Progress and Opportunities for Exsolution in Electrochemistry
Abstract
:1. Introduction
2. Discussion
2.1. Recent Advancements in Exsolution Electrodes
2.2. Future Research Directions
2.2.1. Exsolution from Heteroanionic Ceramics
2.2.2. Exsolution in Photocatalysis
2.2.3. Predetermined Location of Exsolution
2.2.4. Exsolved Core-Shell and Core-Skin Particles
2.2.5. Exsolution from Thin Films Cast on High Surface Area Supports
2.2.6. Exsolution Electrodes for Ambient Temperature Applications
3. Conclusions
Funding
Conflicts of Interest
References
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; de Jong, K.P.; de Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Aikawa, K.; Sato, K.; Uchijima, T. Role of support in reforming of CH4 with CO2 over Rh catalysts. Catal. Lett. 1994, 25, 265–270. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO oxidation over supported gold catalysts—“Inert” and “active” support materials and their role for the oxygen supply during reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Micoud, F.; Maillard, F.; Bonnefont, A.; Job, N.; Chatenet, M. The role of the support in CO ads monolayer electrooxidation on Pt nanoparticles: Pt/WO x vs. Pt/C. Phys. Chem. Chem. Phys. 2010, 12, 1182–1193. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef] [Green Version]
- Ratkovic, S.; Vujicic, D.; Kiss, E.; Boskovic, G.; Geszti, O. Different degrees of weak metal–support interaction in Fe–(Ni)/Al2O3 catalyst governing activity and selectivity in carbon nanotubes’ production using ethylene. Mater. Chem. Phys. 2011, 129, 398–405. [Google Scholar] [CrossRef]
- Park, C.; Keane, M.A. Catalyst support effects in the growth of structured carbon from the decomposition of ethylene over nickel. J. Catal. 2004, 221, 386–399. [Google Scholar] [CrossRef]
- Rosen, B.A.; Singh, S. Fossil Fuels: Coke-Resistant Nanomaterials for Gas-to-Liquid (GTL) Fuels. Nanotechnol. Energy Sustain. 2017, 59–82. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C–H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.M.; Mirzaei, M.Z.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Florez, E.; Mondragon, F. Dry reforming of methane over LaNi1−yByO3 ± δ (B = Mg, Co) perovskites used as catalyst precursor. Appl. Catal. A Gen. 2008, 334, 251–258. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T.S. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Huang, K. An emerging platform for electrocatalysis: Perovskite exsolution. Sci. Bull. 2016, 61, 1783–1784. [Google Scholar] [CrossRef]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef] [Green Version]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Chatzichristodoulou, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef] [Green Version]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef]
- Myung, J.-H.; Neagu, D.; Miller, D.N.; Irvine, J.T.S. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, D.; Kyriakou, V.; Roiban, I.-L.; Aouine, M.; Tang, C.; Caravaca, A.; Kousi, K.; Schreur-Piet, I.; Metcalfe, I.S.; Vernoux, P.; et al. In Situ Observation of Nanoparticle Exsolution from Perovskite Oxides: From Atomic Scale Mechanistic Insight to Nanostructure Tailoring. ACS Nano 2019, 13, 12996–13005. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.-S.; Rahani, E.K.; Neagu, D.; Irvine, J.T.S.; Shenoy, V.B.; Gorte, R.J.; Vohs, J.M. Evidence and Model for Strain-Driven Release of Metal Nanocatalysts from Perovskites during Exsolution. J. Phys. Chem. Lett. 2015, 6, 5106–5110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, J.M.; Barnett, S.A.; Richardson, J.W.; Poeppelmeier, K.R. Structural and Chemical Evolution of the SOFC Anode La0.30Sr0.70Fe0.70Cr0.30O3-δ upon Reduction and Oxidation: An in Situ Neutron Diffraction Study. Chem. Mater. 2010, 22, 3283–3289. [Google Scholar] [CrossRef]
- Buharon, M.; Singh, S.; Komarala, E.P.; Rosen, B.A. Expanding possibilities for solid-phase crystallization by exsolving tunable Pd–NiO core–shell nanostructures. CrystEngComm 2018, 20, 6372–6376. [Google Scholar] [CrossRef]
- Bergamaschini, R.; Rosen, B.A.; Montalenti, F.; Colin, J. Motion of crystalline inclusions by interface diffusion in the proximity of free surfaces. J. Nanopart. Res. 2019, 21, 271. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Z.; You, T.L.; Wang, J.; Xie, L.; He, J.; Ciucci, F. Energetics of Nanoparticle Exsolution from Perovskite Oxides. J. Phys. Chem. Lett. 2018, 9, 3772–3778. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Sun, Y.; Li, J.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.; Behnamian, Y.; Luo, J. A-site deficient perovskite: The parent for in situ exsolution of highly active, regenerable nano-particles as SOFC anodes. J. Mater. Chem. A 2015, 3, 11048–11056. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Wang, M.-N.; Hua, B.; Li, J.; Luo, J.-L. A-site deficient chromite perovskite with in situ exsolution of nano-Fe: A promising bi-functional catalyst bridging the growth of CNTs and SOFCs. J. Mater. Chem. A 2015, 3, 14625–14630. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Cui, L.; Hua, B.; Cui, S.-H.; Li, J.; Luo, J.-L. A-site-deficiency facilitated in situ growth of bimetallic Ni–Fe nano-alloys: A novel coking-tolerant fuel cell anode catalyst. Nanoscale 2015, 7, 11173–11181. [Google Scholar] [CrossRef]
- Kousi, K.; Neagu, D.; Bekris, L.; Papaioannou, E.I.; Metcalfe, I.S. Endogenous Nanoparticles Strain Perovskite Host Lattice Providing Oxygen Capacity and Driving Oxygen Exchange and CH4 Conversion to Syngas. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.E.; Mogni, L.V.; Han, M.; Barnett, S.A. Ni-Substituted Sr(Ti,Fe)O3 SOFC Anodes: Achieving High Performance via Metal Alloy Nanoparticle Exsolution. Joule 2018, 2, 478–496. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Troiani, H.; Mogni, L.V.; Santaya, M.; Han, M.; Barnett, S.A. Exsolution and electrochemistry in perovskite solid oxide fuel cell anodes: Role of stoichiometry in Sr(Ti,Fe,Ni)O3. J. Power Sources 2019, 439, 227077. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, K.; Joo, S.; Jeong, H.Y.; Shin, J.; Han, J.W.; Sengodan, S.; Kim, G. Self-assembled alloy nanoparticles in a layered double perovskite as a fuel oxidation catalyst for solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 15947–15953. [Google Scholar] [CrossRef]
- Chen, X.; Ni, W.; Wang, J.; Zhong, Q.; Han, M.; Zhu, T. Exploration of Co-Fe alloy precipitation and electrochemical behavior hysteresis using Lanthanum and Cobalt co-substituted SrFeO3-δ SOFC anode. Electrochim. Acta 2018, 277, 226–234. [Google Scholar] [CrossRef]
- Zubenko, D.; Singh, S.; Rosen, B.A. Exsolution of Re-alloy catalysts with enhanced stability for methane dry reforming. Appl. Catal. B Environ. 2017, 209, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Hua, B.; Zeng, Y.; Amirkhiz, B.S.; Luo, J.-L. Thermally stable and coking resistant CoMo alloy-based catalysts as fuel electrodes for solid oxide electrochemical cells. J. Mater. Chem. A 2018, 6, 15377–15385. [Google Scholar] [CrossRef]
- Fowler, D.E.; Messner, A.C.; Miller, E.C.; Slone, B.W.; Barnett, S.A.; Poeppelmeier, K.R. Decreasing the Polarization Resistance of (La,Sr)CrO3-δ Solid Oxide Fuel Cell Anodes by Combined Fe and Ru Substitution. Chem. Mater. 2015, 27, 3683–3693. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Meng, J.; Wei, Z.; Liu, X.; Meng, J. Enhanced Anode Performance and Coking Resistance by In Situ Exsolved Multiple-Twinned Co–Fe Nanoparticles for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Wang, L.; Hu, R.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Zhang, Z. Encapsulated Ni@La2O3/SiO2 Catalyst with a One-Pot Method for the Dry Reforming of Methane. Catalysts 2019, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Papargyriou, D.; Miller, D.N.; Irvine, J.T.S. Exsolution of Fe–Ni alloy nanoparticles from (La,Sr)(Cr,Fe,Ni)O3 perovskites as potential oxygen transport membrane catalysts for methane reforming. J. Mater. Chem. A 2019, 7, 15812–15822. [Google Scholar] [CrossRef]
- Singh, S.; Prestat, E.; Huang, L.-F.; Rondinelli, J.M.; Haigh, S.J.; Rosen, B.A. Role of 2D and 3D defects on the reduction of LaNiO3 nanoparticles for catalysis. Sci. Rep. 2017, 7, 10080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Jia, L.; Luo, J.-L.; Chi, B.; Pu, J.; Li, J. CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 2020, 506, 144699. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3 Perovskite Catalysts for Boosting Dry Reforming of CH4 Using CO2 for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Vecino-Mantilla, S.; Gauthier-Maradei, P.; Huvé, M.; Serra, J.M.; Roussel, P.; Gauthier, G.H. Nickel Exsolution-Driven Phase Transformation from an n = 2 to an n = 1 Ruddlesden-Popper Manganite for Methane Steam Reforming Reaction in SOFC Conditions. ChemCatChem 2019, 11, 4631–4641. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. In Materials for Sustainable Energy; Co-Published with Macmillan Publishers Ltd.: London, UK, 2010; pp. 213–223. [Google Scholar]
- Zhu, J.; Zhang, W.; Li, Y.; Yue, W.; Geng, G.; Yu, B. Enhancing CO2 catalytic activation and direct electroreduction on in-situ exsolved Fe/MnOx nanoparticles from (Pr,Ba)2Mn2-yFeyO5 + δ layered perovskites for SOEC cathodes. Appl. Catal. B Environ. 2019, 268, 118389. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Han, H.; Chung, Y.S.; Yoon, W.; Choi, J.; Kim, W.B. In situ exsolved Co nanoparticles on Ruddlesden-Popper material as highly active catalyst for CO2 electrolysis to CO. Appl. Catal. B Environ. 2019, 248, 147–156. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Gao, D.; Song, Y.; Zhou, Y.; Liu, Q.; Wang, G.; Bao, X. In situ exsolved FeNi3 nanoparticles on nickel doped Sr2Fe1.5Mo0.5O6−δ perovskite for efficient electrochemical CO2 reduction reaction. J. Mater. Chem. A 2019, 7, 11967–11975. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. Highly Stable and Efficient Catalyst with In Situ Exsolved Fe–Ni Alloy Nanospheres Socketed on an Oxygen Deficient Perovskite for Direct CO2 Electrolysis. ACS Catal. 2016, 6, 6219–6228. [Google Scholar] [CrossRef]
- Kyriakou, V.; Neagu, D.; Zafeiropoulos, G.; Sharma, R.K.; Tang, C.; Kousi, K.; Metcalfe, I.S.; van de Sanden, M.C.M.; Tsampas, M.N. Symmetrical Exsolution of Rh Nanoparticles in Solid Oxide Cells for Efficient Syngas Production from Greenhouse Gases. ACS Catal. 2019, 1278–1288. [Google Scholar] [CrossRef]
- Bahout, M.; Managutti, P.; Dorcet, V.; la Salle, A.L.G.; Paofai, S.; Hansen, T.C. In situ exsolution of Ni particles on the PrBaMn2O5 SOFC electrode material monitored by high temperature neutron powder diffraction under hydrogen. J. Mater. Chem. A 2020, 8, 3590–3597. [Google Scholar] [CrossRef]
- Lee, J.G.; Myung, J.-H.; Naden, A.B.; Jeon, O.S.; Shul, Y.G.; Irvine, J.T.S. Replacement of Ca by Ni in a Perovskite Titanate to Yield a Novel Perovskite Exsolution Architecture for Oxygen-Evolution Reactions. Adv. Energy Mater. 2020, 1903693. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Zhang, X.; Zhang, H.; Yu, N.; Liu, T.; Wang, Y. Thermal Stability of an in Situ Exsolved Metallic Nanoparticle Structured Perovskite Type Hydrogen Electrode for Solid Oxide Cells. ACS Sustain. Chem. Eng. 2019, 7, 17834–17844. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Lei, Z.; Jin, C.; Liu, Y.; Wang, Y.; Peng, S. Co-substituted Sr2Fe1.5Mo0.5O6-δ as anode materials for solid oxide fuel cells: Achieving high performance via nanoparticle exsolution. J. Power Sources 2019, 438, 226989. [Google Scholar] [CrossRef]
- Wan, Y.; Xing, Y.; Xie, Y.; Shi, N.; Xu, J.; Xia, C. Vanadium-Doped Strontium Molybdate with Exsolved Ni Nanoparticles as Anode Material for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 42271–42279. [Google Scholar] [CrossRef]
- Jo, Y.-R.; Koo, B.; Seo, M.-J.; Kim, J.K.; Lee, S.; Kim, K.; Han, J.W.; Jung, W.; Kim, B.-J. Growth Kinetics of Individual Co Particles Ex-solved on SrTi0.75Co0.25O3-δ Polycrystalline Perovskite Thin Films. J. Am. Chem. Soc. 2019, 141, 6690–6697. [Google Scholar] [CrossRef]
- Qi, H.; Yang, T.; Li, W.; Ma, L.; Hu, S.; Shi, W.; Sabolsky, E.M.; Zondlo, J.W.; Hart, R.; Hackett, G.A.; et al. Reversible In-Situ Exsolution of Fe Catalyst in La0.5Sr1.5Fe1.5Mo0.5O6-δ Anode for SOFCs. ECS Trans. 2019, 91, 1701–1710. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; He, Z.; Meng, W.; Li, Y.; Wang, L. In situ exsolution of PdO nanoparticles from non-stoichiometric LaFePd0.05O3 + δ electrode for impedancemetric NO2 sensor. Sens. Actuators B Chem. 2019, 298, 126827. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; He, Z.; Meng, W.; Li, Y.; Wang, L. Enhancing NH3 sensing performance of mixed potential type sensors by chemical exsolution of Ag nanoparticle on AgNbO3 sensing electrode. Sens. Actuators B Chem. 2019, 298, 126854. [Google Scholar] [CrossRef]
- Kim, K.J.; Rath, M.K.; Kwak, H.H.; Kim, H.J.; Han, J.W.; Hong, S.-T.; Lee, K.T. A Highly Active and Redox-Stable SrGdNi0.2Mn0.8O4 ± δ Anode with in Situ Exsolution of Nanocatalysts. ACS Catal. 2019, 9, 1172–1182. [Google Scholar] [CrossRef]
- Han, H.; Park, J.; Nam, S.Y.; Kim, K.J.; Choi, G.M.; Parkin, S.S.P.; Jang, H.M.; Irvine, J.T.S. Lattice strain-enhanced exsolution of nanoparticles in thin films. Nat. Commun. 2019, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hong, T.; Cheng, J.; Chen, Z.; Xu, C. Enhanced proton conductivity of La0.95Ca0.05NixNb1-xO4 (0.01 ≤ x ≤ 0.3) through in-situ exsolution of metallic nanocatalysts. Mater. Chem. Phys. 2018, 208, 226–236. [Google Scholar] [CrossRef]
- Tan, J.; Lee, D.; Ahn, J.; Kim, B.; Kim, J.; Moon, J. Thermally driven in situ exsolution of Ni nanoparticles from (Ni, Gd)CeO2 for high-performance solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 18133–18142. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Yang, Y.-L.; Chen, J.; Li, M.; Zhang, Y.-Q.; Li, J.-H.; Hua, B.; Luo, J.-L. Toward a rational photocatalyst design: A new formation strategy of co-catalyst/semiconductor heterostructures via in situ exsolution. Chem. Commun. 2018, 54, 1505–1508. [Google Scholar] [CrossRef]
- Arandiyan, H.; Wang, Y.; Scott, J.; Mesgari, S.; Dai, H.; Amal, R. In Situ Exsolution of Bimetallic Rh–Ni Nanoalloys: A Highly Efficient Catalyst for CO2 Methanation. ACS Appl. Mater. Interfaces 2018, 10, 16352–16357. [Google Scholar] [CrossRef]
- Kwak, N.W.; Jeong, S.J.; Seo, H.G.; Lee, S.; Kim, Y.; Kim, J.K.; Byeon, P.; Chung, S.-Y.; Jung, W. In situ synthesis of supported metal nanocatalysts through heterogeneous doping. Nat. Commun. 2018, 9, 4829. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.; Sengodan, S.; Kim, K.; Kim, G.; Jeong, H.Y.; Shin, J.; Ju, Y.-W.; Han, J.W.; Kim, G. Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat. Commun. 2017, 8, 15967. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhou, W.; Ran, R.; Chen, Y.; Shao, Z.; Liu, M. Promotion of Oxygen Reduction by Exsolved Silver Nanoparticles on a Perovskite Scaffold for Low-Temperature Solid Oxide Fuel Cells. Nano Lett. 2016, 16, 512–518. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhang, Y.-Q.; Chen, J.; Li, J.-H.; Zhu, Y.-T.; Zeng, Y.-M.; Amirkhiz, B.S.; Li, J.; Hua, B.; Luo, J.-L. New Opportunity for in Situ Exsolution of Metallic Nanoparticles on Perovskite Parent. Nano Lett. 2016, 16, 5303–5309. [Google Scholar] [CrossRef]
- Fuertes, A. Chemistry and applications of oxynitride perovskites. J. Mater. Chem. 2012, 22, 3293–3299. [Google Scholar] [CrossRef]
- Yang, M.; Oró-Solé, J.; Rodgers, J.A.; Jorge, A.B.; Fuertes, A.; Attfield, J.P. Anion order in perovskite oxynitrides. Nat. Chem. 2011, 3, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padi, S.P.; Shelly, L.; Komarala, E.P.; Schweke, D.; Hayun, S.; Rosen, B.A. Coke-free methane dry reforming over nano-sized NiO-CeO2 solid solution after exsolution. Catal. Commun. 2020, 138, 105951. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.; Saccoccio, M.; Lu, Z.; Ciucci, F. From material design to mechanism study: Nanoscale Ni exsolution on a highly active A-site deficient anode material for solid oxide fuel cells. Nano Energy 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Singh, S.; Zubenko, D.; Rosen, B.A. Influence of LaNiO3 Shape on Its Solid-Phase Crystallization into Coke-Free Reforming Catalysts. ACS Catal. 2016, 6, 4199–4205. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.; Zhao, P.; Chen, C.; Li, X.; Meng, J.; Liu, G.; Chen, L.; Wan, H.; Guan, G. In situ exsolution of Co/CoOx core-shell nanoparticles on double perovskite porous nanotubular webs: A synergistically active catalyst for soot efficient oxidation. Chem. Eng. J. 2019, 372, 752–764. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Zhang, Y.-Q.; Hua, B.; Luo, J.-L. Bifunctional Catalyst of Core–Shell Nanoparticles Socketed on Oxygen-Deficient Layered Perovskite for Soot Combustion: In Situ Observation of Synergistic Dual Active Sites. ACS Catal. 2016, 6, 2710–2714. [Google Scholar] [CrossRef]
- Ruban, A.V.; Skriver, H.L.; Nørskov, J.K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59, 15990–16000. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Foucher, A.C.; Ji, Y.; Curran, C.D.; Stach, E.A.; McIntosh, S.; Gorte, R.J. “Intelligent” Pt Catalysts Studied on High-Surface-Area CaTiO3 Films. ACS Catal. 2019, 9, 7318–7327. [Google Scholar] [CrossRef]
- Lai, K.-Y.; Manthiram, A. Evolution of Exsolved Nanoparticles on a Perovskite Oxide Surface during a Redox Process. Chem. Mater. 2018, 30, 2838–2847. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, Z.; Sun, Y.; Wang, X.; Huang, K.; Cong, Y.; Shi, F.; Wang, Y.; Zhang, W.; Feng, S. Highly Efficient B-Site Exsolution Assisted by Co Doping in Lanthanum Ferrite toward High-Performance Electrocatalysts for Oxygen Evolution and Oxygen Reduction. ACS Sustain. Chem. Eng. 2019, 8, 302–310. [Google Scholar] [CrossRef]

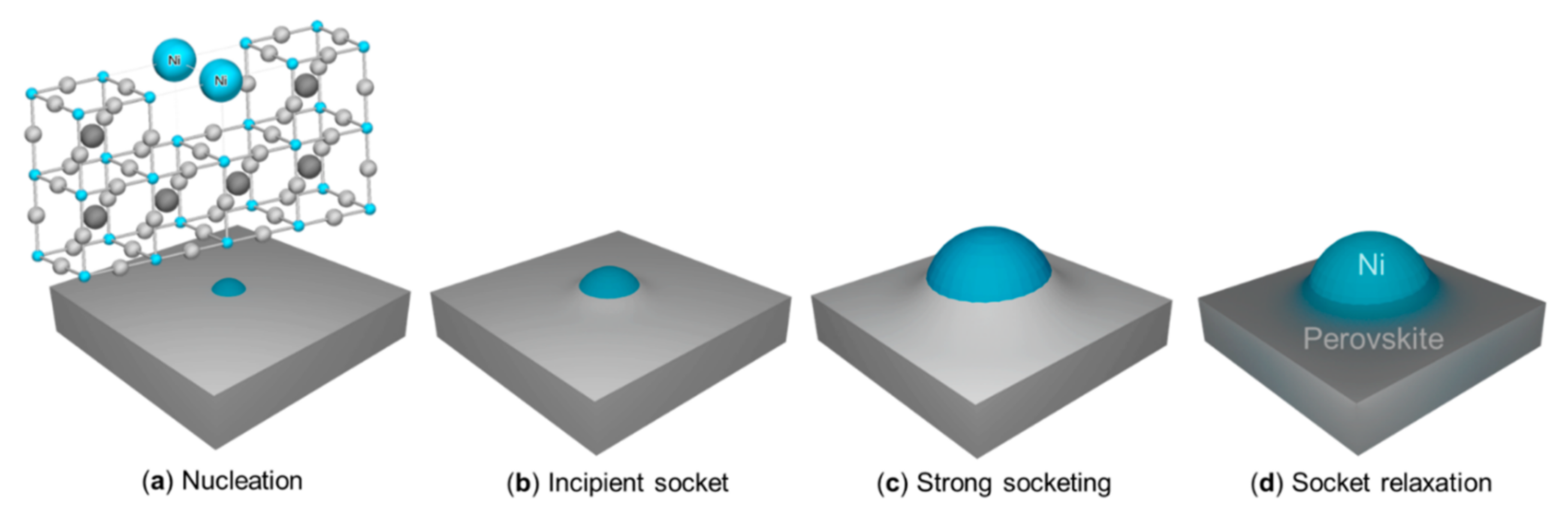
| Material | System | Notes | Year | Ref |
|---|---|---|---|---|
| Pr0.65Ba0.35Mn0.975Ni0.025O3 | H2 SOFC | In-situ neutron diffraction of Ni exsolution process | 2020 | [53] |
| CaTi0.94Ni0.04O3−δ | Oxygen reduction | A-site exsolution | 2020 | [54] |
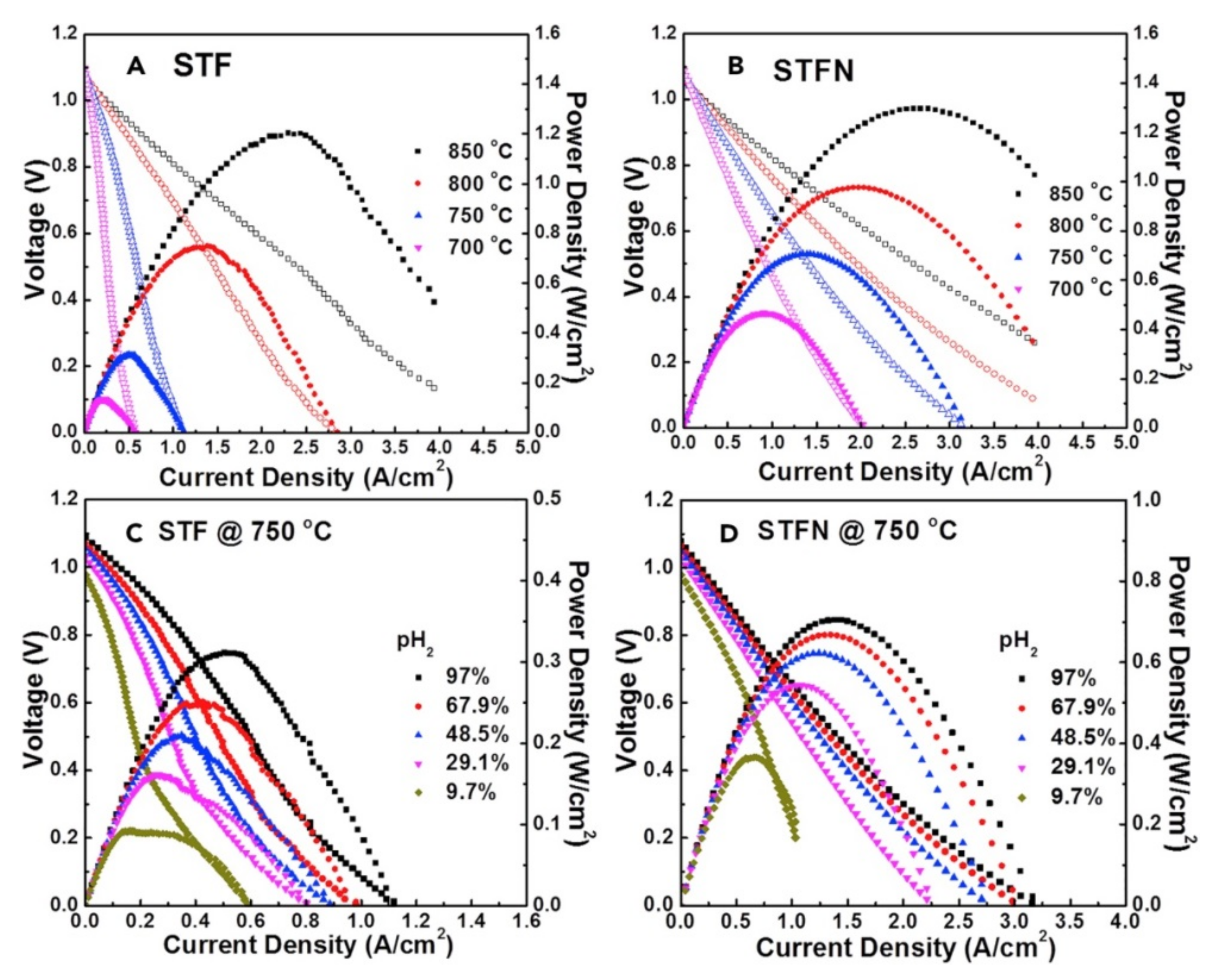
| Sr0.95(Ti0.3Fe0.63Ni0.07)O3−δ | H2 SOFC | Effect of non-stoichiometry in Sr(Ti,Fe,Ni)O3 | 2019 | [34] |
| (Pr,Ba)2Mn2-yFeyO5+δ | CO2 SOEC | Exsolution of Fe/MnOx for CO2 reduction | 2019 | [48] |
| Sr2CoMo0.95Fe0.05O6−δ | H2/CH4 SOFC | Twinning in Co-Fe exsolution | 2019 | [40] |
| Sr2Fe1.4Ni0.1Mo0.5O6 | H2 SOFC | Thermal stability of Ni exsolution | 2019 | [55] |
| Sr2Fe1.5Mo0.5O6−δ | H2 SOFC | Co exsolution from double-perovskites | 2019 | [56] |
| SrV0.5Mo0.5Ni0.1O4−δ | H2 SOFC | B-site excess doping | 2019 | [57] |
| La1.5Sr1.5Mn1.5Ni0.5O7±δ | H2 SOFC | Exsolution in Ruddlesden Popper Phases | 2019 | [46] |
| SrTi0.75Co0.25O3−δ | CO oxidation | Particle density and growth kinetics | 2019 | [58] |
| La0.5Sr1.5Fe1.5Mo0.5O6−δ | H2 SOFC | Reversible Fe exsolution | 2019 | [59] |
| La0.6Sr0.4Co0.7Mn0.3O3 | CO2 SOEC | Exsolution in Ruddlesden Popper Phases | 2019 | [49] |
| La0.43Ca0.37Ni0.06Ti0.94O3 | Fundamental | In-situ TEM of Ni exsolution | 2019 | [22] |
| LaFePd0.05O3+δ | CO sensor | Pd exsolution | 2019 | [60] |
| AgNbO3 | NH3 sensor | Ag exsolution | 2019 | [61] |
| SrGdNi0.2Mn0.8O4±δ | H2 SOFC | Improved redox stability | 2019 | [62] |
| La0.2Sr0.7Ni0.1Ti0.9O3−δ | Fundamental | Strain enhanced exsolution | 2019 | [63] |
| Sr0.95(Ti0.3Fe0.63Ni0.07)O3 | H2 SOFC | High current density | 2018 | [33] |
| La0.95Ca0.05NixNb1−xO4 | H2 SOFC | Enhanced proton conductivity | 2018 | [64] |
| (Gd0.2−xNixCe0.8O2−δ | H2 SOFC | Exsolution in Gd doped perovskites | 2018 | [65] |
| SrTiWO3 | H2 Production | Exsolution in photocatalysis | 2018 | [66] |
| Rh/3DOM LaNi0.08Al0.92O3 | CO2 reduction | Rh-Ni exsolution for methanation | 2018 | [67] |
| Many | Co oxidation | Predetermined location | 2018 | [68] |
| LaNiO3 | Fundamental | Role of extended defects | 2017 | [43] |
| Pr0.5Ba0.5Mn0.85T0.15O3-δ | H2 SOFC | Exsolution in layered perovskites | 2017 | [69] |
| Sr0.95Ag0.05Nb0.1Co0.9O3−δ | LT-SOFC | Oxygen reduction at low temperature | 2016 | [70] |
| Co-doped Pr0.5Ba0.5MnOx | SOFC/SOEC | High population, dual use | 2016 | [71] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosen, B.A. Progress and Opportunities for Exsolution in Electrochemistry. Electrochem 2020, 1, 32-43. https://doi.org/10.3390/electrochem1010004
Rosen BA. Progress and Opportunities for Exsolution in Electrochemistry. Electrochem. 2020; 1(1):32-43. https://doi.org/10.3390/electrochem1010004
Chicago/Turabian StyleRosen, Brian A. 2020. "Progress and Opportunities for Exsolution in Electrochemistry" Electrochem 1, no. 1: 32-43. https://doi.org/10.3390/electrochem1010004
APA StyleRosen, B. A. (2020). Progress and Opportunities for Exsolution in Electrochemistry. Electrochem, 1(1), 32-43. https://doi.org/10.3390/electrochem1010004





