Theoretical Investigation of Ru-Doped Wurtzite Zno: Insights into Electronic Structure and Photocatalytic Potential
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
4. Conclusions
5. Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaghoubi, S.; Mousavi, S.M.; Babapoor, A.; Binazadeh, M.; Lai, C.W.; Althomali, R.H.; Rahman, M.M.; Chiang, W.H. Photocatalysts for solar energy conversion: Recent advances and environmental applications. Renew. Sustain. Energy Rev. 2024, 200, 114538. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and bandgap engineering using ZnO nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 934587. [Google Scholar] [CrossRef]
- Bhapkar, A.R.; Bhame, S. A review on ZnO and its modifications for photocatalytic degradation of prominent textile effluents: Synthesis, mechanisms, and future directions. J. Environ. Chem. Eng. 2024, 12, 112553. [Google Scholar] [CrossRef]
- Dasuki, D.; Habanjar, K.; Awad, R. Effect of growth and calcination temperatures on the optical properties of ruthenium-doped ZnO nanoparticles. Condens. Matter 2023, 8, 102. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.D.; Luo, S.; Vovchok, D.; Llorca, J.; Sallis, S.; Kattel, S.; Xu, W.; Piper, L.F.; Polyansky, D.E.; Senanayake, S.D.; et al. Three-dimensional ruthenium-doped TiO2 sea urchins for enhanced visible-light-responsive H2 production. Phys. Chem. Chem. Phys. 2016, 18, 15972–15979. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Singh, N.; Singh, M.; Singh, S.K.; Tandon, P. Development and characterization of varying percentages of Ru-doped ZnO (x Ru: ZnO; 1% ≤ x ≤ 5%) as a potential material for LPG sensing at room temperature. Appl. Phys. A 2020, 126, 321. [Google Scholar] [CrossRef]
- Krishnan, B.; Shaji, S.; Acosta-Enríquez, M.C.; Acosta-Enríquez, E.B.; Castillo-Ortega, R.; Zayas, M.E.; Castillo, S.J.; Palamà, I.E.; D’Amone, E.; Pech-Canul, M.I.; et al. Group II–VI Semiconductors. In Semiconductors: Synthesis, Properties and Applications; Springer: Cham, Switzerland, 2019; pp. 397–464. [Google Scholar]
- John, R.; Padmavathi, S. Ab initio calculations on structural, electronic and optical properties of ZnO in wurtzite phase. Cryst. Struct. Theory Appl. 2016, 5, 24. [Google Scholar] [CrossRef]
- Witkowski, B.S. Applications of ZnO Nanorods and Nanowires—A Review. Acta Phys. Pol. A 2018, 134, 24. [Google Scholar] [CrossRef]
- Shabatina, T.; Vernaya, O.; Shumilkin, A.; Semenov, A.; Melnikov, M. Nanoparticles of bioactive metals/metal oxides and their nanocomposites with antibacterial drugs for biomedical applications. Materials 2022, 15, 3602. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.Y.; Feng, Y.; Yuan, Z.Y.; Su, B.L. One-dimensional metal oxide nanotubes, nanowires, nanoribbons, and nanorods: Synthesis, characterizations, properties and applications. Crit. Rev. Solid State Mater. Sci. 2012, 37, 1–74. [Google Scholar] [CrossRef]
- Abdelmohsen, A.; Ismail, N. Morphology transition of ZnO and Cu2O nanoparticles to 1D, 2D, and 3D nanostructures: Hypothesis for engineering of micro and nanostructures (HEMNS). J. Sol-Gel Sci. Technol. 2020, 94, 213–228. [Google Scholar] [CrossRef]
- Chauhan, V.; Singh, P.; Kumar, R. Ion beam-induced modifications in ZnO nanostructures and potential applications. In Nanostructured Zinc Oxide; Elsevier: Amsterdam, The Netherlands, 2021; pp. 117–155. [Google Scholar]
- Teng, F.; Hu, K.; Ouyang, W.; Fang, X. Photoelectric detectors based on inorganic p-type semiconductor materials. Adv. Mater. 2018, 30, 1706262. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Teng, F.; He, J.H.; Fang, X. Enhancing the photoelectric performance of photodetectors based on metal oxide semiconductors by charge-carrier engineering. Adv. Funct. Mater. 2019, 29, 1807672. [Google Scholar] [CrossRef]
- Zafar, A.; Younas, M.; Fatima, S.A.; Qian, L.; Liu, Y.; Sun, H.; Shaheen, R.; Nisar, A.; Karim, S.; Nadeem, M.; et al. Frequency stable dielectric constant with reduced dielectric loss of one-dimensional ZnO-ZnS heterostructures. Nanoscale 2021, 13, 15711–15720. [Google Scholar] [CrossRef] [PubMed]
- Goktas, A.; Aslan, F.; Yeşilata, B.; Boz, İ. Physical properties of solution processable n-type Fe and Al co-doped ZnO nanostructured thin films: Role of Al doping levels and annealing. Mater. Sci. Semicond. Process. 2018, 75, 221–233. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Singh, R.K. Progress on transition metal-doped ZnO nanoparticles and its application. Ind. Eng. Chem. Res. 2019, 58, 17130–17163. [Google Scholar] [CrossRef]
- Kayani, A.B.A.; Kuriakose, S.; Monshipouri, M.; Khalid, F.A.; Walia, S.; Sriram, S.; Bhaskaran, M. UV photochromism in transition metal oxides and hybrid materials. Small 2021, 17, 2100621. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Sarkar, T.; Bazuev, G.V.; Kuznetsov, M.V.; Nordblad, P.; Mathieu, R. Modification of the structure and magnetic properties of ceramic La2CoMnO6 with Ru doping. J. Alloys Compd. 2018, 752, 420–430. [Google Scholar] [CrossRef]
- Al Shomar, S.M. Investigation of the effect of doping concentration in Ruthenium-doped TiO2 thin films for solar cells and sensors applications. Mater. Res. Express 2020, 7, 036409. [Google Scholar] [CrossRef]
- Medhi, R.; Li, C.H.; Lee, S.H.; Marquez, M.D.; Jacobson, A.J.; Lee, T.C.; Lee, T.R. Uniformly spherical and monodisperse antimony-and zinc-doped tin oxide nanoparticles for optical and electronic applications. ACS Appl. Nano Mater. 2019, 2, 6554–6564. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, C.Y.; Yang, X.H.; Yang, X.J.; Li, Y.P.; Feng, C.M.; Xu, Z.A. Effect of ruthenium doping on superconductivity in Nb2PdS5. J. Phys. Conf. Ser. 2017, 807, 052002. [Google Scholar] [CrossRef]
- Papadimitriou, D.N. Engineering of optical and electrical properties of electrodeposited highly doped Al: ZnO and In: ZnO for cost-effective photovoltaic device technology. Micromachines 2022, 13, 1966. [Google Scholar] [CrossRef]
- Vinoditha, U.; Sarojini, B.K.; Sandeep, K.M.; Narayana, B.; Maidur, S.R.; Patil, P.S.; Balakrishna, K.M. Defects-induced nonlinear saturable absorption mechanism in europium-doped ZnO nanoparticles synthesized by facile hydrothermal method. Appl. Phys. A 2019, 125, 436. [Google Scholar] [CrossRef]
- Vikal, S.; Meena, S.; Gautam, Y.K.; Kumar, A.; Sethi, M.; Meena, S.; Gautam, D.; Singh, B.P.; Agarwal, P.C.; Meena, M.L. Visible-light induced effective and sustainable remediation of nitro organics pollutants using Pd-doped ZnO nanocatalyst. Sci. Rep. 2024, 14, 22430. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, Z.; Li, X.; Geng, Y.; Tian, X.; Du, Y.; Qian, Z. Enhanced acetone-sensing properties to ppb detection level using Au/Pd-doped ZnO nanorod. Sens. Actuators Chem. 2020, 310, 127129. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Ranjbari, A.; Demeestere, K.; Kim, K.H.; Heynderickx, P.M. Oxygen vacancy modification of commercial ZnO by hydrogen reduction for the removal of thiabendazole: Characterization and kinetic study. Appl. Catal. B Environ. 2023, 324, 122265. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Leprince-Wang, Y. Advancements in ZnO-based photocatalysts for water treatment: A comprehensive review. Crystals 2024, 14, 611. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Hu, Z.; Yan, H. First-principles study of Fe-doped ZnO: A potential diluted magnetic semiconductor. Phys. B 2008, 403, 3752–3756. [Google Scholar]
- Lin, T.; Tomaz, M.A.; Schwickert, M.M.; Harp, G.R. Structure and magnetic properties of Ru/Fe (001) multilayers. Phys. Rev. B 1998, 58, 862. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Li, J.; Yu, H.; Yu, J. Constructing Surface Oxygen Vacancies on ZnO Nanosheets for Boosting Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2022, 314, 122265. [Google Scholar]
- Tveten, E.G.; Müller, T.; Linder, J.; Brataas, A. Intrinsic magnetization of antiferromagnetic textures. Phys. Rev. B 2016, 93, 1044081. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native Point Defects in ZnO. Phys. Rev. B 2009, 100, 121201. [Google Scholar] [CrossRef]
- Panigrahi, S.; Nanda, K.K. Density Functional Study of Transition Metal Doped ZnO Nanoclusters. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 60, 65–70. [Google Scholar]
- Gupta, S.M.; Tripathi, M. A Study on the Visible Light Photocatalytic Activity of Transition Metal Doped ZnO Nanoparticles. Mater. Sci. Semicond. Process. 2013, 16, 1743–1748. [Google Scholar]
- Zhao, Y.; Ma, J.; Li, Y. Effect of Ruthenium Doping on the Photocatalytic Performance of TiO2 Nanoparticles. Appl. Surf. Sci. 2013, 200, 222–228. [Google Scholar]
- Zhang, W.; Li, H.; Zhang, Z. Facile Synthesis of RuO2-ZnO Composite and Its Enhanced Photocatalytic Activity under Visible Light. J. Mol. Catal. Chem. 2014, 390, 142–148. [Google Scholar]
- Thi, V.H.-T.; Lee, B.-K. Effective photocatalytic degradation of paracetamol using La-doped ZnO photocatalyst under visible light irradiation. Mater. Res. Bull. 2017, 96, 171–182. [Google Scholar] [CrossRef]
- Ruterana, P.; Abouzaid, M.; Béré, A.; Chen, J. Formation of a low energy grain boundary in ZnO: The structural unit concept in hexagonal symmetry materials. J. Appl. Phys. 2008, 103, 033501. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Han, S.; Yun, Q.; Tu, S.; Zhu, L.; Cao, W.; Lu, Q. Metallic ruthenium-based nanomaterials for electrocatalytic and photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 24691–24714. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, Y. Crystal-phase and surface-structure engineering of ruthenium nanocrystals. Nat. Rev. Mater. 2020, 5, 440–459. [Google Scholar] [CrossRef]
- Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Recent development in defects engineered photocatalysts: An overview of the experimental and theoretical strategies. Energy Environ. Mater. 2022, 5, 68–114. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Photocatalytic removal of methyl orange azo dye with simultaneous hydrogen production using Ru-modified ZnO photocatalyst. Catalysts 2019, 9, 964. [Google Scholar] [CrossRef]
- Maiti, S.; Maiti, K.; Curnan, M.T.; Kim, K.; Noh, K.J.; Han, J.W. Engineering electrocatalyst nanosurfaces to enrich the activity by inducing lattice strain. Energy Environ. Sci. 2021, 14, 3717–3756. [Google Scholar] [CrossRef]
- Fang, X.; Choi, J.Y.; Stodolka, M.; Pham, H.T.; Park, J. Advancing Electrically Conductive Metal–Organic Frameworks for Photocatalytic Energy Conversion. Acc. Chem. Res. 2024, 57, 2316–2325. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Lei, H.; Tu, Q.; Huang, C.; Cheng, X.; Yang, X.; Liu, H.; Huo, C. Regulating oxygen vacancies by Zn atom doping to anchor and disperse promoter Ba on MgO support to improve Ru-based catalysts activity for ammonia synthesis. RSC Adv. 2024, 14, 13157–13167. [Google Scholar] [CrossRef]
- Gopal, P.; Spaldin, N.A. Magnetic Interactions in Transition Metal Doped ZnO: An Ab Initio Study. Phys. Rev. B 2006, 74, 094418. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Wu, J.; Li, Y. Electronic Structure and Magnetic Properties of Co-Doped ZnO: A First-Principles Study. J. Phys. Chem. C 2019, 123, 4562–4570. [Google Scholar]
- Ren, M.; Guo, X.; Huang, S. Transition metal atoms (Fe, Co, Ni, Cu, Zn) doped RuIr surface for the hydrogen evolution reaction: A first-principles study. Appl. Surf. Sci. 2021, 556, 149801. [Google Scholar] [CrossRef]
- Nigam, R.; Pan, A.V.; Dou, S.X. Explanation of magnetic behavior in Ru-based superconducting ferromagnets. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 77, 134509. [Google Scholar] [CrossRef]
- Yuan, H.K.; Chen, H.; Kuang, A.L.; Wu, B.; Wang, J.Z. Structural and magnetic properties of small 4d transition metal clusters: Role of spin orbit coupling. J. Phys. Chem. A 2012, 116, 11673–11684. [Google Scholar] [CrossRef]
- Chhowalla, M.; Jena, D.; Zhang, H. Two-dimensional semiconductors for transistors. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Li, R.; Qiu, L.P.; Cao, S.Z.; Li, Z.; Gao, S.L.; Zhang, J.; Ramakrishna, S.; Long, Y.Z. Research advances in magnetic field-assisted photocatalysis. Adv. Funct. Mater. 2024, 34, 2316725. [Google Scholar] [CrossRef]
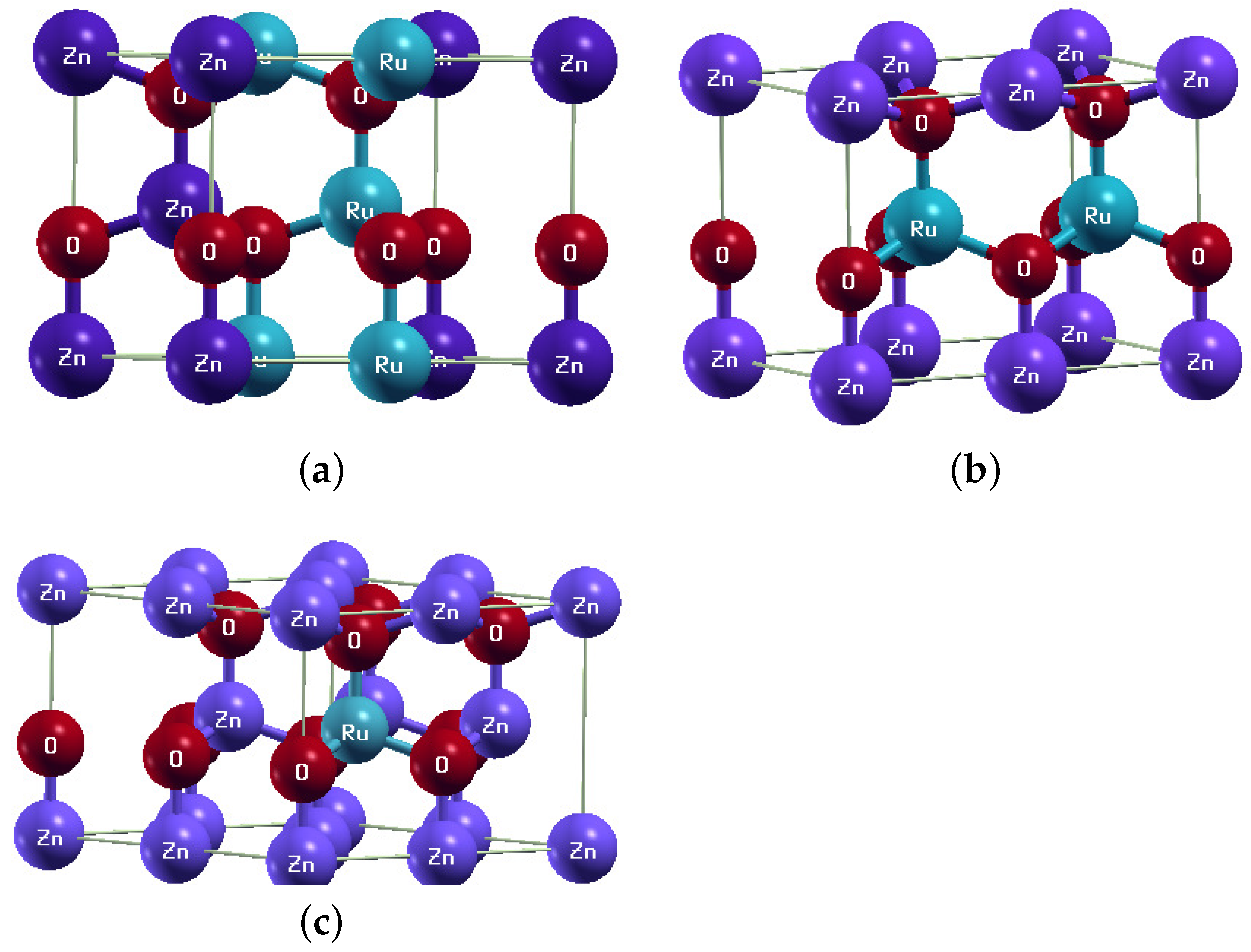

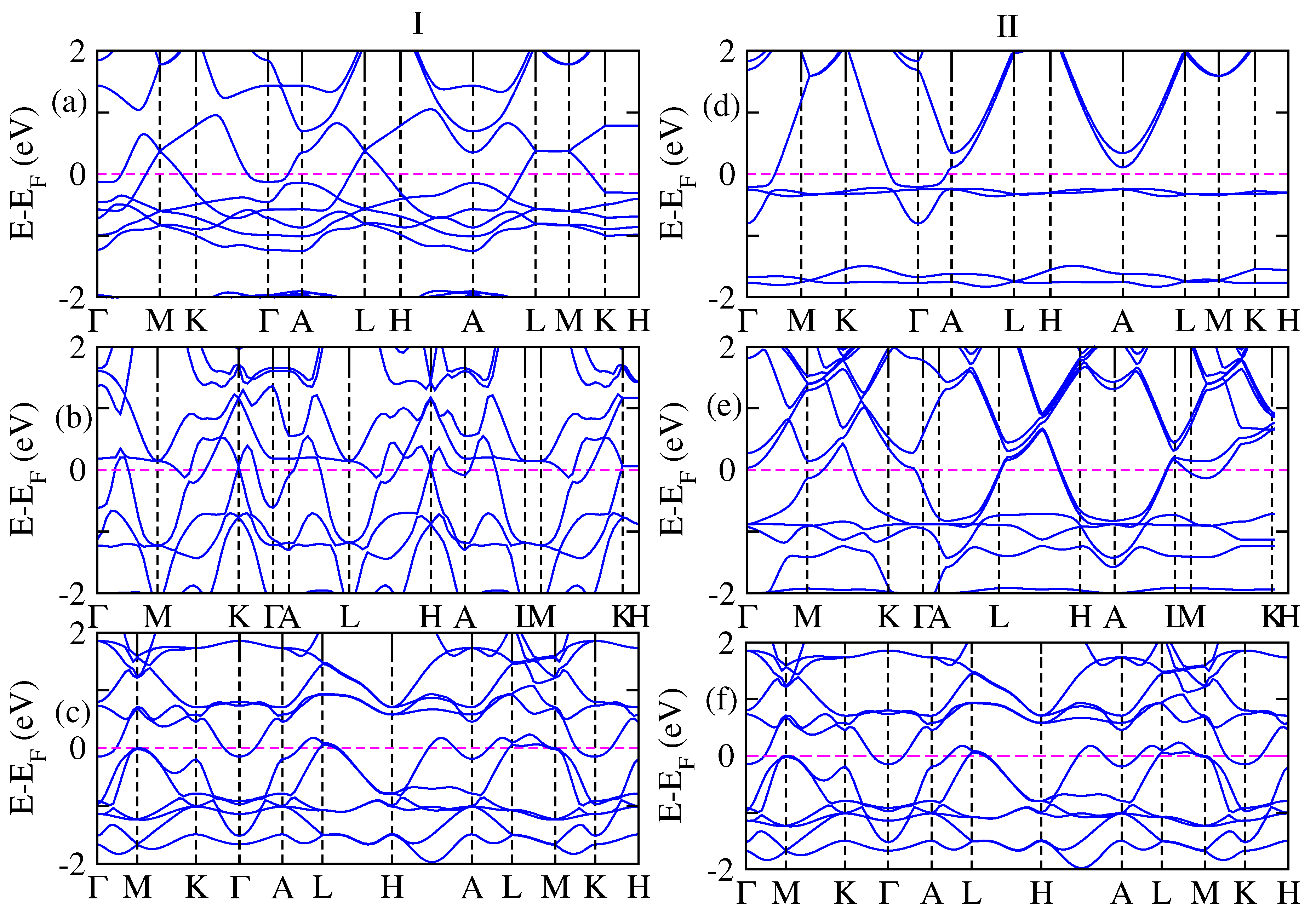
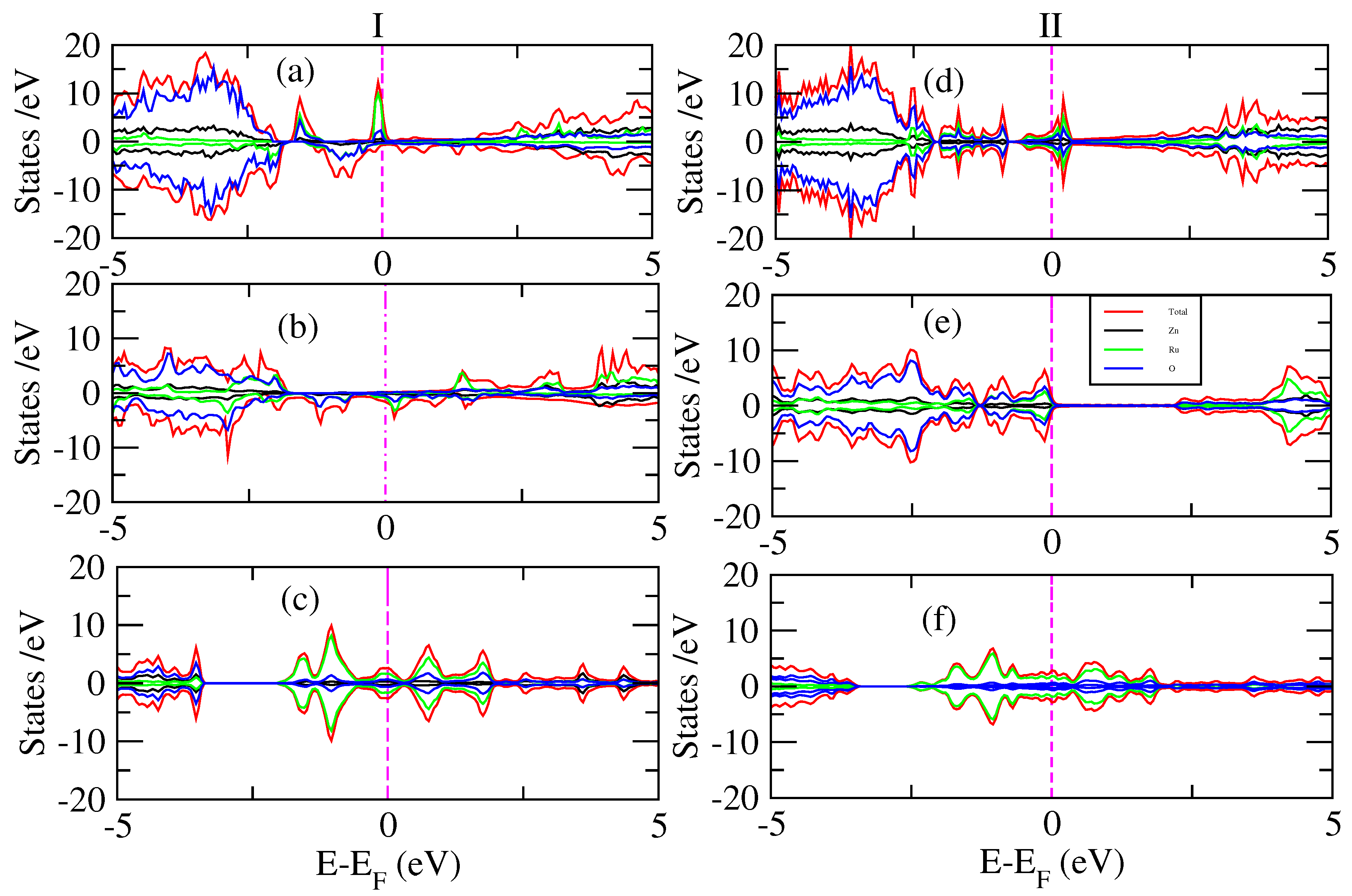
| Composition | Fermi Energy | Total Energy of FM | Lattice Parameter | Volume |
|---|---|---|---|---|
| 0.125 | 9.695 | −1489.211 | 12.431 | 1332.843 |
| 0.25 | 10.593 | −767.574 | 12.431 | 666.421 |
| 0.5 | 13.250 | −406.796 | 6.095 | 316.713 |
| Composition | EF of FM (eV) | TE of FM (Ry) | EF of AFM (eV) | TE of AFM (Ry) | E |
|---|---|---|---|---|---|
| 0.125 | 9.627 | −297.627 | 9.803 | −2977.641 | 0.014 |
| 0.25 | 10.888 | −1534.436 | 9.889 | −1534.564 | 0.127 |
| 0.5 | 12.944 | −812.799 | 12.703 | −812.812 | 0.013 |
| Ferromagnetic (FM) | Antiferromagnetic (AFM) | |||
|---|---|---|---|---|
| Composition | Total Mag. | Absolute Mag. | Total Mag. | Absolute Mag. |
| 0.125 | 3.85 | 4.38 | 0.0000 | 5.31 |
| 0.25 | 5.51 | 5.74 | 0.0000 | 7.34 |
| 0.5 | 8.44 | 8.53 | 0.0000 | 7.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golja, D.R.; Dinka, M.O. Theoretical Investigation of Ru-Doped Wurtzite Zno: Insights into Electronic Structure and Photocatalytic Potential. Optics 2025, 6, 45. https://doi.org/10.3390/opt6040045
Golja DR, Dinka MO. Theoretical Investigation of Ru-Doped Wurtzite Zno: Insights into Electronic Structure and Photocatalytic Potential. Optics. 2025; 6(4):45. https://doi.org/10.3390/opt6040045
Chicago/Turabian StyleGolja, Desta Regassa, and Megersa Olumana Dinka. 2025. "Theoretical Investigation of Ru-Doped Wurtzite Zno: Insights into Electronic Structure and Photocatalytic Potential" Optics 6, no. 4: 45. https://doi.org/10.3390/opt6040045
APA StyleGolja, D. R., & Dinka, M. O. (2025). Theoretical Investigation of Ru-Doped Wurtzite Zno: Insights into Electronic Structure and Photocatalytic Potential. Optics, 6(4), 45. https://doi.org/10.3390/opt6040045









