1. Introduction
Magnetic nanoparticles (MNPs) of magnetite and maghemite (Fe
3O
4, γ-Fe
2O
3) in aqueous solution are called ferrofluids, which have colloidal properties of interest for biomedical applications. They also have magnetic properties very similar to NPM powder [
1,
2]. In recent years, researchers have shown growing interest in the magneto-optical properties of ferrofluids, especially because of their potential in innovative technologies like tunable photonic devices, holographic tweezers, and smart sensors [
3].
Understanding the structural, magnetic, morphological, and optical characteristics of MNPs is essential for identifying the most suitable applications for these nanoparticles. Some of the key advantages of these materials include their compatibility with biological systems, their ability to break down naturally in the body, and their generally low toxicity. These qualities make them highly suitable for a variety of biomedical uses, including magnetic labeling of cells, cancer hyperthermia, phototherapy, targeted drug delivery, and as contrast agents in magnetic resonance imaging [
4,
5,
6,
7,
8,
9,
10].
The study of light transmittance through ferrofluids is of great interest both experimentally and theoretically. The first studies reported were by Philip et al., in 2008 [
11], who studied the magneto-optic effects of magnetic nanoparticles in the ferrofluid. Their results showed that the intensity of light transmitted in ferrofluids would decline through a magnetic field where the incident light goes along the orientation of the field. It was also observed that the transmitted intensity recovered to its initial phase while the external field disappeared. Based on a detailed analysis of the results, the authors suggested that the intriguing effects observed were likely caused by resonances occurring within the embedded nanoparticles [
12,
13].
Numerous studies have explored the fascinating interplay between magnetic fields and light transmission in ferrofluids, revealing promising avenues for both fundamental science and biomedical applications. For instance, Rablau et al. (2008) [
14] investigated how light intensity changes over time as it passes through a ferrofluid under a magnetic field. They observed that the transmitted light initially drops sharply to zero—due to the rapid formation of chain-like aggregates of nanoparticles aligned with the field—and then gradually increases as the system reaches equilibrium, a behavior driven by dynamic light scattering processes. Building on this, Philip and Laskar (2012) [
15] reviewed the diverse optical properties of ferrofluids. They emphasized their potential in biomedical fields, particularly in imaging and targeted therapy, due to their tunable optical response under magnetic stimuli.
More recent contributions have expanded our understanding of this magneto-optical phenomenon. Vales-Pinzón et al. (2014) [
16] demonstrated that adding carbon nanotubes to ferrofluids enhances polarized light transmission under magnetic influence, indicating a potential route for improving anisotropic optical materials. Shulyma and colleagues (2016) [
17] reported a striking inversion of extinction trends in ferrofluids when exposed to magnetic fields, revealing a complex balance between particle interactions and field strength. Jin et al. (2018) [
18] further analyzed the wavelength-dependent scattering behavior of ferrofluids, showing how external fields modulate light diffusion in real-time, which is crucial for optical device design. Finally, Lakic et al. (2019) [
19] provided compelling optical evidence for magnetic-field-induced aggregation in different ferrite-based ferrofluids, comparing cobalt ferrite, magnetite, and magnesium ferrite systems. Their findings underscore how the composition of nanoparticles influences their collective optical behavior, particularly under external stimuli. Together, these studies underscore the dynamic and responsive nature of ferrofluids, laying the groundwork for their integration into smart optical systems, biosensing platforms, and magnetically controlled biomedical devices.
MNPs are used to create ferrofluids (FFs) of nanoparticles as polar or non-polar liquid carriers, having the property of magnetism as characteristics. Ferrofluids exhibit properties such as optical anisotropy, birefringence in the presence of a magnetic field and field-dependent transmission. All these properties make the study of FFs of great interest for developing optoelectronic devices with various applications, mainly in the biomedical area [
20].
Although numerous studies have examined the optical applications of ferrofluids, the magneto-optic interactions—particularly those involving nanoparticle aggregation—remain insufficiently explored. Similarly, the influence of different types of coatings on magnetic nanoparticles has not been extensively investigated.
In this study, we systematically investigate the influence of chitosan surface functionalization on the physicochemical, magnetic, and optical properties of magnetite nanoparticles (MNPs), with a particular emphasis on their magneto-optical behavior and suitability for biomedical applications. Chitosan, a biocompatible and biodegradable polysaccharide, was selected as the coating agent due to its proven ability to stabilize nanoparticle suspensions and modulate interfacial interactions under physiological conditions.
We hypothesize that the incorporation of a chitosan shell will mitigate surface oxidation, preserve the superparamagnetic behavior, and induce a measurable shift in optical absorption characteristics due to changes in surface energy and particle–matrix interactions. These modifications are anticipated to enhance the functional performance of the resulting ferrofluids, positioning them as promising candidates for applications such as magnetic hyperthermia, biosensing, and targeted drug delivery.
2. Materials and Methods
2.1. Synthesis of the Magnetic Nanoparticles
The method of co-precipitation in situ used in this work to obtain magnetic nanoparticles was detailed by Guzman-Rocha et al., 2022 [
21]. Magnetic nanoparticles (MNPs) were synthesized via a co-precipitation method using stoichiometric amounts of FeCl
3 (0.4 M, 11 mL) and FeCl
2 (0.2 M, 11 mL) dissolved in 100 mL of deionized water containing 10 mL of a 10%
w/
w chitosan solution. The chitosan used had a medium molecular weight (approximately 190–310 kDa) and a degree of deacetylation of about <75% (Sigma-Aldrich, Saint Louis, MO, USA). These characteristics were selected based on prior work by our research group [
22], where chitosan with similar specifications has been shown to provide effective steric and electrostatic stabilization, enhancing colloidal dispersion and maintaining biocompatibility in magnetite-based nanomaterials. The mixture was stirred for 5 min at room temperature and then slowly poured into 19 mL of 0.3 M ammonium hydroxide under vigorous stirring. The reaction was conducted under an inert atmosphere and the temperature was gradually increased to 90 °C and maintained for 90 min. During the reaction, a black precipitate formed, indicating the generation of magnetite (Fe
3O
4) nanoparticles. The resulting ferrofluid was washed three times with deionized water, separated by magnetic decantation, and stored in aqueous suspension. In the case of chitosan-coated nanoparticles (MNP-Q), the presence of low-molecular-weight chitosan in the reaction medium facilitated polymer adsorption onto the surface of the forming nanoparticles, enhancing their stability and biocompatibility.
2.2. Characterization Structural and Morphological
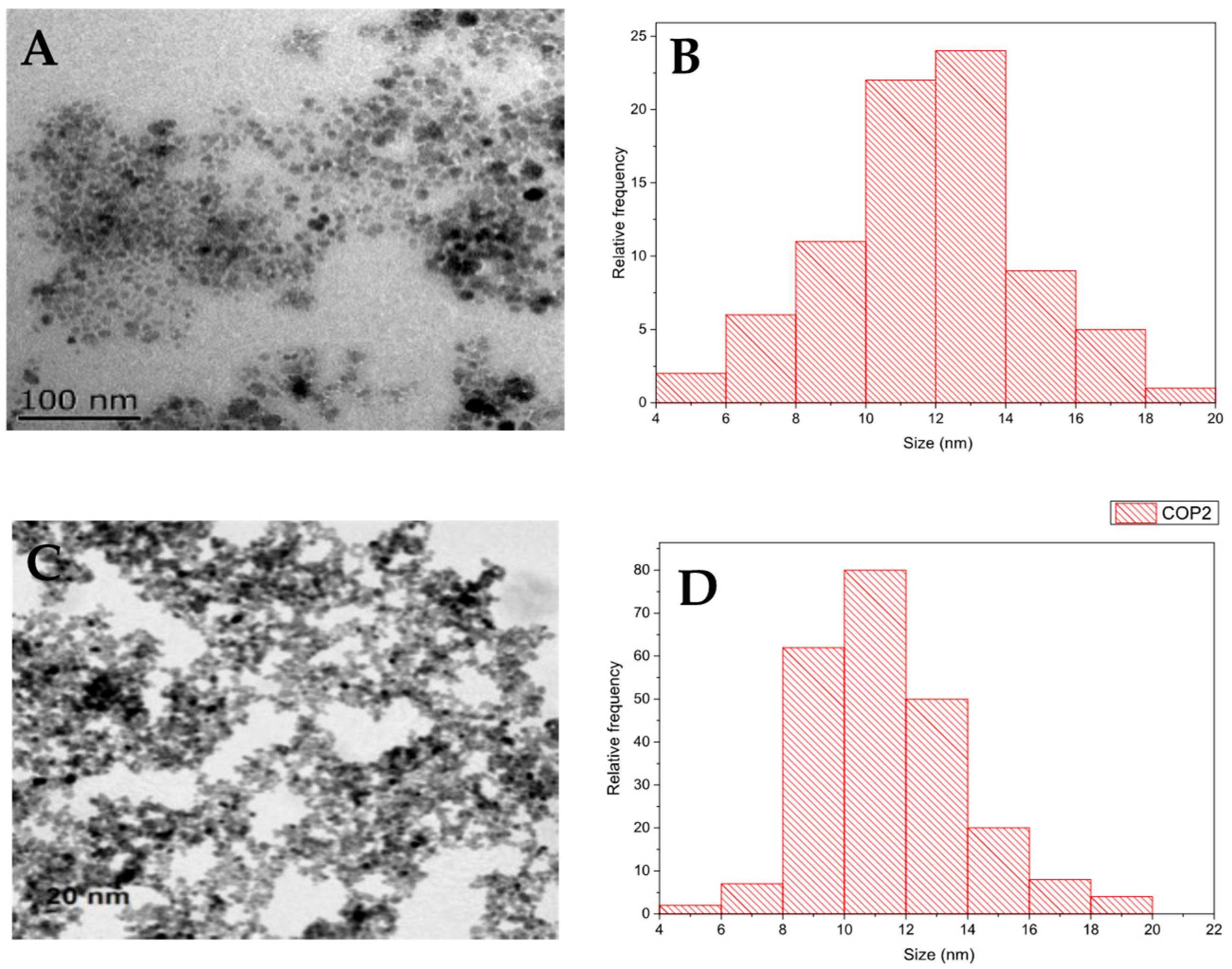
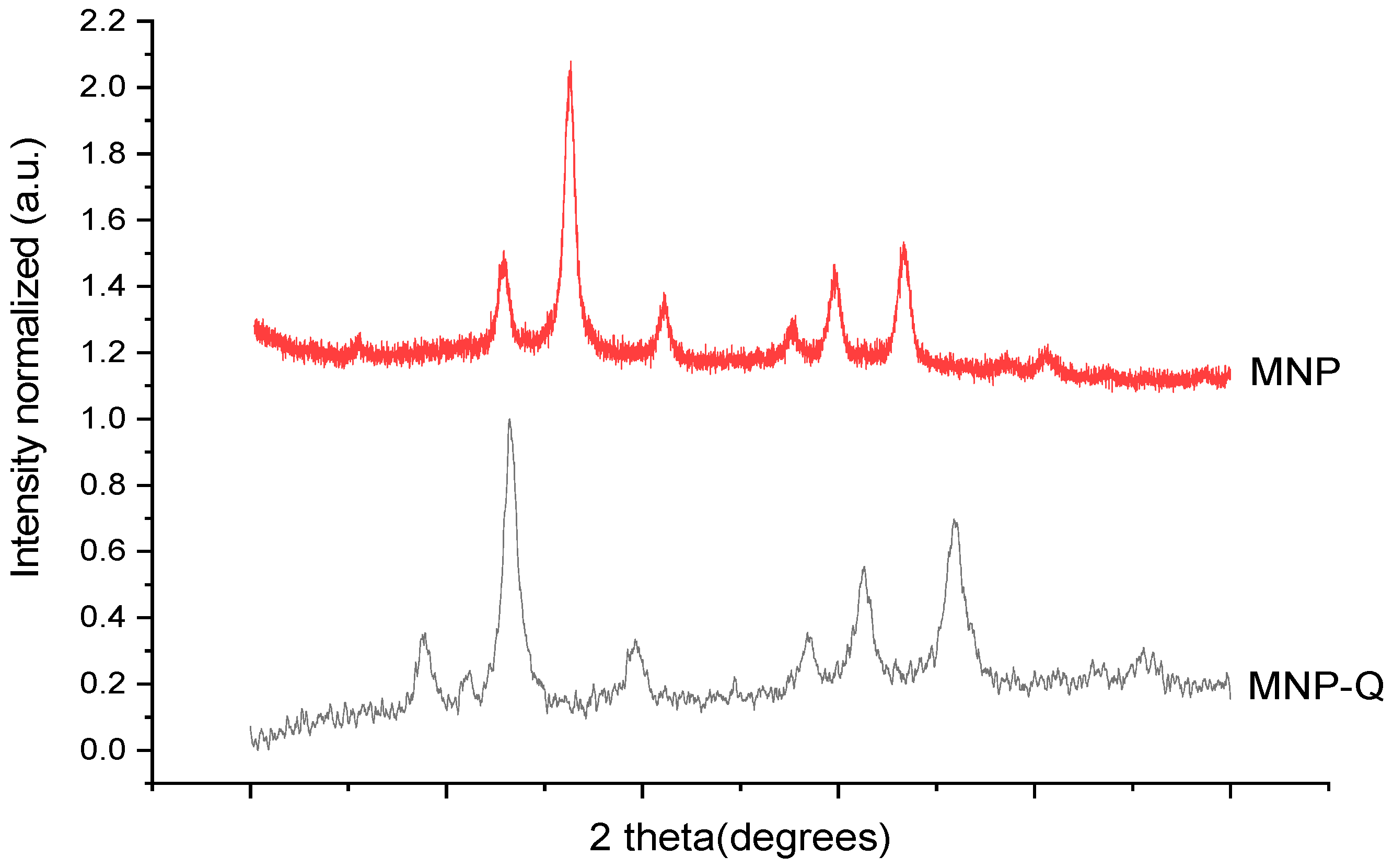
The crystalline structure of the MNP and MNP-Q samples was identified by X-ray powder diffraction (XRD) performed in a Bruker D8 Advance powder diffractometer (Billerica, MA, USA) using Cu Kα radiation. The patterns were recorded between 20° and 80°. For this analysis, part of the sample is dried at 50 °C for 24 h, and then 40 mg is placed in the sample holder of the X-ray diffraction equipment. Particle size and shape were determined by transmission electron microscopy (TEM) using a JEOL-JEM1010 (100 keV) microscope (JEOL, Tokyo, Japan), with a digital Camera Gatan SC200 (Gatan, Pleasanton, CA, USA). To determine the average particle size and distribution, the longest internal dimension of at least 200 individual nanoparticles was measured using the ImageJ software (version 1.54p, National Institutes of Health, Bethesda, MD, USA).
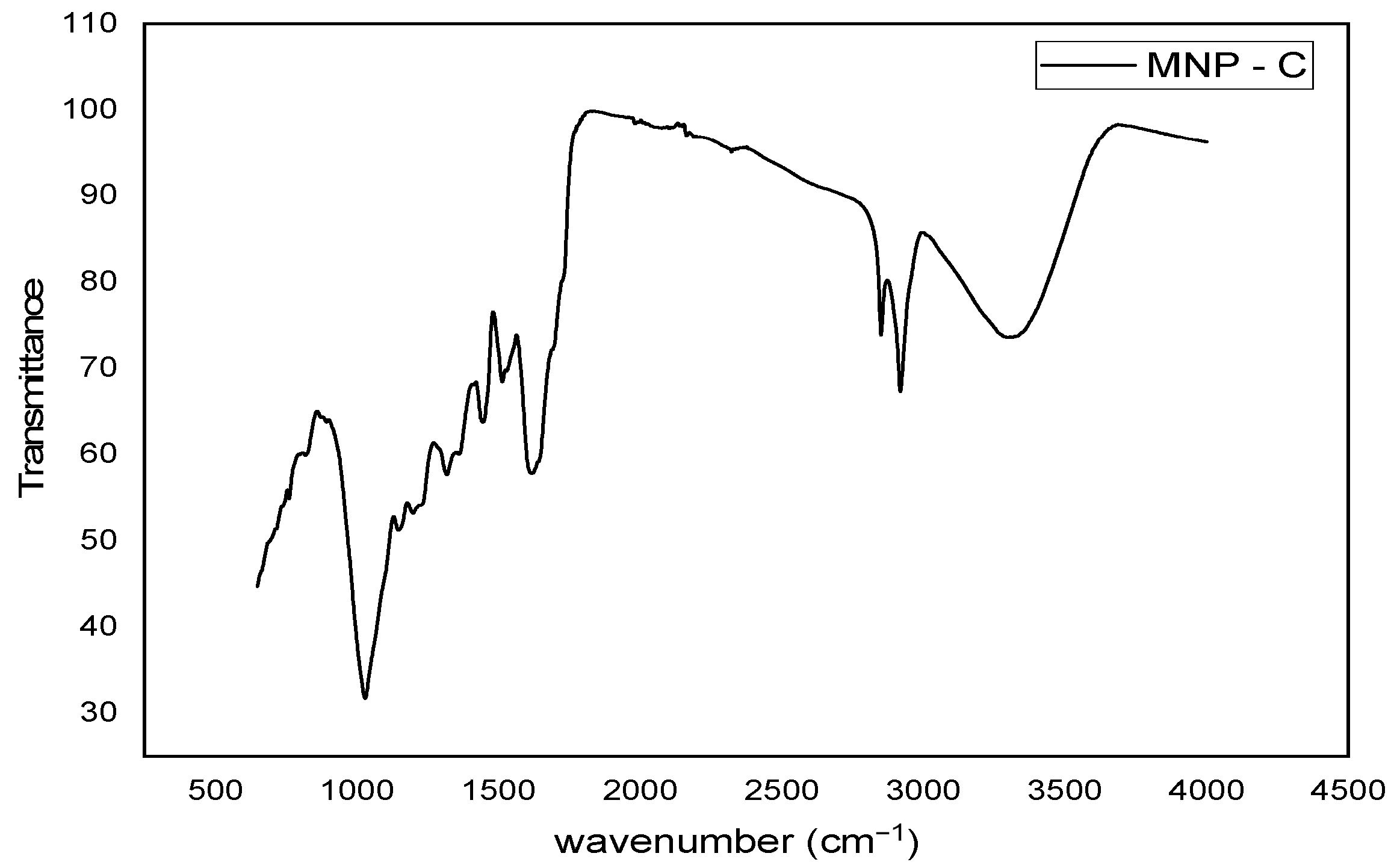
2.3. Infrared Spectroscopy with Fourier Transform (FTIR)
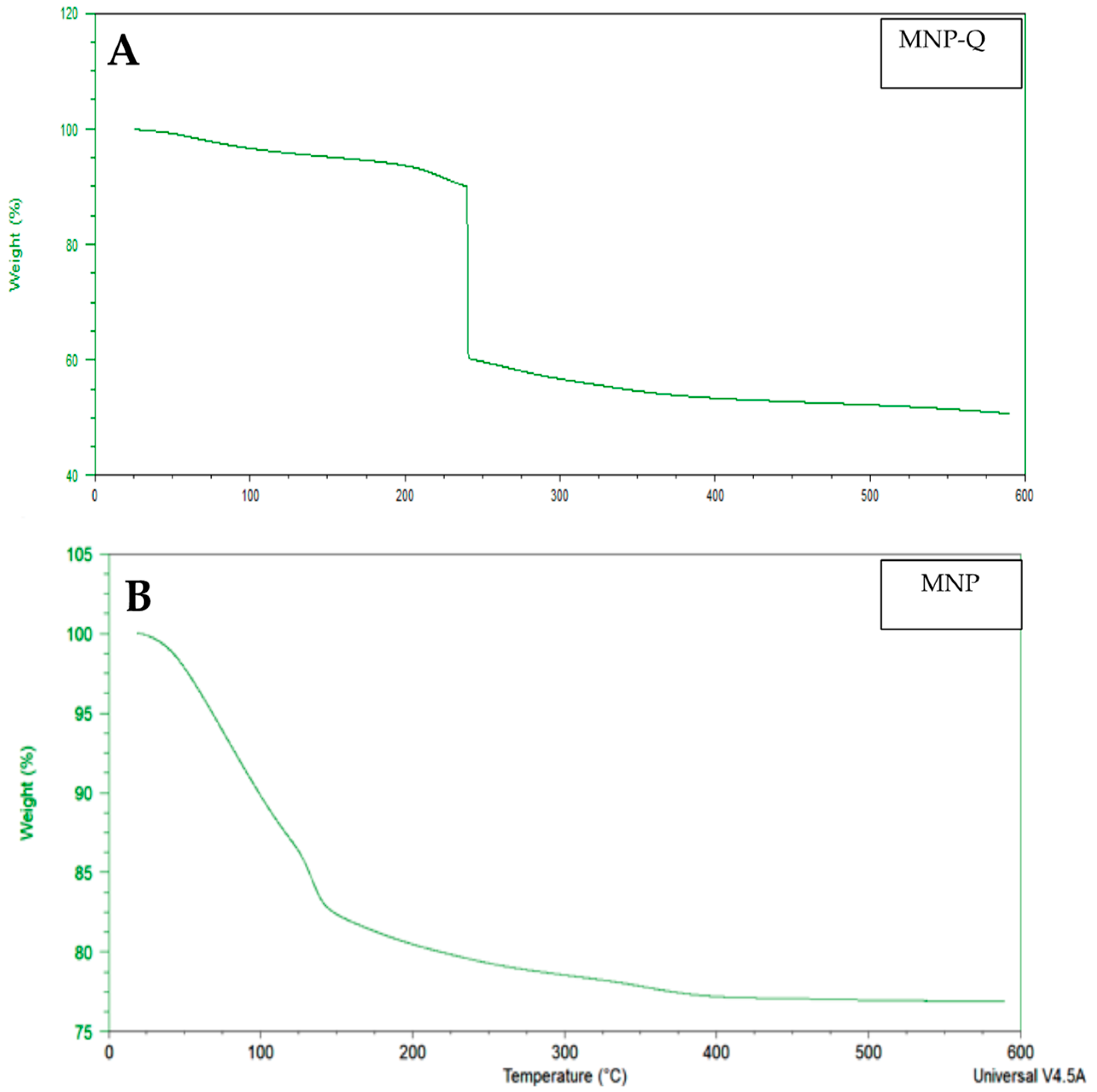
The FTIR was used, utilizing an ATR (Attenuated Total Reflection) accessory to evaluate the interaction between the sample and infrared radiation. The samples were desiccated at 50 °C to subtract excess water for analysis. The FTIR spectra were logged in transmittance units within the 4000–400 cm−1 range in a Bruker Vertex 70 V spectrometer (Billerica, MA, USA). At the same time, thermogravimetric (TG) analysis and differential thermal analysis were performed on instrument SDT Q600 (New Castle, De, USA). Samples were heated from room temperature to 900 °C at 10 °C/min under an air flow of 100 mL/min.
2.4. Magnetization Measurements
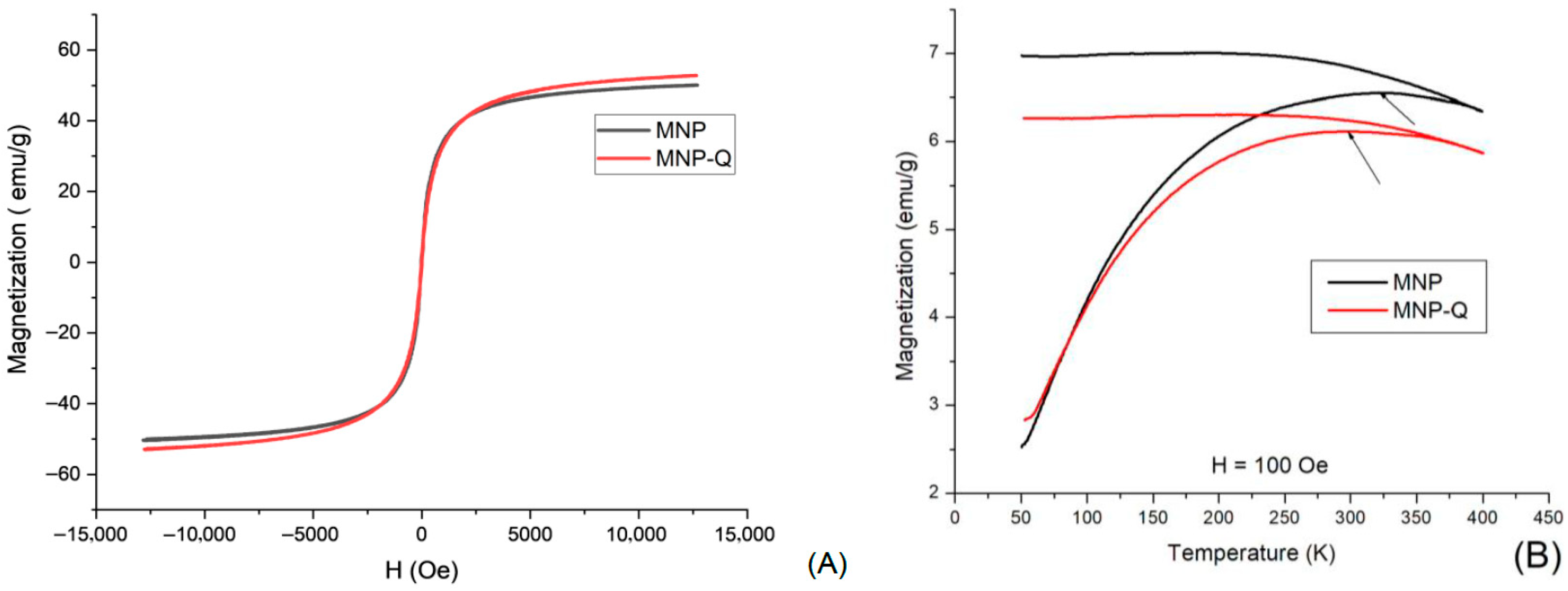
Magnetic properties of the samples were evaluated at room temperature using a vibrating sample magnetometer (VSM) system (Quantum Design VersaLab and MagLabVSM, Oxford Instruments, Abingdon, Oxfordshire, UK). Powder samples of MNP and MNP-Q were pre-dried at 50 °C for 24 h, accurately weighed, and compacted into sample holders to ensure reproducibility and comparability. Hysteresis curves (M vs. H) were recorded at 290 K, with applied magnetic fields ranging from −12,500 to +12,500 Oe for general characterization, and up to ±15,000 Oe in selected tests. This allowed for a detailed analysis of the superparamagnetic behavior before and after chitosan grafting. Additionally, measurements of magnetization covering the temperature interval from 50 to 400 K were performed, following the ZFC/FC protocol and using 100 Oe of static magnetic field.
2.5. Optical Measurements
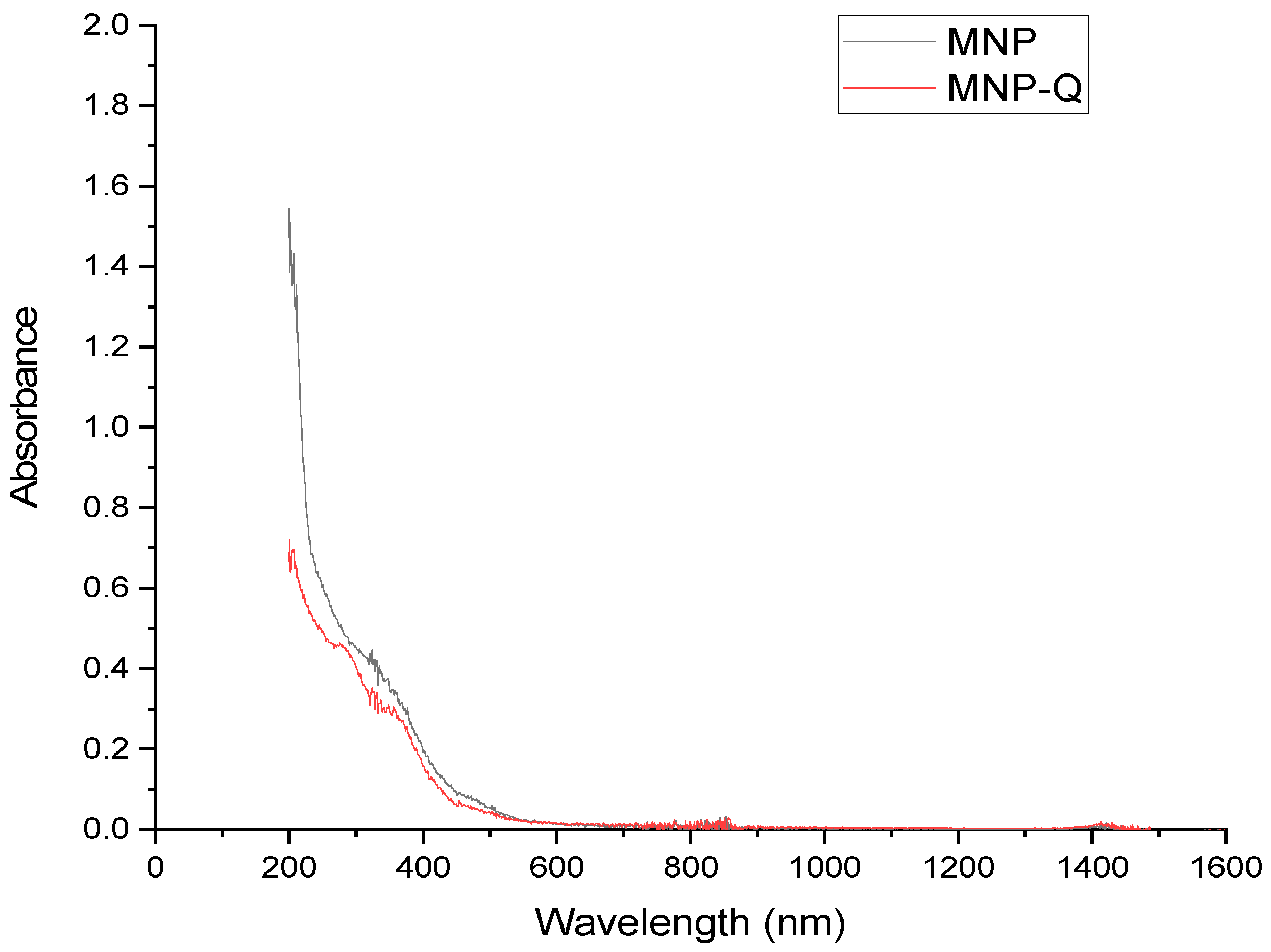
The optical properties of the magnetite nanoparticles (MNP) and chitosan-coated magnetite nanoparticles (MNP-Q) were characterized using a PerkinElmer Lambda 900 UV-Vis-NIR spectrophotometer (Waltham, MA, USA). Absorbance spectra were collected in the spectral range of 200–1600 nm with a resolution of 1 nm, at room temperature. Before measurement, aqueous suspensions of both samples were prepared and placed in 1 cm path length quartz cuvettes to ensure consistency and minimize scattering effects.
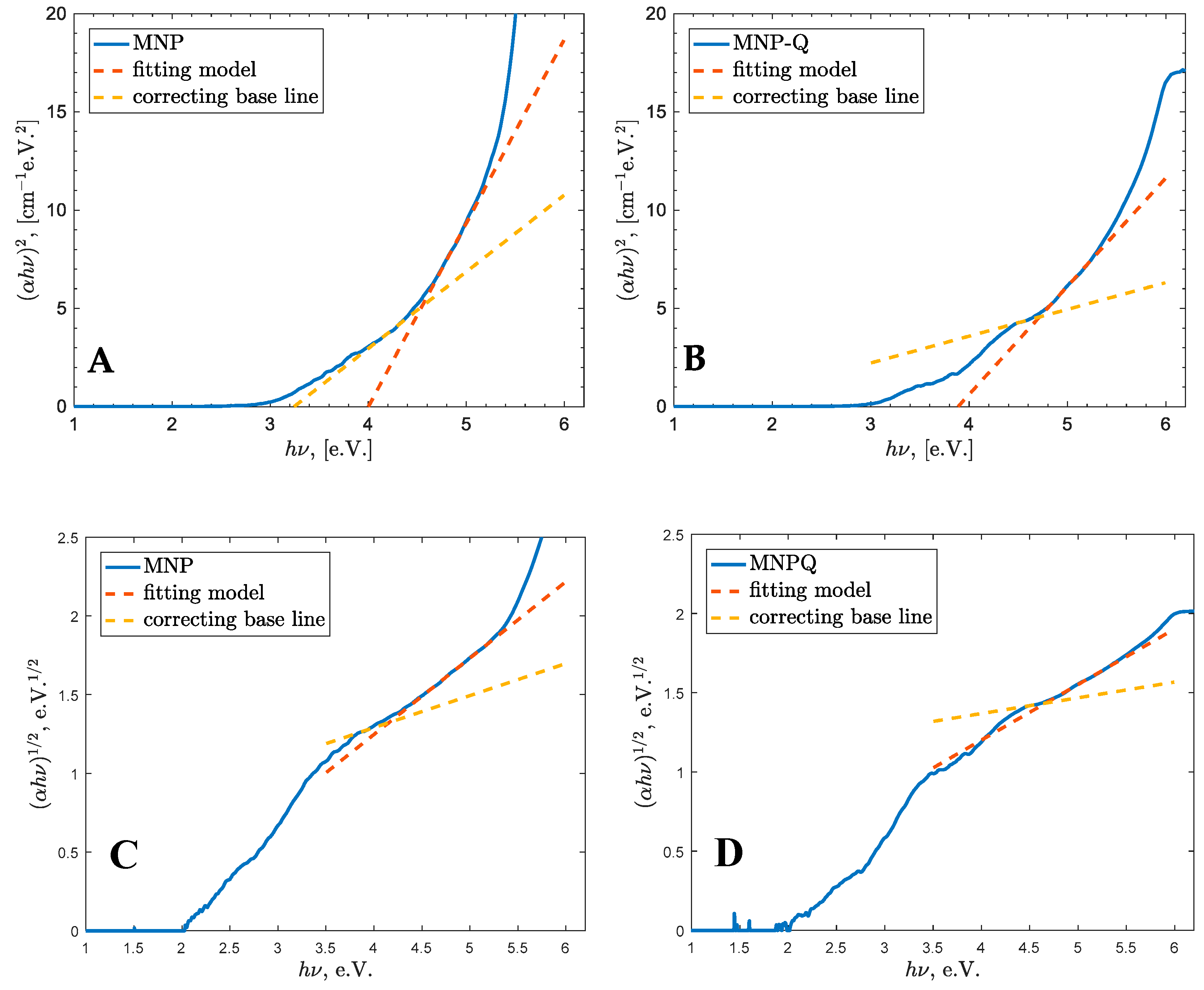
The optical band gap energy (Eg) was estimated using the Tauc plot method, and direct and indirect electronic transitions were present (UV-VIS, Thermo Fisher, Waltham, MA, USA). The absorption coefficient (α) was calculated from the absorbance data, and (αhν)2 was plotted against the photon energy (hν), for direct and indirect electronic transition, respectively. The Eg value for each sample was determined by extrapolating the linear region of the curve to the crossing of a corrective base line (OriginPro 2022, OriginLab Corporation, Northampton, MA, USA).
This analysis allowed for a comparison of the electronic structures of MNP and MNP-Q and an assessment of how chitosan coating influences the optical band gap through surface modifications and changes in nanoparticle dispersion.
4. Discussion
The synthesis of magnetic nanoparticles coated with chitosan (MNP-Q) demonstrated a successful approach to modulating both magnetic and optical properties of ferrofluids for potential biomedical applications. The structural analysis via XRD confirmed that both MNPs and MNP-Qs retained the typical spinel structure of magnetite, indicating that the chitosan coating does not disrupt the crystalline framework [
23]. TEM analysis showed spherical morphology in both samples, with a slight reduction in average size for MNP-Qs, likely due to steric stabilization during nucleation provided by the chitosan matrix [
21].
Thermogravimetric analysis revealed a significant mass loss (~40%) in the MNP-Q sample, confirming the presence of an organic chitosan layer. In contrast, the uncoated MNP sample exhibited minimal loss, attributable mainly to adsorbed moisture. This supports the effectiveness of chitosan in modifying surface chemistry and enhancing thermal responsiveness [
5,
20].
Magnetic measurements revealed that both samples exhibit superparamagnetic behavior, characterized by negligible magnetic remanence and coercive magnetic field and confirmed by the determination of the blocking temperatures (T
B). Whereas the uncoated MNP exhibits T
B = 321 K, this temperature was diminished to T
B = 299 K, due to the cover of chitosan. The saturation magnetization of 53 emu/g in MNP-Q was slightly higher than that of bare MNPs (51 emu/g), possibly due to the chitosan shell preventing surface oxidation and preserving the magnetic domains [
6,
7,
21]. The differences in magnetic saturation, remanence, and coercivity observed in the MNP-Q sample compared to the uncoated MNPs are attributed to the interaction with the chitosan biopolymer. Through electrostatic interactions involving hydroxyl and amino functional groups, chitosan enhances nanoparticle stabilization within the ferrofluid, which in turn contributes to the observed increase in magnetic properties. In simpler terms, surfactants, polymers, or biopolymers can stick to the surface of magnetic nanoparticles either chemically or physically, forming one or two protective layers around them. These layers help create a kind of “personal space” for each nanoparticle, preventing them from clumping together—mainly through a force called steric repulsion. This keeps the nanoparticles well-dispersed in liquid suspensions, which is essential for their effectiveness. Thanks to these properties, these nanoparticles are especially promising for medical uses, such as delivering drugs directly to specific areas of the body, heating tumors through magnetic hyperthermia, or serving as contrast agents in magnetic resonance imaging (MRI) [
4,
8,
9].
In terms of optical properties, UV-Vis spectra showed strong absorption in the UV region for both samples, with lower absorbance in the MNP-Q sample. This attenuation is likely due to changes in the refractive index and reduced aggregation caused by the chitosan shell [
15,
17]. The reduced absorption is also favorable for applications where optical transparency or tunability is desired, such as in biosensors or optical switches [
3,
16].
Band gap energy analysis using Tauc plots demonstrated a slight blue shift in MNP-Q (4.72 eV) compared to MNP (4.55 eV), indicating a modification of the electronic structure induced by surface functionalization and MNP size reduction. This result is consistent with previous reports where polymer coatings influenced surface states and quantum confinement effects in iron oxide nanoparticles [
13,
18,
19]. The increase in E
g may also suggest that chitosan limits the formation of smaller nanoparticles (<10 nm), which are formed due to fragmentation during collisions events.
Overall, these results confirm that chitosan acts not only as a stabilizing biopolymer but also as a modulator of magnetic and optical behavior, enhancing the biocompatibility and application range of the ferrofluids [
10,
11,
12,
20]. The results further support the hypothesis that coating magnetic nanoparticles (MNPs) with chitosan modifies their physicochemical, magnetic, and optical properties without altering their crystalline structure. TEM images show preserved spherical morphology with a slight size reduction in MNP-Q, while XRD analysis confirms the retention of the typical spinel structure in both samples. FTIR and TGA analyses verify the presence of the chitosan coating, and magnetization curves indicate superparamagnetic behavior, with a slight increase in saturation magnetization in MNP-Q. Additionally, UV-Vis spectacle reveals reduced absorbance in the coated sample, and Tauc plot analysis demonstrates a modest increase in band gap energy (
Eg), attributed to surface modification and MNP size reduction. Together, these findings highlight the potential of the MNP-Q system for magneto-optical biomedical applications, including biosensors, smart drug delivery systems, and optical contrast agents.
The broad versatility of chitosan-coated magnetite nanoparticles (MNPs) enables their integration into multiple biomedical applications due to the combined advantages of magnetic responsiveness, biocompatibility, and tunable surface chemistry. In magnetic hyperthermia, the superparamagnetic nature of the coated MNPs allows localized heat generation under an alternating magnetic field, potentially enhancing cancer treatment while minimizing damage to surrounding tissues [
24]. In targeted drug delivery, the chitosan shell provides reactive amine and hydroxyl groups for covalent or electrostatic binding of therapeutic molecules, while the magnetic core enables remote guidance to specific anatomical sites [
25]. For biosensing, chitosan-coated MNPs can immobilize biomolecules such as enzymes, antibodies, or nucleic acids, enhancing detection sensitivity through magnetic concentration and improved signal transduction [
26]. Additionally, the coating’s antimicrobial and mucoadhesive properties expand their potential toward wound healing materials [
27] and oral or mucosal delivery systems [
28]. Furthermore, their biocompatibility and hemocompatibility profiles, combined with low cytotoxicity reported in vitro, support their prospective use in regenerative medicine applications [
29]. However, their clinical translation faces significant challenges, including immune surveillance, reticuloendothelial system uptake, and long-term retention in organs such as the liver and spleen, which is influenced by nanoparticle size, surface coating, and administration route [
13].
This study advances our understanding of how chitosan surface functionalization influences the physicochemical stability and magneto-optical behavior of magnetite-based ferrofluids. By clarifying the relationship between nanoparticle aggregation dynamics and field-induced optical responses, our findings address a key challenge in designing ferrofluids with predictable and tunable properties. The demonstrated ability of chitosan to modulate magnetic and optical characteristics without altering the crystalline structure highlights its dual role as both a stabilizing and functionalizing agent in smart nanomaterials. Future work will include the assessment of colloidal stability and dispersion behavior in physiological solutions, which will be undertaken once the necessary equipment for zeta potential measurements becomes available. These results open new opportunities for multifunctional magnetic nanoparticles in biomedical applications such as targeted drug delivery, magnetic hyperthermia, biosensing, and theranostic platforms that integrate diagnosis and therapy. Future research should focus on biocompatibility assessment through in vitro and in vivo studies, optimization for magneto-optical biosensors, and further functionalization with specific ligands or biopolymers to expand their potential in precision medicine.
5. Conclusions
Magnetic nanoparticles coated with chitosan (MNP-Q) were successfully synthesized via the in situ co-precipitation method, yielding a stable ferrofluid in an aqueous medium. Structural analysis through X-ray diffraction confirmed a face-centered cubic spinel structure, while transmission electron microscopy revealed a spherical morphology with particle diameters ranging from 10 to 14 nm.
Magnetically, the MNP-Q sample exhibited a saturation magnetization of 53 emu/g, slightly higher than the 51 emu/g observed in uncoated MNPs. This enhancement is attributed to the protective chitosan shell, which minimizes surface oxidation and helps preserve the magnetic properties. Optical characterization disclosed a blue shift in the band gap energy from 4.55 eV (MNP) to 4.72 eV (MNP-Q), suggesting that the chitosan coating modifies the surface states and electronic structure of the nanoparticles. Moreover, Tauc analysis indicates that chitosan suppresses the formation of particles below 10 nm, influencing quantum confinement effects.
The magneto-optical properties of the chitosan-coated ferrofluid are promising, positioning this material as a potential candidate for biomedical applications such as biosensing, drug delivery, and photothermal therapy.