Corneal Astigmatism After Cataract Surgery: A Review of Mechanisms, Outcomes, and Surgical Considerations
Abstract
1. Introduction
2. Materials and Methods
3. Discussion
3.1. Pathophysiology of Postoperative Corneal Astigmatism
3.2. Assessment of Astigmatism
3.2.1. Visual Acuity
3.2.2. Keratometry
3.2.3. Autorefractometry
3.2.4. Pachymetry
3.2.5. Anterior Segment Optical Coherence Tomography (AS-OCT)
3.2.6. Corneal Topography
3.2.7. Wavefront Aberrometry
3.2.8. Ray Tracing
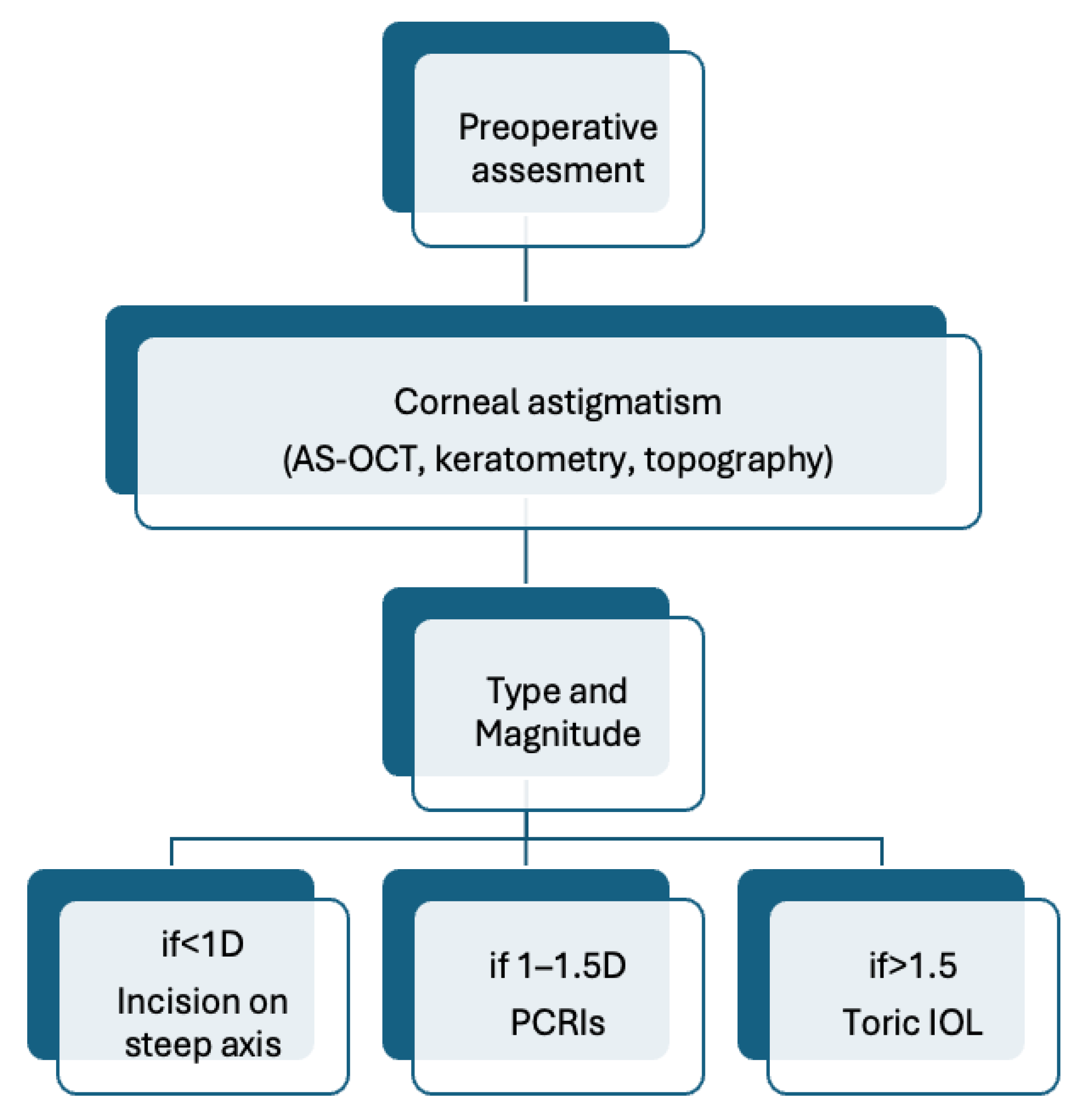
3.3. Prevention and Management Strategies
3.4. Visual Outcomes and Quality of Life
3.5. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, T.; Romano, M.R.; Iannetta, D.; Romano, V.; Gualdi, L.; D’AGostino, I.; Ripandelli, G. Cataract surgery practice patterns worldwide: A survey. BMJ Open Ophthalmol. 2021, 6, e000464. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. “Phacoemulsification”, StatPearls, June 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576419/ (accessed on 25 August 2025).
- Hashemi, H.; Fotouhi, A.; Yekta, A.; Pakzad, R.; Ostadimoghaddam, H.; Khabazkhoob, M. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J. Curr. Ophthalmol. 2018, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Kaur, K. “Astigmatism”, StatPearls, June 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK582142/ (accessed on 6 May 2025).
- Mohammadi, S.-F.; Khorrami-Nejad, M.; Hamidirad, M. Posterior corneal astigmatism: A review article. Clin. Optom. 2019, 11, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J.; Carney, L.G. A review of astigmatism and its possible genesis. Clin. Exp. Optom. 2007, 90, 5–19. [Google Scholar] [CrossRef]
- Read, S.A.; Vincent, S.J.; Collins, M.J. The visual and functional impacts of astigmatism and its clinical management. Ophthalmic Physiol. Opt. 2014, 34, 267–294. [Google Scholar] [CrossRef]
- Næser, K. Assessment and Statistics of Surgically Induced Astigmatism. Acta Ophthalmol. 2008, 86, 1–28. [Google Scholar] [CrossRef]
- Namba, H.; Sugano, A.; Murakami, T.; Utsunomiya, H.; Nishitsuka, K.; Ishizawa, K.; Kayama, T.; Yamashita, H. Age-Related Changes in Astigmatism and Potential Causes. Cornea 2020, 39, S34–S38. [Google Scholar] [CrossRef]
- Comparison of Surgically Induced Astigmatism of Temporal Versus Superior Clear Corneal Incisions|Request PDF. Available online: https://www.researchgate.net/publication/6372904_Comparison_of_surgically_induced_astigmatism_of_temporal_versus_superior_clear_corneal_incisions (accessed on 6 May 2025).
- Angermann, R.; Palme, C.; Segnitz, P.; Dimmer, A.; Schmid, E.; Hofer, M.; Steger, B. Surgically induced astigmatism and coupling effect-mediated keratometric changes after conventional phacoemulsification cataract surgery. Spektrum Der Augenheilkd. 2021, 35, 235–240. [Google Scholar] [CrossRef]
- Wan, T.; Chen, H.; Wu, S.; Jin, H. Analysis of surgically induced astigmatism of the anterior, posterior, and total cornea after implantable collamer lens implantation: A comparative study between temporal and superior clear corneal incisions. BMC Ophthalmol. 2024, 24, 252. [Google Scholar] [CrossRef]
- Sigireddi, R.R.; Weikert, M.P. How much astigmatism to treat in cataract surgery. Curr. Opin. Ophthalmol. 2020, 31, 10–14. [Google Scholar] [CrossRef]
- Alpins, N.; Ong, J.K.Y.; Stamatelatos, G. Asymmetric Corneal Flattening Effect After Small Incision Cataract Surgery. J. Refract. Surg. 2016, 32, 598–603. [Google Scholar] [CrossRef]
- Nielsen, P.J. Prospective evaluation of surgically induced astigmatism and astigmatic keratotomy effects of various self-sealing small incisions. J. Cataract. Refract. Surg. 1995, 21, 43–48. [Google Scholar] [CrossRef]
- Beltrame, G.; Salvetat, M.L.; Chizzolini, M.; Driussi, G. Corneal topographic changes induced by different oblique cataract incisions. J. Cataract. Refract. Surg. 2001, 27, 720–727. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Nakao, F.; Hayashi, F. The Correlation between Incision Size and Corneal Shape Changes in Sutureless Cataract Surgery. Ophthalmology 1995, 102, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.C.; Brodstein, R.S.; Richards, W.L.; Olson, R.J.; Combe, P.H.; Crowell, K.E. Long-term course of surgically induced astigmatism. J. Cataract. Refract. Surg. 1988, 14, 270–276. [Google Scholar] [CrossRef]
- Cravy, T.V. Routine use of a lateral approach to cataract extraction to achieve rapid and sustained stabilization of postoperative astigmatism. J. Cataract. Refract. Surg. 1991, 17, 415–423. [Google Scholar] [CrossRef]
- Sun, H.; Wang, C.; Wu, H. Recent advances and current challenges in suture and sutureless scleral fixation techniques for intraocular lens: A comprehensive review. Eye Vis. 2024, 11, 49. [Google Scholar] [CrossRef]
- Kondrot, E.C. Keratometric cylinder and visual recovery following phacoemulsification and intraocular lens implantation using a self-sealing cataract incision. J. Cataract. Refract. Surg. 1991, 17, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Ernest, P.; Tipperman, R.; Eagle, R.; Kardasis, C.; Lavery, K.; Sensoli, A.; Rhem, M. Is there a difference in incision healing based on location? J. Cataract. Refract. Surg. 1998, 24, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.; Dahan, E. Opposite clear corneal incisions to correct pre-existing astigmatism in cataract surgery. J. Cataract. Refract. Surg. 2000, 26, 803–805. [Google Scholar] [CrossRef]
- Łabuz, G.; Varadi, D.; Khoramnia, R.; Auffarth, G.U. Central and mid-peripheral corneal astigmatism in an elderly population: A retrospective analysis of Scheimpflug topography results. Sci. Rep. 2021, 11, 7968. [Google Scholar] [CrossRef]
- Łabuz, G.; Varadi, D.; Khoramnia, R.; Auffarth, G.U. Progressive-toric IOL design reduces residual astigmatism with increasing pupil size: A ray-tracing simulation based on corneal topography data. Biomed. Opt. Express 2021, 12, 1568–1576. [Google Scholar] [CrossRef]
- Hennelly, M.L. How to detect myopia in the eye clinic. Community Eye Health/Int. Cent. Eye Health 2019, 32, 15–16. [Google Scholar]
- Tay, E.; Mengher, L.; Lin, X.-Y.; Ferguson, V. The impact of off the visual axis retinoscopy on objective central refractive measurement in adult clinical practice: A prospective, randomized clinical study. Eye 2011, 25, 888–892. [Google Scholar] [CrossRef][Green Version]
- Lim, T.-C.; Chattopadhyay, S.; Acharya, U.R. A survey and comparative study on the instruments for glaucoma detection. Med. Eng. Phys. 2012, 34, 129–139. [Google Scholar] [CrossRef]
- Emmerich, L.; Ohlendorf, A.; Leube, A.; Suchkov, N.; Wahl, S. Development and Testing of a Compact Autorefractor Based on Double-Pass Imaging. Sensors 2022, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; DeBoer, C.; Xu, B.Y. Anterior Segment Optical Coherence Tomography: Applications for Clinical Care and Scientific Research. Asia Pac. J. Ophthalmol. 2019, 8, 146–157. [Google Scholar] [CrossRef]
- Ruggeri, F.; Rullo, D.; Maugliani, E.; Trotta, N.; Ciancimino, C.; Di Pippo, M.; Guglielmelli, F.; Abdolrahimzadeh, S. The role of anterior segment optical coherence tomography in post-cataract surgery Descemet membrane detachment. Int. Ophthalmol. 2025, 45, 1–20. [Google Scholar] [CrossRef]
- Wong, A.L.; Leung, C.K.-S.; Weinreb, R.N.; Cheng, A.K.C.; Cheung, C.Y.L.; Lam, P.T.-H.; Pang, C.P.; Lam, D.S.C. Quantitative assessment of lens opacities with anterior segment optical coherence tomography. Br. J. Ophthalmol. 2008, 93, 61–65. [Google Scholar] [CrossRef]
- Chan, T.C.; Li, E.Y.; Yau, J.C. Application of anterior segment optical coherence tomography to identify eyes with posterior polar cataract at high risk for posterior capsule rupture. J. Cataract. Refract. Surg. 2014, 40, 2076–2081. [Google Scholar] [CrossRef]
- Belin, M.W.; Khachikian, S.S. New devices and clinical implications for measuring corneal thickness. Clin. Exp. Ophthalmol. 2006, 34, 729–731. [Google Scholar] [CrossRef]
- Mrochen, M.; Kaemmerer, M.; Seiler, T. Wavefront-guided Laser in situ Keratomileusis: Early Results in Three Eyes. J. Refract. Surg. 2000, 16, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mrochen, M.; Bueeler, M.; Donitzky, C.; Seiler, T. Optical Ray Tracing for the Calculation of Optimized Corneal Ablation Profiles in Refractive Treatment Planning. J. Refract. Surg. 2008, 24, S446–S451. [Google Scholar] [CrossRef]
- Yin, X.-L.; Ji, Z.-Y.; Li, X.-X.; Liang, X.-M.; Ji, S.-X. Surgical approaches to correct corneal astigmatism at time of cataract surgery: A mini-review. Int. J. Ophthalmol. 2024, 17, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Icoz, M.; Yildirim, B.; Icoz, S.G.G. Comparison of different methods of correcting astigmatism in cataract surgery. Clin. Exp. Optom. 2023, 107, 409–414. [Google Scholar] [CrossRef]
- Orts, P.; Piñero, D.P.; Aguilar, S.; Tañá, P. Efficacy of astigmatic correction after femtosecond laser-guided cataract surgery using intraoperative aberrometry in eyes with low-to-moderate levels of corneal astigmatism. Int. Ophthalmol. 2020, 40, 1181–1189. [Google Scholar] [CrossRef]
- Derakhshan, A.; Bamdad, S.; Kheiri, H.; Yasemi, M. Correlation between keratometry and corneal incision before and after phaco surgery. Folia Medica 2021, 63, 527–532. [Google Scholar] [CrossRef]
- Donnenfeld, E.D.; Olson, R.J.; Solomon, R.; Finger, P.T.; Biser, S.A.; Perry, H.D.; Doshi, S. Efficacy and wound-temperature gradient of WhiteStar phacoemulsification through a 1.2 mm incision. J. Cataract. Refract. Surg. 2003, 29, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Lichter, P.R. Sutures, Cylinder, and Straw Men. Ophthalmology 1991, 98, 415–416. [Google Scholar] [CrossRef]
- Hepokur, M.; Kizilay, E.B.; Durmus, E.; Aykut, V.; Esen, F.; Oguz, H. The influence of corneal incision size on endothelial cell loss and surgically induced astigmatism following phacoemulsification cataract surgery. North. Clin. Istanb. 2022, 9, 385–390. [Google Scholar] [CrossRef]
- Langenbucher, A.; Szentmáry, N.; Cayless, A.; Casaza, M.; Weisensee, J.; Hoffmann, P.; Wendelstein, J.; Gasazza, M. Surgically Induced Astigmatism after Cataract Surgery—A Vector Analysis. Curr. Eye Res. 2022, 47, 1279–1287. [Google Scholar] [CrossRef]
- Masket, S.; Wang, L.; Belani, S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J. Refract. Surg. 2009, 25, 21–24. [Google Scholar] [CrossRef]
- Abdelghany, A.A.; Alio, J.L. Surgical options for correction of refractive error following cataract surgery. Eye Vis. 2014, 1, 2. [Google Scholar] [CrossRef]
- Alpins, N.A. Vector analysis of astigmatism changes by flattening, steepening, and torque. J. Cataract. Refract. Surg. 1997, 23, 1503–1514. [Google Scholar] [CrossRef]
- Lavanya, R.; Wong, T.Y.; Aung, T.; Tan, D.T.H.; Saw, S.-M.; Tay, W.T.; Wang, J.J.; for the SiMES team. Prevalence of cataract surgery and post-surgical visual outcomes in an urban Asian population: The Singapore Malay Eye Study. Br. J. Ophthalmol. 2008, 93, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, C.; Sun, J.; Yang, J.; Wen, K.; Chen, X.; Zhao, F.; Sun, X.; Tian, F. Assessing the interchangeability of keratometry measurements from four biometric devices in intraocular lens power calculations: Insights into the predictive accuracy of five modern IOL formulas. BMC Ophthalmol. 2025, 25, 236. [Google Scholar] [CrossRef] [PubMed]
- Keel, S.; Xie, J.; Foreman, J.; Taylor, H.R.; Dirani, M. Population-based assessment of visual acuity outcomes following cataract surgery in Australia: The National Eye Health Survey. Br. J. Ophthalmol. 2018, 102, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Kanthan, G.L.; Mitchell, P.; Burlutsky, G.; Wang, J.J. Intermediate- and longer-term visual outcomes after cataract surgery: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2010, 39, 201–206. [Google Scholar] [CrossRef]
- Schuster, A.K.; Schlichtenbrede, F.C.; Harder, B.C.; Beutelspacher, S.C.; Jonas, J.B. Target refraction for best uncorrected distance and near vision in cataract surgery. Eur. J. Ophthalmol. 2013, 24, 509–515. [Google Scholar] [CrossRef]
- Son, H.-S.; Kim, S.H.; Auffarth, G.U.; Choi, C.Y. Prospective comparative study of tolerance to refractive errors after implantation of extended depth of focus and monofocal intraocular lenses with identical aspheric platform in Korean population. BMC Ophthalmol. 2019, 19, 187. [Google Scholar] [CrossRef]
- Park, C.Y.; Chuck, R.S. Residual refractive error and visual outcome after cataract surgery using spherical Versus Aspheric IOLs. Ophthalmic Surgery, Lasers Imaging Retin. 2011, 42, 37–43. [Google Scholar] [CrossRef]
- Fernández-Vega, L.; Alfonso, J.F.; Montés-Micó, R.; Amhaz, H. Visual acuity tolerance to residual refractive errors in patients with an apodized diffractive intraocular lens. J. Cataract. Refract. Surg. 2008, 34, 199–204. [Google Scholar] [CrossRef]
- de Vries, N.E.; Webers, C.A.; Touwslager, W.R.; Bauer, N.J.; de Brabander, J.; Berendschot, T.T.; Nuijts, R.M. Dissatisfaction after implantation of multifocal intraocular lenses. J. Cataract. Refract. Surg. 2011, 37, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pesala, V.; Garg, P.; Bharadwaj, S.R. Relation between uncorrected astigmatism and visual acuity in pseudophakia. Optom. Vis. Sci. 2013, 90, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Gjerdrum, B.; Gundersen, K.G.; Lundmark, P.O.; Aakre, B.M. Repeatability of OCT-Based versus Scheimpflug- and Reflection-Based Keratometry in Patients with Hyperosmolar and Normal Tear Film. Clin. Ophthalmol. 2020, 14, 3991–4003. [Google Scholar] [CrossRef]
- Lin, X.; Ma, D.; Yang, J. Insights into the rotational stability of toric intraocular lens implantation: Diagnostic approaches, influencing factors and intervention strategies. Front. Med. 2024, 11, 1349496. [Google Scholar] [CrossRef]
- Light Adjustable Intraocular Lenses—EyeWiki. Available online: https://eyewiki.org/Light_Adjustable_Intraocular_Lenses#cite_note-FDA-3 (accessed on 6 May 2025).
- Jun, J.H.; Lieu, A.; Afshari, N.A. Light adjustable intraocular lenses in cataract surgery: Considerations. Curr. Opin. Ophthalmol. 2023, 35, 44–49. [Google Scholar] [CrossRef]
- Kaufman, A.R.; Pineda, R.I. Intraoperative aberrometry: An update on applications and outcomes. Curr. Opin. Ophthalmol. 2022, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Sakai, Y.; Toda, H.; Kato, Y.; Ichikawa, K. Visual Outcomes After Cataract Surgery With the Light Adjustable Lens in Japanese Patients With and Without Prior Corneal Refractive Surgery. J. Refract. Surg. 2024, 40, e854–e862. [Google Scholar] [CrossRef]
- Moshirfar, M.; Martin, D.J.; Jensen, J.L.; Payne, C.J. Light adjustable intraocular lenses: An updated platform for cataract surgery. Curr. Opin. Ophthalmol. 2022, 34, 78–83. [Google Scholar] [CrossRef]
- Gaur, S.; Dhull, C.; Khokhar, S.K. Intra-operative aberrometry versus conventional biometry for intraocular lens power calculation: A prospective observational study from a tertiary referral center. Indian J. Ophthalmol. 2023, 71, 530–534. [Google Scholar] [CrossRef]
- Nuliqiman, M.; Xu, M.; Sun, Y.; Cao, J.; Chen, P.; Gao, Q.; Xu, P.; Ye, J. Artificial Intelligence in Ophthalmic Surgery: Current Applications and Expectations. Clin. Ophthalmol. 2023, 17, 3499–3511. [Google Scholar] [CrossRef]
- Kumari, B.; Tidake, P. Robotic Integration in the Field of Opthalmology and Its Prospects in India. Cureus 2022, 14, e30482. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Tadano, K.; Sonoda, K.-H. Advancements in robotic surgery for vitreoretinal diseases: Current trends and the future. Jpn. J. Ophthalmol. 2025, 69, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Leng, T. Artificial intelligence and ophthalmic surgery. Curr. Opin. Ophthalmol. 2021, 32, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sarofim, M. Devil’s advocate: Exploring the potential negative impacts of artificial intelligence on the field of surgery. J. Med. Artif. Intell. 2024, 7, 7. [Google Scholar] [CrossRef]
- Lindegger, D.J.; Wawrzynski, J.; Saleh, G.M. Evolution and Applications of Artificial Intelligence to Cataract Surgery. Ophthalmol. Sci. 2022, 2, 100164. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Weerasinghe, K.; Mathugamage, M.D.D.E.; Odetayo, A.; Aderinto, N.; Teke, J.; Boussios, S. Enhancing Ophthalmic Diagnosis and Treatment with Artificial Intelligence. Medicina 2025, 61, 433. [Google Scholar] [CrossRef]
- Lu, L.; Rocha-De-Lossada, C.; Rachwani-Anil, R.; Flikier, S.; Flikier, D. The role of posterior corneal power in 21st century biometry: A review. J. Francais D Ophtalmol. 2021, 44, 1052–1058. [Google Scholar] [CrossRef]
- Adegbesan, A.; Akingbola, A.; Aremu, O.; Adewole, O.; Amamdikwa, J.C.; Shagaya, U. From Scalpels to Algorithms: The Risk of Dependence on Artificial Intelligence in Surgery. J. Med. Surg. Public Health 2024, 3, 100140. [Google Scholar] [CrossRef]
- Lee, B.; Narsey, N. Introduction to Artificial Intelligence for General Surgeons: A Narrative Review. Cureus 2025, 17, e79871. [Google Scholar] [CrossRef]
- Koch, D.D.; Ali, S.F.; Weikert, M.P.; Shirayama, M.; Jenkins, R.; Wang, L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J. Cataract. Refract. Surg. 2012, 38, 2080–2087. [Google Scholar] [CrossRef]
- Ma, S.; Li, C.; Sun, J.; Yang, J.; Wen, K.; Chen, X.; Zhao, F.; Sun, X.; Tian, F. Comparative Analysis of Eighteen IOL Power Calculation Formulas Using a Modified Formula Performance Index Across Diverse Biometric Parameters. Am. J. Ophthalmol. 2025, 273, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Feiz, V. Intraocular Lens Power Calculation After Corneal Refractive Surgery. Middle East Afr. J. Ophthalmol. 2010, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.D. The Enigmatic Cornea and Intraocular Lens Calculations: The LXXIII Edward Jackson Memorial Lecture. Arch. Ophthalmol. 2016, 171, xv–xxx. [Google Scholar] [CrossRef] [PubMed]
- Mallareddy, V.; Daigavane, S.; Iii, V.M. Innovations and Outcomes in Astigmatism Correction During Cataract Surgery: A Comprehensive Review. Cureus 2024, 16, e67828. [Google Scholar] [CrossRef]
- Núñez, M.X.; Henriquez, M.A.; Escaf, L.J.; Ventura, B.V.; Srur, M.; Newball, L.; Espaillat, A.; Centurion, V.A. Consensus on the management of astigmatism in cataract surgery. Clin. Ophthalmol. 2019, 13, 311–324. [Google Scholar] [CrossRef]
- CRSToday|Preoperative Surgical Planning. Available online: https://crstoday.com/articles/mar-2021/preoperative-surgical-planning (accessed on 5 September 2025).
- Personalized Planning of Cataract Surgery and Intraoperative Fine-Tuning of Astigmatism Makes Emmetropia Concrete! Available online: https://www.researchgate.net/publication/337171583_Personalized_planning_of_cataract_surgery_and_intraoperative_fine-tuning_of_astigmatism_makes_emmetropia_concrete (accessed on 5 September 2025).
- Reinstein, D.Z.; Archer, T.J.; Srinivasan, S.; Mamalis, N.; Kohnen, T.; Dupps, W.J.; Randleman, J.B. Standard for Reporting Refractive Outcomes of Intraocular Lens–Based Refractive Surgery. J. Refract. Surg. 2017, 33, 218–222. [Google Scholar] [CrossRef]

| Modality | Measures | Advantages | Limitations | Application in Cataract Surgery |
|---|---|---|---|---|
| Keratometry | Anterior Curvature | Simple, quick | No posterior data, limited area | Basic evaluation |
| Topography | Anterior (± posterior curvature) | Maps large area, detects irregularities | Variates by system | Preoperative planning |
| AS-OCT | Full anterior segment | High resolution, posterior data | Expensive media clarity dependent | Detailed planning |
| Scheimplug Imaging | Anterior and posterior surface | Full thickness evaluation of the cornea | Susceptible to corneal opacities, cannot evaluate structures beyond the iris or anterior chamber angle | IOL power calculations |
| Wavefront aberrometry | Total optical system | Detects high order aberrations | Requires clear media | Toric IOL alignment and fine-tuning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muşat, A.-A.-M.; Tãtaru, C.-P.; Muşat, G.-C.; Bubulac, L.; Preda, M.-A.; Muşat, O. Corneal Astigmatism After Cataract Surgery: A Review of Mechanisms, Outcomes, and Surgical Considerations. Optics 2025, 6, 42. https://doi.org/10.3390/opt6030042
Muşat A-A-M, Tãtaru C-P, Muşat G-C, Bubulac L, Preda M-A, Muşat O. Corneal Astigmatism After Cataract Surgery: A Review of Mechanisms, Outcomes, and Surgical Considerations. Optics. 2025; 6(3):42. https://doi.org/10.3390/opt6030042
Chicago/Turabian StyleMuşat, Andreea-Alexandra-Mihaela, Cãlin-Petru Tãtaru, Gabriela-Cornelia Muşat, Lucia Bubulac, Mihai-Alexandru Preda, and Ovidiu Muşat. 2025. "Corneal Astigmatism After Cataract Surgery: A Review of Mechanisms, Outcomes, and Surgical Considerations" Optics 6, no. 3: 42. https://doi.org/10.3390/opt6030042
APA StyleMuşat, A.-A.-M., Tãtaru, C.-P., Muşat, G.-C., Bubulac, L., Preda, M.-A., & Muşat, O. (2025). Corneal Astigmatism After Cataract Surgery: A Review of Mechanisms, Outcomes, and Surgical Considerations. Optics, 6(3), 42. https://doi.org/10.3390/opt6030042







