600 | 0.131 | 72.48 | 0.172 | 65.74 |
| 800 | 0.090 | 81.09 | 0.139 | 72.31 |
| 1000 | 0.070 | 85.29 | 0.098 | 80.48 |
| Inhibitor Concentration (ppm) | Corrosion Current Density (icorr) of AV Alkaloid | Efficiency (%) of AV Alkaloid | Corrosion Current Density (icorr) of ST Alkaloid | Efficiency (%) of ST Alkaloid |
|---|---|---|---|---|
| 1 M H2SO4 | 0.243 | - | 0.292 | - |
| 200 | 0.102 | 58.03 | 0.115 | 60.62 |
| 400 | 0.070 | 71.19 | 0.098 | 66.44 |
| 600 | 0.041 | 83.13 | 0.078 | 73.29 |
| 800 | 0.034 | 86.01 | 0.052 | 82.19 |
| 1000 | 0.019 | 92.18 | 0.026 | 91.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parajuli, D.; Sharma, S.; Oli, H.B.; Bohara, D.S.; Bhattarai, D.P.; Tiwari, A.P.; Yadav, A.P. Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia vulgaris and Solanum tuberosum in Mild Steel Samples in 1 M Sulphuric Acid. Electrochem 2022, 3, 416-433. https://doi.org/10.3390/electrochem3030029
Parajuli D, Sharma S, Oli HB, Bohara DS, Bhattarai DP, Tiwari AP, Yadav AP. Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia vulgaris and Solanum tuberosum in Mild Steel Samples in 1 M Sulphuric Acid. Electrochem. 2022; 3(3):416-433. https://doi.org/10.3390/electrochem3030029
Chicago/Turabian StyleParajuli, Davilal, Srijana Sharma, Hari Bhakta Oli, Dilip Singh Bohara, Deval Prasad Bhattarai, Arjun Prasad Tiwari, and Amar Prasad Yadav. 2022. "Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia vulgaris and Solanum tuberosum in Mild Steel Samples in 1 M Sulphuric Acid" Electrochem 3, no. 3: 416-433. https://doi.org/10.3390/electrochem3030029
APA StyleParajuli, D., Sharma, S., Oli, H. B., Bohara, D. S., Bhattarai, D. P., Tiwari, A. P., & Yadav, A. P. (2022). Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia vulgaris and Solanum tuberosum in Mild Steel Samples in 1 M Sulphuric Acid. Electrochem, 3(3), 416-433. https://doi.org/10.3390/electrochem3030029










