Catalysts for Oxygen Reduction Reaction in the Polymer Electrolyte Membrane Fuel Cells: A Brief Review
Abstract
1. Introduction
2. Platinum Group Metal Catalyst (PGM)
2.1. Platinum and Pt-Based Catalysts
2.2. Core-Shell Catalysts
2.3. Nanocrystals
2.4. Carbon and Non-Carbon Supports for PGM Catalyst
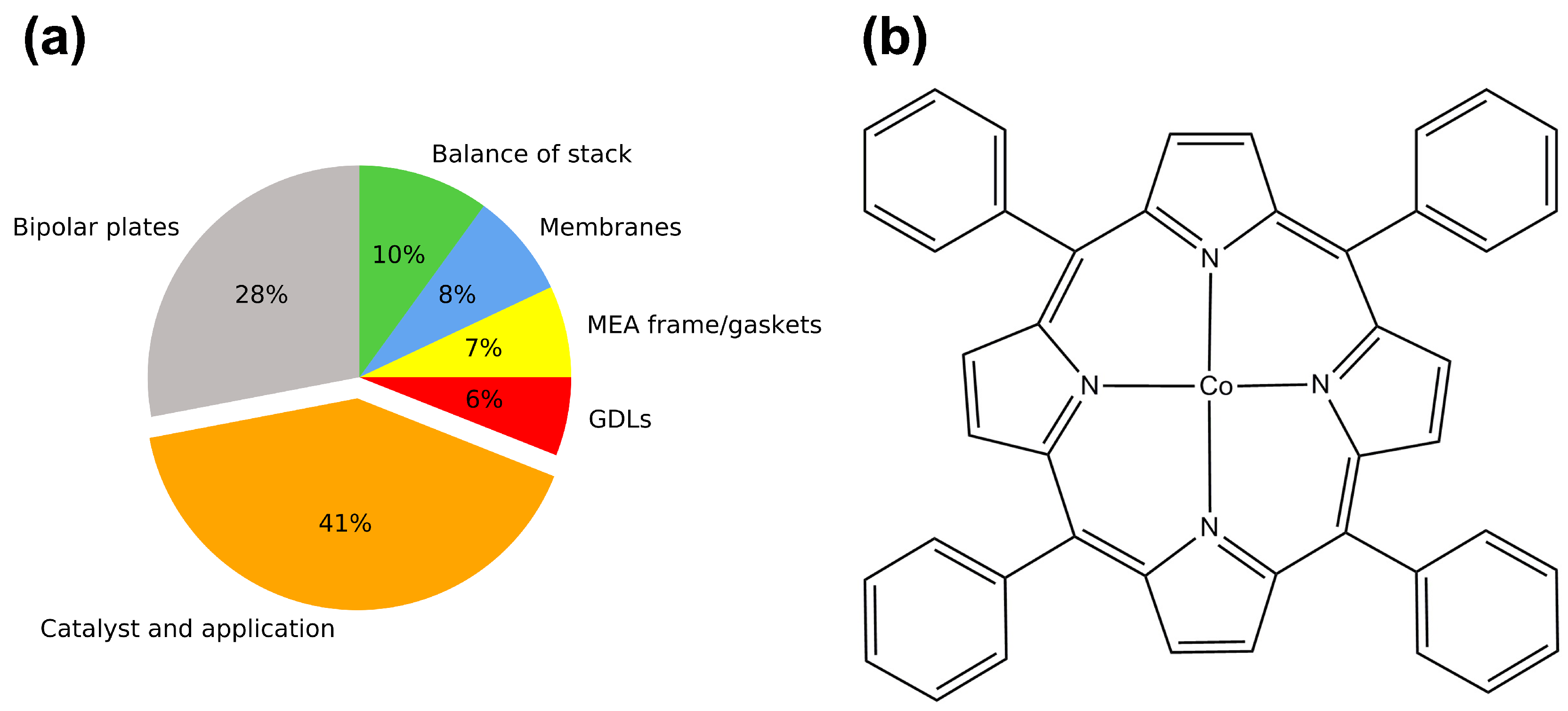
3. Non-Precious Metal Catalyst
4. Perspectives on Stability of PGM Catalyst and NPMC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEMFC | Proton exchange membrane fuel cells |
| PGM | Platinum group metals |
| ORR | Oxygen reduction reaction |
| HOR | Hydrogen oxidation reaction |
| GDL | Gas diffusion layer |
| NHE | Normal Hydrogen Electrode |
| ICE | Internal combustion engines |
| MEA | Membrane electrode assembly |
| BoL | Beginning of Life |
| NPMC | Non-precious metal catalyst |
| DoE | Department of Energy (USA) |
| RHE | Reversible Hydrogen Electrode |
| CNT | Carbon nanotubes |
| NC | Nanocluster |
References
- He, W.; King, M.; Luo, X.; Dooner, M.; Li, D.; Wang, J. Technologies and economics of electric energy storages in power systems: Review and perspective. Adv. Appl. Energy 2021, 4, 100060. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yan, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Olabi, A.G.; Onumaegbu, C.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Al-Alami, A.H. Critical review of energy storage systems. Energy 2021, 214, 118987. [Google Scholar] [CrossRef]
- Irfan, M.; Abas, N.; Saleem, M.S. Thermal performance analysis of net zero energy home for sub zero temperature areas. Case Stud. 2018, 12, 789–796. [Google Scholar] [CrossRef]
- Khatib, F.N. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew. Sust. Energ. Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Marsik, F.; Weigand, B.; Tomas, M.; Tucek, O.; Novotny, P. On the Efficiency of Electrochemical Devices from the Perspective of Endoreversible Thermodynamics. J. Non-Equilib. Thermodyn. 2019, 44, 425–437. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current Status of Automotive Fuel Cells for Sustainable Transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Aili, D.; Lu, S.; Li, Q.; Jiang, S.P. Advancement toward Polymer Electrolyte Membrane Fuel Cells at Elevated Temperatures. Research 2020, 2020, 9089405. [Google Scholar] [CrossRef] [PubMed]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent Developments in Catalyst-related PEM Fuel Cell Durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (O•, OH• and OOH•) Formation and Ionomer Degradation in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2011, 158, B755–B769. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Pivovar, B. Catalysts for Fuel Cell Transportation and Hydrogen Related Uses. Nat. Catal. 2019, 2, 562–565. [Google Scholar] [CrossRef]
- David, M.; Lyth, S.M.; Lindner, R.; Harrington, G.F. Future-Proofing Fuel Cells, Critical Raw Material Governance in Sustainable Energy; Palgrave Macmillan: London, UK, 2021; ISBN 978-3-030-76806-5. [Google Scholar]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current Progress of Pt and Pt-based Electrocatalysts Used for Fuel Cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Stoddart, J.F. Molecular Triangles: A New Class of Macrocycles. Acc. Chem. Res. 2021, 54, 2027–2039. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Alloy Catalysts Designed from First Principles. Nat. Mater. 2004, 3, 810–815. [Google Scholar] [CrossRef]
- Seselj, N.; Engelbrekt, C.; Zhang, J. Graphene-supported Platinum Catalysts for Fuel Cells. Sci. Bull. 2015, 60, 864–876. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontierin Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Kim, D.Y.; Zhang, H.; Xia, Y. Platinum Concave Nanocubes with High-Index Facets and Their Enhanced Activity for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 123, 2825–2829. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Cui, Z. Strategies to enhance the electrochemical performances of Pt-based intermetallic catalysts. Chem. Commun. 2021, 57, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Rößner, L.; Armbrüster, M. Electrochemical Energy Conversion on Intermetallic Compounds: A Review. ACS Catal. 2019, 9, 2018–2062. [Google Scholar] [CrossRef]
- Sievers, G.W.; Jensen, A.W.; Quinson, J.; Zana, A.; Bizzotto, F.; Oezaslan, M.; Dworzak, A.; Kirkensgaard, J.J.; Smitshuysen, T.E.; Kadkhodazadeh, S.; et al. Self-supported Pt–CoO Networks Combining High Specific Activity with High Surface Area for Oxygen Reduction. Nat. Mater. 2021, 20, 208–213. [Google Scholar] [CrossRef]
- Ioroi, T.; Yasuda, K. Platinum-Iridium Alloys as Oxygen Reduction Electrocatalystsfor Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2005, 152, A1917–A1924. [Google Scholar] [CrossRef]
- Ganesan, A.; Narayanasamy, M. Ultra low Loading of Platinum in Proton Exchange Membrane based Fuel Cells: A Brief Review. Mater. Renew. Sustain. Energy 2019, 8, 18. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Chen, S. Pt-W Bimetallic Alloys as CO-tolerant PEMFC Anode Catalysts. Electrochim. Acta 2013, 89, 744–748. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Wang, Z.; Yao, X.; Wei, Y.; Kang, Y.; Du, H.; Li, J.; Gan, L. Mitigating Metal Dissolution and Redeposition of Pt-Co Catalysts in PEM Fuel Cells: Impacts of Structural Ordering and Particle Size. J. Electrochem. Soc. 2020, 167, 064520. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M. Just a Dream—Or Future Reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef]
- Luna, A.C.; Bonesi, A.; Triaca, W.E.; Di Blasi, A.; Stassi, A.; Baglio, V.; Antonucci, V.; Aricò, A.S. Investigation of a Pt–Fe/C Catalyst for Oxygen Reduction Reaction in Direct Ethanol Fuel Cells. J. Nanopart. Res. 2010, 12, 357–365. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.D.; et al. Engineering Bunched Pt-Ni Alloy Nanocages for Efficient Oxygen Reduction in Practical Fuel Cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Zignani, S.C.; Baglio, V.; Sebastián, D.; Saccà, A.; Gatto, I.; Aricò, A.S. Towards Highly Performing and Stable PtNi Catalysts in Polymer Electrolyte Fuel Cells for Automotive Application. Materials 2017, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z. Recent Advancements in Pt and Pt-free Catalysts for Oxygen Reduction Reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Chung, D.Y.; Kim, H.I.; Chung, Y.H.; Lee, M.J.; Yoo, S.J.; Bokare, A.D.; Choi, W.; Sung, Y.E. Inhibition of CO Poisoning on Pt Catalyst Coupled with the Reduction of Toxic Hexavalent Chromium in a Dual-Functional Fuel Cell. Sci. Rep. 2014, 4, 7450. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Jeffery, A.A.; Kim, Y.; Jung, N. Electrochemical Analysis for Demonstrating CO Tolerance of Catalysts in Polymer Electrolyte Membrane Fuel Cells. Nanomaterials 2019, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Vignarooban, K.; Lin, J.; Arvay, A.; Kolli, S.; Kruusenberg, I.; Tammeveski, K.; Munukutla, L.; Kannan, A.M. Nano-electrocatalyst Materials for Low Temperature Fuel Cells: A Review. Chin. J. Catal. 2015, 36, 458–472. [Google Scholar] [CrossRef]
- Walker, J.S.; Rees, N.V.; Mendes, P.M. Progress Towards the Ideal Core@shell Nanoparticle for Fuel Cell Electrocatalysis. J. Exp. Nanosci. 2018, 13, 258–271. [Google Scholar] [CrossRef]
- Kuttiyiel, K.A.; Choi, Y.; Hwang, S.M.; Park, G.G.; Yang, T.H.; Su, D.; Sasaki, K.; Liu, P.; Adzic, R.R. Enhancement of the Oxygen Reduction on Nitride Stabilized pt-M (M=Fe, Co and Ni) Core–shell Nanoparticle Electrocatalysts. Nano Energy 2015, 13, 442–449. [Google Scholar] [CrossRef]
- Santhosh Kumar, K.; Bhooshan Kumar, V.; Paik, P. Recent Advancement in Functional Core-Shell Nanoparticles of Polymers: Synthesis, Physical Properties and Applications in Medical Biotechnology. J. Nanopart. 2013, 2013, 672059. [Google Scholar]
- Yurukcu, M.; Badradeen, E.; Bilnoski, S.; Yurtsever, F.; Begum, M. The Effect of the Core/shell Nanostructure Arrays on PEM Fuel Cells: A Short Review. Mater. Sci. Eng. 2018, 2, 58–64. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Ruban, A.V.; Mavrikakis, M. Adsorption and Dissociation of O2 on Pt-Co and Pt-Fe Alloys. J. Am. Chem. Soc. 2004, 126, 4717–4725. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V.; Yang, Y.; Thi, C.M.; Van Minh, N.; Cao, Y.; Nogami, M. The Development of Mixture, Alloy and Core-shell Nanocatalysts with Nanomaterial Supports for Energy Conversion in Low-temperature Fuel Cells. Nano Energy 2013, 2, 636–676. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yoo, S.J.; Shin, J.; Cho, Y.H.; Jang, J.H.; Cho, E.; Sung, Y.E.; Nam, S.W.; Lim, T.H.; Lee, S.C.; et al. Supported Core@Shell Electrocatalysts for Fuel Cells: Close Encounter with Reality. Sci. Rep. 2013, 3, 1309. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, M.; Kim, Y.; Gok, S.; Lee, E.; Hwang, J.Y.; Jang, J.H.; Cho, Y.H.; Lim, T.; Sung, Y.E.; Kwon, O.J. A Highly Durable Carbon-nanofiber-supported Pt–C Core–shell Cathode Catalyst for Ultra-low Pt Loading Proton Exchange Membrane Fuel Cells: Facile Carbon Encapsulation. Energy Environ. Sci. 2019, 12, 2820–2829. [Google Scholar] [CrossRef]
- Oezaslan, M.; Hasché, F.; Strasser, P. Pt-Based Core–Shell Catalyst Architectures for Oxygen Fuel Cell Electrodes. J. Phys. Chem. Lett. 2013, 4, 3273–3291. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Luo, F.; Liao, S. Core-Shell-Structured Low-Platinum Electrocatalysts for Fuel Cell Applications. Electrochem. Energy Rev. 2018, 1, 324–387. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.L.; Zboril, R.; Varma, R.S. Core-shell Nanoparticles: Synthesis and Applications in Catalysis and Electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef]
- Tao, L.; Huang, B.; Jin, F.; Yang, Y.; Luo, M.; Sun, M.; Liu, Q.; Gao, F.; Guo, S. Atomic PdAu Interlayer Sandwiched into Pd/Pt Core/Shell Nanowires Achieves Superstable Oxygen Reduction Catalysis. ACS Nano 2020, 14, 11570–11578. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, L.; Kuttiyiel, K.A.; Goodman, K.R.; Zhang, C.; Zhu, Y.; Vukmirovic, M.B.; White, M.G.; Sasaki, K.; Adzic, R.R. Increasing Stability and Activity of Core-shell Catalysts by Preferential Segregation of Oxide on Edges and Vertexes: Oxygen Reduction on Ti-Au@Pt/C. J. Am. Chem. Soc. 2016, 138, 9294–9300. [Google Scholar] [CrossRef]
- Miyazaki, K.; Mori, H. Origin of High Oxygen Reduction Reaction Activity of Pt12 and Strategy to Obtain Better Catalyst Using Sub-nanosized Pt-alloy Clusters. Sci. Rep. 2017, 7, 45381. [Google Scholar] [CrossRef]
- Tian, N.; Lu, B.A.; Yang, X.D.; Huang, R.; Jiang, Y.X.; Zhou, Z.Y.; Sun, S.G. Rational Design and Synthesis of Low Temperature Fuel Cell Electrocatalysts. Electrochem. Energy Rev. 2018, 1, 54–83. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Yersak, T.; Yang, D.; Xiao, Q.; Zhang, J.; Zhang, C. Recent Advances in Pt-based Octahedral Nanocrystals as High Performance Fuel Cell Catalysts. J. Mater. Chem. A 2016, 4, 11559–11581. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, E.; Chen, Y.; Li, Y.; Chiu, C.Y.; Xu, Y.; Lin, Z.; Duan, X.; Huang, Y. A Facile Strategy to Pt3Ni Nanocrystals with Highly Porous Features as an Enhanced Oxygen Reduction Reaction Catalyst. Adv. Mater. 2013, 25, 2974–2979. [Google Scholar] [CrossRef]
- Choi, S.I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X.; et al. Synthesis and Characterization of 9-nm Pt-Ni Octahedra with a Record High Activity of 3.3 A/mgPt for the Oxygen Reduction Reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef]
- Wang, Y.J.; Long, W.; Wang, L.; Yuan, R.; Ignaszak, A.; Fang, B.; Wilkinson, D.P. Unlocking the Door to Highly Active ORR Catalysts for PEMFC Applications: Polyhedron-engineered Pt-based Nanocrystals. Energy Environ. Sci. 2018, 11, 258–275. [Google Scholar] [CrossRef]
- Zhu, J.; Elnabawy, A.O.; Lyu, Z.; Xie, M.; Murray, E.A.; Chen, Z.; Jin, W.; Mavrikakis, M.; Xia, Y. Facet-controlled Pt–Ir Nanocrystals with Substantially Enhanced Activity and Durability Towards Oxygen Reduction. Mater. Today 2020, 35, 69–77. [Google Scholar] [CrossRef]
- Lori, O.; Elbaz, L. Advances in Ceramic Supports for Polymer Electrolyte Fuel Cells. Catalysts 2015, 5, 1445–1464. [Google Scholar] [CrossRef]
- Chen, M.; Hwang, S.; Li, J.; Karakalos, S.; Chen, K.; He, Y.; Mukherjee, S.; Su, D.; Wu, G. Pt alloy nanoparticles decorated on large-size nitrogen-doped graphene tubes for highly stable oxygen-reduction catalysts. Nanoscale 2018, 10, 17318–17326. [Google Scholar] [CrossRef]
- Yang, X.D.; Chen, C.; Zhou, Z.Y.; Sun, S.G. Advances in Active Site Structure of Carbon-Based Non-Precious Metal Catalysts for Oxygen Reduction Reaction. Acta Phys. Chim. Sin. 2019, 35, 472–485. [Google Scholar] [CrossRef]
- Ren, G.; Gao, L.; Teng, C.; Li, Y.; Yang, H.; Shui, J.; Lu, X.; Zhu, Y.; Dai, L. Ancient Chemistry “Pharaoh’s Snakes” for Efficient Fe-/N-Doped Carbon Electrocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 10778–10785. [Google Scholar] [CrossRef] [PubMed]
- Bau, V.M.; Bo, X.; Guo, L. Nitrogen-doped Cobalt Nanoparticles/nitrogen-doped Plate-like Ordered Mesoporous Carbons Composites as Noble-metal Free Electrocatalysts for Oxygen Reduction Reaction. J. Energy Chem. 2017, 26, 63–71. [Google Scholar] [CrossRef]
- Wang, L.; Wan, X.; Liu, S.; Xu, L.; Shui, J. Fe-N-C Catalysts for PEMFC: Progress Towards the Commercial Application under DOE Reference. J. Energy Chem. 2019, 39, 77–87. [Google Scholar] [CrossRef]
- Thompson, S.T.; James, B.D.; Huya-Kouadio, J.M.; Houchins, C.; DeSantis, D.A.; Ahluwalia, R.; Wilson, A.R.; Kleen, G.; Papageorgopoulos, D. Direct Hydrogen Fuel Cell Electric Vehicle Cost Analysis: System and High-volume Manufacturing Description, Validation and Outlook. J. Power Sources 2018, 399, 304–313. [Google Scholar] [CrossRef]
- James, B.D.; Huya-Kouadio, J.M.; Houchins, C. Mass Production Cost Estimation of Direct H2 PEM Fuel Cell Systems for Transportation Applications: 2015 Update. Report, US Department of Energy, Strategic Analysis. 2015. Available online: https://www.energy.gov/sites/prod/files/2016/11/f34/fcto_sa_2015_pemfc_transportation_cost_analysis.pdf (accessed on 20 October 2021).
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Zagal, J.H.; Koper, M.T.M. Reactivity Descriptors for the Activity of Molecular MN4 Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Edit. 2016, 55, 14510–14521. [Google Scholar] [CrossRef]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen Reduction Reaction Mechanism and Kinetics on M-NxCy and M@N-C Active Sites Present in Model M-N-C Catalysts under Alkaline and Acidic Conditions. J. Solid State Chem. 2021, 25, 45–56. [Google Scholar] [CrossRef]
- Song, M.; Song, Y.; Sha, W.; Xu, B.; Guo, J.; Wu, Y. Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs. Catalysts 2020, 10, 141. [Google Scholar] [CrossRef]
- Yamazaki, S. Metalloporphyrins and Related Metallomacrocycles as Electrocatalysts for Use in Polymer Electrolyte Fuel Cells and Water Electrolyzers. Coordin. Chem. Rev. 2018, 373, 148–166. [Google Scholar] [CrossRef]
- Othman, R.; Dicks, A.L.; Zhu, Z. Non Precious Metal Catalysts for the PEM Fuel Cell Cathode. Int. J. Hydrog. Energy 2012, 37, 357–372. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A Review on Non-precious Metal Electrocatalysts for PEM Fuel Cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Banham, D.; Kishimoto, T.; Zhou, Y.; Sato, T.; Bai, K.; Ozaki, J.I.; Imashiro, Y.; Ye, S. Critical Advancements in Achieving High Power and Stable Nonprecious Metal Catalyst–based MEAs for Real-world Proton Exchange Membrane Fuel Cell Applications. Sci. Adv. 2018, 4, eaar7180. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Zheng, L.; Shui, J. The Solid-Phase Synthesis of an Fe-N-C Electrocatalyst for High-Power Proton-Exchange Membrane Fuel Cells. Angew. Chem. Int. Edit. 2018, 57, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Huang, J.; Li, J.; Wu, Y.; Huang, G.; Jin, Y.Q.; Zeng, H.; Zhang, H.; Chen, J.; Jin, Y.; et al. Improving the Catalytic Performance of Co/N/C Catalyst for Oxygen Reduction Reaction by Alloying with Fe. J. Electrochem. Soc. 2020, 167, 104502. [Google Scholar] [CrossRef]
- Xia, D.; Liu, S.; Wang, Z.; Chen, G.; Zhang, L.; Zhang, L.; Hui, S.R.; Zhang, J. Methanol-tolerant MoN Electrocatalyst Synthesized Through Heat Treatment of Molybdenum Tetraphenylporphyrin for Four-electron Oxygen Reduction Reaction. J. Power Sources 2008, 177, 296–302. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Wang, X.X.; Cullen, D.A.; Pan, Y.T.; Hwang, S.; Wang, M.; Feng, Z.; Wang, J.; Engelhard, M.H.; Zhang, H.; He, Y.; et al. Nitrogen-Coordinated Single Cobalt Atom Catalysts for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. Adv. Mater. 2018, 30, 1706758. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Roy, A.; Silvioli, L.; Cullen, D.A.; Zitolo, A.; Sougrati, M.T.; Oguz, I.C.; Mineva, T.; Teschner, D.; Wagner, S.; et al. P-Block Single-Met. Tin/nitrogen-Doped Carbon Fuel Cell Cathode Catal. Oxyg. Reduct. Reaction. Nat. Mater. 2020, 19, 1215–1223. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A Review of Pt-based Electrocatalysts for Oxygen Reduction Reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef]
- Zhang, C.; Hwang, S.Y.; Trout, A.; Peng, Z. Solid-State Chemistry-Enabled Scalable Production of Octahedral Pt–Ni Alloy Electrocatalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2014, 136, 7805–7808. [Google Scholar] [CrossRef]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.H.; Vej-Hansen, U.G.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiøtz, J.; Rossmeisl, J.; Stephens, I.E.; Chorkendorff, I. Tuning the Activity of Pt Alloy Electrocatalysts by Means of the Lanthanide Contraction. Science 2016, 352, 73–76. [Google Scholar] [CrossRef]
- Lindahl, N.; Zamburlini, E.; Feng, L.; Grönbeck, H.; Escudero-Escribano, M.; Stephens, I.E.; Chorkendorff, I.; Langhammer, C.; Wickman, B. High Specific and Mass Activity for the Oxygen Reduction Reaction for Thin Film Catalysts of Sputtered Pt3Y. Adv. Mater. Interfaces 2017, 4, 1700311. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine Jagged Platinum Nanowires Enable Ultrahigh Mass Activity for the Oxygen Reduction Reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 137, 15478–15485. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, D.; Strmcnik, D.; Paulikas, A.P.; Markovic, N.M.; Stamenkovic, V.R. Recent Advances in the Design of Tailored Nanomaterials for Efficient Oxygen Reduction Reaction. Nano Energy 2016, 29, 149–165. [Google Scholar] [CrossRef]
- Zhang, M.; Miao, S.; Xu, B.Q. Core@Shell Nanostructured Au-d@NimPtm for Electrochemical Oxygen Reduction Reaction: Effect of the Core Size and Shell Thickness. Catal. Sci. Technol. 2019, 9, 4668–4677. [Google Scholar] [CrossRef]
- Jackson, A.; Strickler, A.; Higgins, D.; Jaramillo, T.F. Engineering Ru@Pt Core-Shell Catalysts for Enhanced Electrochemical Oxygen Reduction Mass Activity and Stability. Nanomaterials 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, J.; Feng, B.; Xu, T.; Wang, F. Enhanced Electrocatalytic Activity and Stability toward the Oxygen Reduction Reaction with Unprotected Pt Nanoclusters. Nanomaterials 2018, 8, 955. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Zhang, W.; Li, Z.; Lv, L.; Ruan, Y.; Wu, H.H.; Chiang, W.H.; Wang, C.; Liu, M.; Zeng, X.C. Unraveling the High-activity Nature of Fe-N-C Electrocatalysts for the Oxygen Reduction Reaction: The Extraordinary Synergy between Fe-N4 and Fe4N4. J. Mater. Chem. A 2019, 7, 11792–11801. [Google Scholar] [CrossRef]
- Kong, Z.; Maswadeh, Y.; Vargas, J.A.; Shan, S.; Wu, Z.P.; Kareem, H.; Leff, A.C.; Tran, D.T.; Chang, F.; Yan, S.; et al. Origin of High Activity and Durability of Twisty Nanowire Alloy Catalysts under Oxygen Reduction and Fuel Cell Operating Conditions. J. Am. Chem. Soc. 2020, 142, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Deng, Y.; Zeng, J.; Song, H.; Liao, S. A High-performance Composite ORR Catalyst Based on the Synergy between Binary Transition Metal Nitride and Nitrogen-doped Reduced Graphene Oxide. J. Mater. Chem. A 2017, 5, 5829–5837. [Google Scholar] [CrossRef]
- Li, J.; Sharma, S.; Liu, X.; Pan, Y.T.; Spendelow, J.S.; Chi, M.; Jia, Y.; Zhang, P.; Cullen, D.A.; Xi, Z.; et al. Hard-Magnet L10-CoPt Nanoparticles Advance Fuel Cell Catalysis. Joule 2019, 3, 124–135. [Google Scholar] [CrossRef]
- Kirsanova, M.A.; Okatenko, V.D.; Aksyonov, D.A.; Forslund, R.P.; Mefford, J.T.; Stevenson, K.J.; Abakumov, A.M. Bifunctional OER/ORR catalytic activity in the tetrahedral YBaCo4O7.3 oxide. J. Mater. Chem. A 2019, 7, 330–341. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Du, L.; Zhang, J.; Chiang, F.K.; Wen, Y.; Wang, X.; Wu, Y.; Chen, N.; Sun, S. PGM-Free Fe/N/C and Ultralow Loading Pt/C Hybrid Cathode Catalysts with Enhanced Stability and Activity in PEM Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 13739–13749. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, H.; Jin, S.; Choi, S.M.; Kim, H.J.; Seo, M.H.; Kim, W.B. A Review on Recent Progress in the Aspect of Stability of Oxygen Reduction Electrocatalysts for Proton-Exchange Membrane Fuel Cell: Quantum Mechanics and Experimental Approaches. Energy Technol. 2019, 7, 1900312. [Google Scholar] [CrossRef]
- Zhao, X.; Takao, S.; Higashi, K.; Kaneko, T.; Samjeskè, G.; Sekizawa, O.; Sakata, T.; Yoshida, Y.; Uruga, T.; Iwasawa, Y. Simultaneous Improvements in Performance and Durability of an Octahedral PtNix/C Electrocatalyst for Next-Generation Fuel Cells by Continuous, Compressive and Concave Pt Skin Layers. ACS Catal. 2017, 7, 4642–4654. [Google Scholar] [CrossRef]
- Lopes, P.P.; Li, D.; Lv, H.; Wang, C.; Tripkovic, D.; Zhu, Y.; Schimmenti, R.; Daimon, H.; Kang, Y.; Snyder, J.; et al. Eliminating dissolution of platinum-based electrocatalysts at the atomic scale. Nat. Mater. 2020, 19, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sougrati, M.T.; Zitolo, A.; Ablett, J.M.; Oğuz, I.C.; Mineva, T.; Matanovic, I.; Atanassov, P.; Huang, Y.; Zenyuk, I.; et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 2021, 4, 10–19. [Google Scholar] [CrossRef]
- Mølmen, L.; Eiler, K.; Fast, L.; Leisner, P.; Pellicer, E. Recent advances in catalyst materials for proton exchange membrane fuel cells. APL Mater. 2021, 9, 040702. [Google Scholar] [CrossRef]

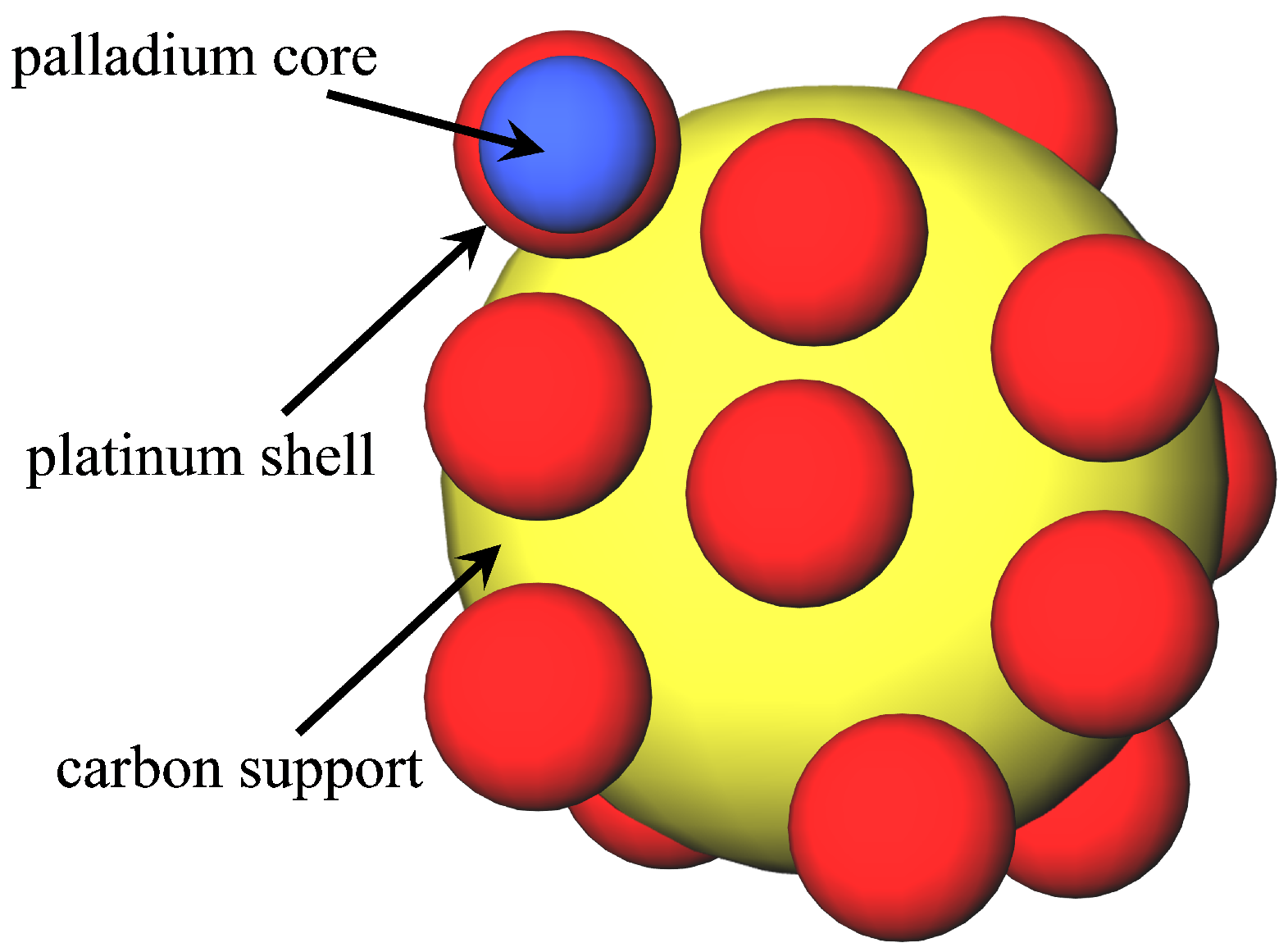
| Catalyst | Surface Activity/ | Mass Activity/ | Ref. |
|---|---|---|---|
| commercial Pt/C | 0.23 | 0.15 | [83] |
| DoE 2020 target | - | 0.44 | [83] |
| 18 | - | [84] | |
| 3.99 | 1.96 | [85] | |
| alloy | - | 3.6 | [86] |
| thin layer | 13.4 | 3.5 | [87] |
| Pt/NiO core-shell nanowires | 11.5 | 13.6 | [88] |
| PtFe N-doped carbon shell | - | 1.6 | [89] |
| octahedrons | - | 2.35 | [89] |
| Pt-Ni octahedrons | - | 3.3 | [90] |
| - | 1.6 | 0.496 | [91] |
| 0.7 | 0.5 | [92] | |
| NCs/CNTs (Pt loading 10%) | 0.194 | 0.167 | [93] |
| Fe-containing nanocrystals | 6.18 | - | [94] |
| PtFe nanowires | - | 3.4 | [95] |
| /N-rGO | 2.51 | - | [96] |
| magnet core-shell CoPt/Pt | - | 2.26 | [97] |
| tetrahedral | 2.76 | - | [98] |
| Fe-N-C+Pt/C | - | 0.22 | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomas, M.; Gholami, F.; Gholami, Z.; Sedlacek, J. Catalysts for Oxygen Reduction Reaction in the Polymer Electrolyte Membrane Fuel Cells: A Brief Review. Electrochem 2021, 2, 590-603. https://doi.org/10.3390/electrochem2040037
Tomas M, Gholami F, Gholami Z, Sedlacek J. Catalysts for Oxygen Reduction Reaction in the Polymer Electrolyte Membrane Fuel Cells: A Brief Review. Electrochem. 2021; 2(4):590-603. https://doi.org/10.3390/electrochem2040037
Chicago/Turabian StyleTomas, Martin, Fatemeh Gholami, Zahra Gholami, and Jan Sedlacek. 2021. "Catalysts for Oxygen Reduction Reaction in the Polymer Electrolyte Membrane Fuel Cells: A Brief Review" Electrochem 2, no. 4: 590-603. https://doi.org/10.3390/electrochem2040037
APA StyleTomas, M., Gholami, F., Gholami, Z., & Sedlacek, J. (2021). Catalysts for Oxygen Reduction Reaction in the Polymer Electrolyte Membrane Fuel Cells: A Brief Review. Electrochem, 2(4), 590-603. https://doi.org/10.3390/electrochem2040037









