1. Introduction
Single-crystal diamond (SCD) and nanocrystalline diamond (NCD) present remarkable disparities in microstructure, physical properties, and application domains [
1,
2,
3,
4,
5,
6,
7,
8,
9]. SCD exhibits a perfect crystal structure, characterized by extremely high hardness, outstanding thermal conductivity, and exceptional optical transparency. Due to its long-range-ordered lattice arrangement, SCD demonstrates relatively high electron mobility. Consequently, it finds extensive applications in semiconductors, optical windows, and high-precision mechanical processing [
10,
11,
12,
13,
14,
15,
16]. NCD, comprising nanoscale grains, generally exhibits a lower defect density and higher isotropy. These properties confer upon it distinct advantages in fields such as tribology, biomedical coatings, and flexible electronic devices [
17,
18,
19,
20].
SCD and NCD can both undergo graphitization under laser irradiation. This transformation occurs as the laser-induced thermal effect elevates the temperature sufficiently to convert the sp
3-hybridized carbon structure of diamond into the sp
2-hybridized carbon structure characteristic of graphite. Wang et al. investigated the effects of nanosecond and picosecond laser irradiation on SCD. Their findings revealed that pulse durations of 8 ns, 50 ns, and 120 ns resulted in graphitization, cracking, and fragmentation, respectively. Additionally, at laser energy densities below 40 J/cm
2, periodic ripple structures with wavelengths ranging from 500 to 1100 nm were observed on the sidewalls [
21]. Zhang et al. explored the effects of a nanosecond pulsed laser on the crack propagation and surface morphology of SCD. It was discovered that a transition layer with the coexistence of sp
3/sp
2 was formed at an angle of 60–80° in the laser scanning direction, and the generation and expansion mechanism of cracks was elucidated by the thermoelastic bending model. This study also disclosed that the deposition metamorphic layer (DML) of the diamond material underwent a process from carbon island nucleation to the formation of the deposition layer [
22]. Bakhtiar Ali et al. conducted a study on the influence of an ultrashort pulsed femtosecond (fs) laser (30 fs, 800 nm, 1 kHz) on the graphitization of SCD. It was discovered that within the laser fluence range of 3.3 to 3.9 J/cm
2, the sp
3 structure gradually transformed into the sp
2 structure, and amorphous carbon was formed when the fluence exceeded 3.9 J/cm
2. Moreover, a 20% increase in the laser fluence (from 3.3 to 3.9 J/cm
2) resulted in an approximately threefold increase in the depth of the sp
2 graphitization region, suggesting that the nonlinear interaction of the femtosecond laser is a crucial factor for this phenomenon [
23].
In recent years, the phase transformation mechanisms of SCD and NCD under laser irradiation have garnered significant attention. Researchers worldwide have conducted in-depth studies on the phase transformation processes of these two types of diamond materials. Khomich et al. investigated the graphitization mechanism induced by a single pulse of a Ti:sapphire laser (100 fs, 266 nm) on the (111) surface of SCD. The experiments demonstrated that under optimal laser fluence conditions, highly ordered graphite (HOG) layers can be achieved; however, both the lower and higher laser energies lead to structural defects that disrupt the formation of HOG. Raman spectroscopy analysis revealed three distinct graphitization modes: non-ablative graphitization, conventional ablative graphitization, and bulk graphitization [
24]. Wang et al. investigated the ablation characteristics and material removal mechanisms of SCD using nanosecond and picosecond laser irradiation. This study determined the ablation thresholds under different laser pulse conditions (nanosecond laser: ~10 J/cm
2; picosecond laser: ~2 J/cm
2). It was found that nanosecond laser treatment primarily induces graphitization through thermal mechanisms, whereas picosecond laser treatment triggers non-thermal electron–phonon interactions, thereby reducing the formation of the heat-affected zone [
21]. Wu et al. conducted a finite element simulation to analyze the temperature evolution during the nanosecond pulsed laser ablation of diamond. They found that graphitization is the primary phase transition induced by thermal accumulation [
25].
However, despite the important insights provided by the aforementioned studies into the phase transition mechanisms of SCD and NCD, existing research predominantly focuses on individual material systems. There is a notable lack of systematic comparative analyses regarding their phase transition behaviors under identical laser irradiation conditions. In particular, the phase transition pathways, kinetic characteristics, and underlying microscopic mechanisms induced by nanosecond lasers remain insufficiently elucidated. Therefore, there is an urgent need to conduct a systematic comparative study on the phase transition mechanisms of SCD and NCD under the influence of nanosecond laser irradiation.
Molecular dynamics (MD) simulations, as a crucial method for elucidating the structural evolution and phase transition mechanisms of materials at the nanoscale, have demonstrated unique advantages in the study of diamond graphitization phase transitions. Zhao et al. demonstrated via molecular dynamics simulations that diamond undergoes the sp
3-to-sp
2 phase transition under ultraviolet nanosecond pulsed laser irradiation (43.8 J/cm
2, 45 ps pulse), initiating graphite formation at temperatures exceeding 1475 K with sp
2-hybridized atoms increasing linearly with fluence. Simulations revealed transient thermal gradients ranging from 3773 K at amorphous–graphite interfaces to 1475 K at graphite–diamond interfaces, along with a reduction in graphitization activation energy under higher fluence, collectively promoting the growth of thicker oriented graphite layers [
26]. Meanwhile, Zemojtel et al. conducted a comprehensive study on the chemical reactions at the diamond–glass interface under infrared laser irradiation and analyzed the changes in the sp
2/sp
3 ratio using MD simulation [
27]. Additionally, Cui et al. investigated the formation mechanism of chemically deposited diamond surfaces under laser irradiation conditions through MD simulation and compared the results of samples with different particle sizes. They determined the significant influence of GBs and particle size on the laser-induced graphitization process [
28]. Furthermore, Zhao et al. utilized MD simulation to study the local effects of ultrashort laser scribing on the microstructure, phase transformation, stress–strain distribution, and mechanical properties of diamond, thereby providing important insights into the phase transformation and structural changes in diamond during the laser irradiation [
29].
In this study, the MD method was employed to systematically investigate and compare the dynamic responses of SCD and NCD under nanosecond laser irradiation. Particular emphasis was placed on the graphitization process induced by laser irradiation and its underlying mechanisms. By analyzing the evolution of the microstructure, temperature distribution, and stress fields, this research elucidated the key driving factors and microscopic mechanisms governing the graphitization transformation in both types of diamonds. Consequently, this work enhances our understanding of the phase transformation behavior of diamond materials under nanosecond laser irradiation.
3. Results and Discussion
3.1. SCD and NCD Phase Transition Differences Under Nanosecond Pulsed Laser Irradiation
Figure 2 presents the results of the MD simulations conducted on nanosecond pulsed laser irradiation at an energy density of 20 J/cm
2. The cross-sectional snapshots of both SCD and NCD are color-coded based on their coordination numbers. The coordination number is defined as the count of neighboring atoms surrounding a central atom within a specified cutoff distance. The cutoff distance for carbon–carbon bonds is set at 1.6 Å, which slightly exceeds the first nearest neighbor distances of 1.5 Å in diamond and 1.4 Å in graphite. The coordination numbers for the yellow, green, blue, white, and black atoms are, respectively, 0, 1, 2, 3, and 4. It has been established that carbon atoms in solids typically have three or four neighboring atoms. For instance, the coordination numbers of sp
2- and sp
3-hybridized carbon allotropes are three and four, respectively. Therefore, atoms with a coordination number not exceeding three exist in gaseous or liquid states [
33].
It is found from
Figure 2a,b that SCD and NCD both exhibit a phenomenon of phase transition from diamond to graphite under laser irradiation, accompanied by the scattering of some diamond atoms due to high temperatures. In SCD, graphitization primarily initiates from the region subjected to laser ablation and gradually expands outward. This transformation demonstrates a strong directional characteristic, exhibiting diffusion from the center of ablation outward. In contrast, during the laser irradiation process of NCD, the behavior of graphitization is more complex. It occurs not only at the surface layer but also along GBs where graphite phases are formed, subsequently extending into the interior of grains. This indicates that GBs in nanocrystalline structures play a significant role in influencing the graphitization process under laser exposure.
It is found from
Figure 2c that NCD exhibits a phase transition from diamond to graphite during the laser irradiation process. This phenomenon can be attributed to the intense non-equilibrium state experienced by the atomic structure of diamond under laser irradiation. In the case of diamond’s cubic crystal structure, carbon atoms are bonded in a stable tetrahedral configuration through sp
3 hybridization [
14,
21]. In SCD, the local temperature rise caused by laser ablation is relatively uniform. Consequently, the propagation of graphitization is primarily controlled by thermal diffusion, manifesting as a spread from the ablation zone to the surrounding areas. In contrast, in NCD, the presence of numerous GBs leads to a relatively disordered atomic arrangement at these boundaries and weaker bonding interactions. This structural characteristic facilitates phase transitions under high-temperature conditions. Moreover, GBs serve as diffusion pathways with lower energy barriers that promote graphitization along these interfaces and further penetrate into the grains themselves. Therefore, in nanocrystalline systems, graphite not only grows inward from the surface but also preferentially precipitates along GBs before ultimately extending into the interior of individual grains. This results in a more complex phase transformation structure.
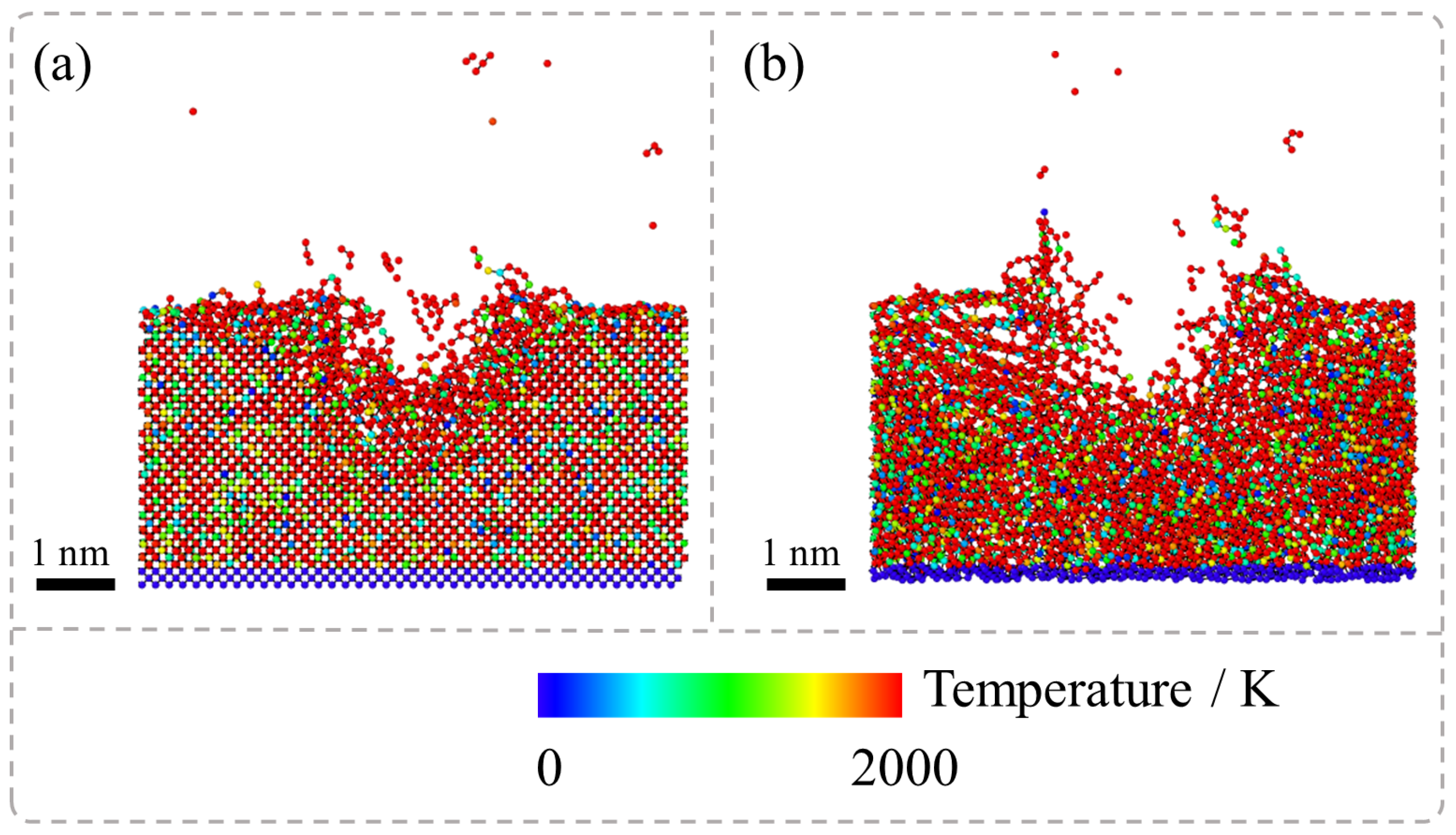
Figure 3 presents the results of MD simulations regarding the temperature evolution during the nanosecond pulsed laser irradiation of SCD and NCD captured at 15 ps during the laser irradiation stage. The system was initially equilibrated at 300 K for 20 ps before ablation. However, the localized temperature spikes induced by laser irradiation led to rapid bond breaking, graphitization, and material evaporation. It is observed that under laser exposure, the temperature of diamond atoms within the ablation pit surpasses 2000 K. This high-temperature environment facilitates the transformation of diamond into the graphite phase. However, a comparison between the temperature distribution in SCD and NCD reveals that during the ablation process of NCD, not only does the temperature in the ablation pit region exceed 2000 K but atomic temperatures at GB regions also surpass this threshold. In contrast, SCD exhibits high-temperature phase transitions solely within the ablation pit area, whereas in NCD, both the extent and overall temperature of high-temperature regions are significantly greater.
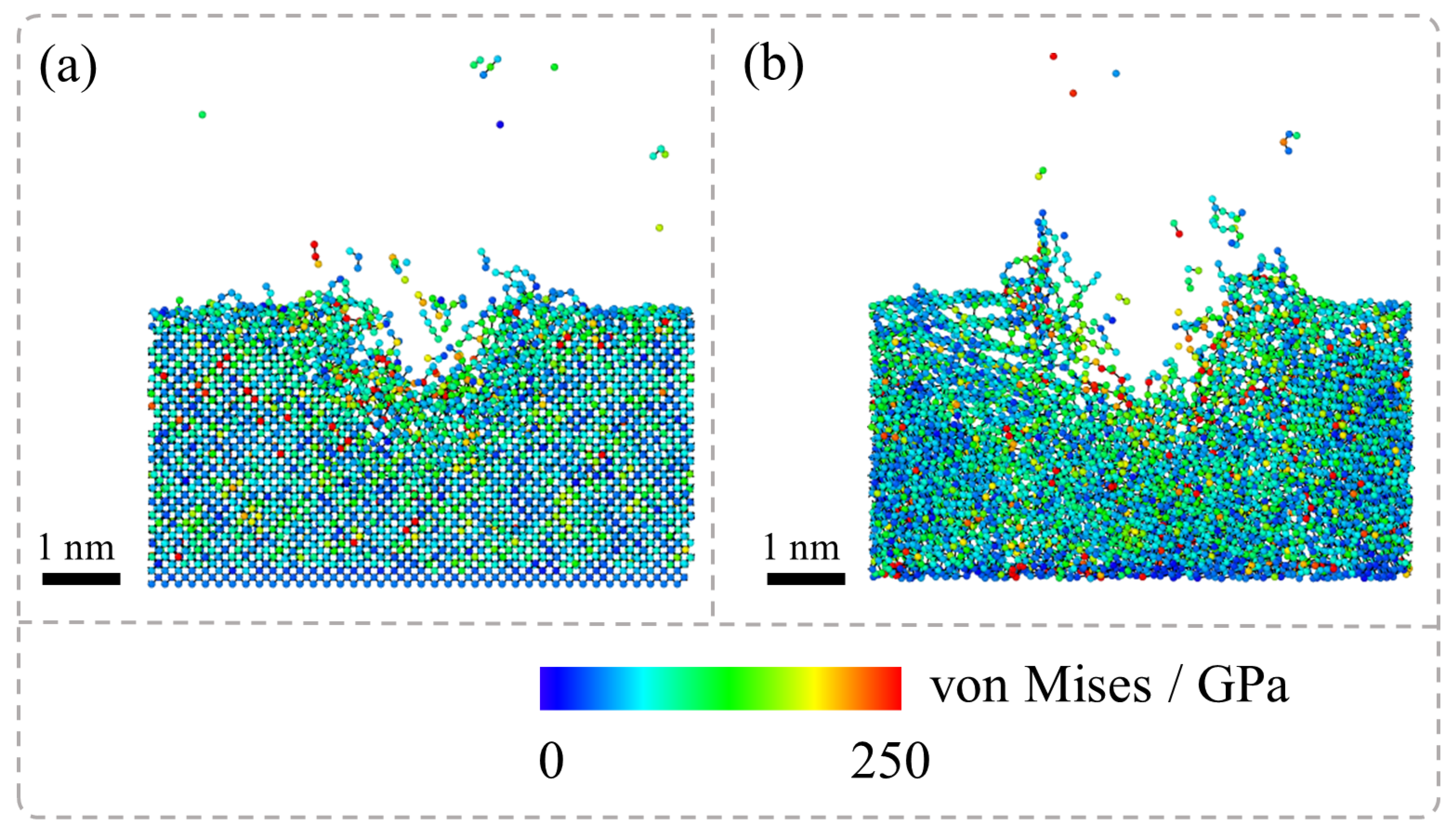
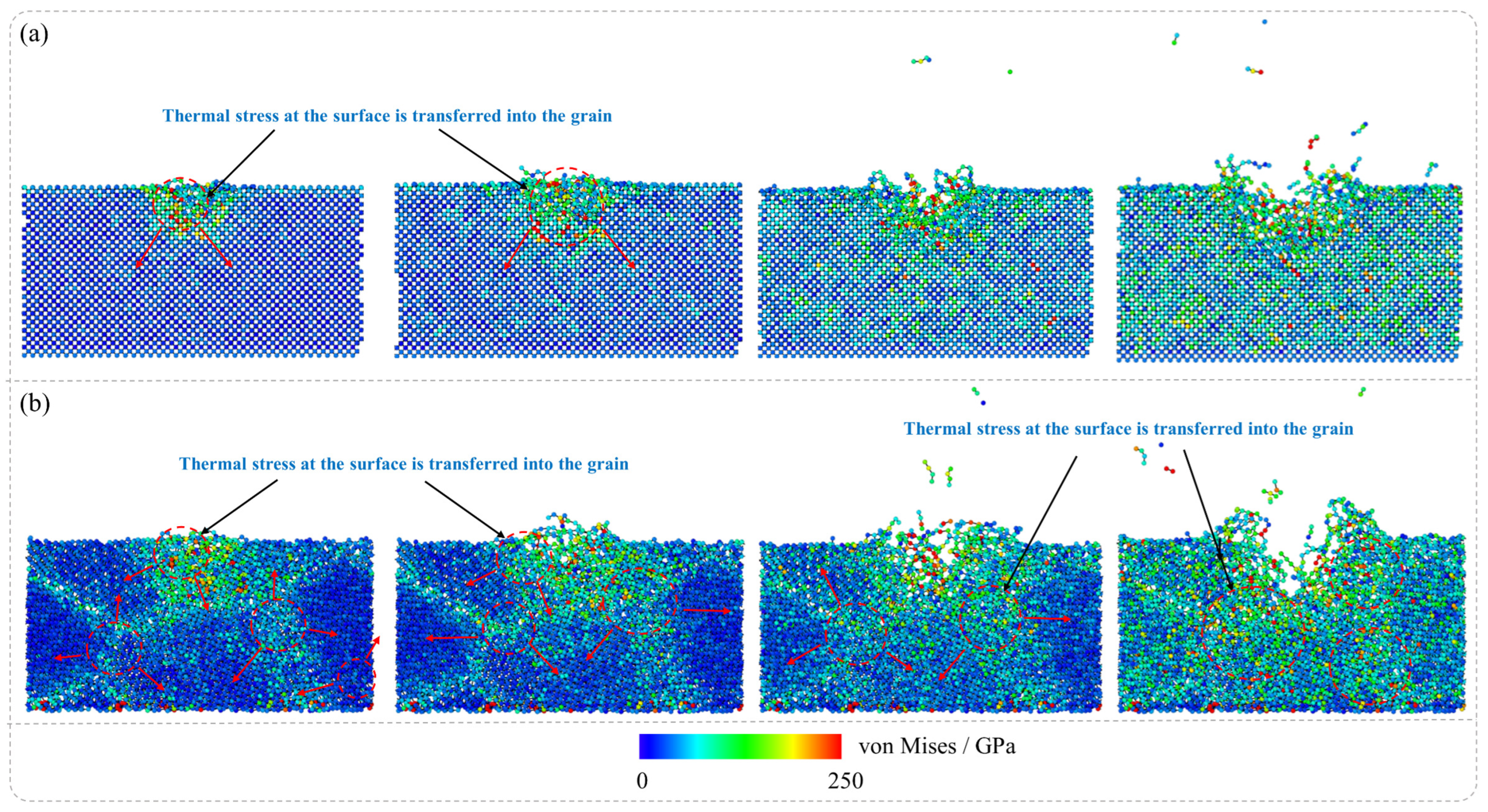
Figure 4 presents the von Mises stress distribution during the laser irradiation stage of the nanosecond pulsed laser irradiation of SCD and NCD, and it has been observed that during the laser irradiation of SCD and NCD, the simulation results indicate significant differences in their stress distribution characteristics. Specifically, in the laser machining process of SCD, high stress is localized primarily around the area near the ablation pit, while regions farther from the pit exhibit relatively low stress distribution. This suggests that the stress concentration in SCD under laser irradiation is confined to a limited local area. In contrast, for NCD, both the ablation pit and its surrounding boundaries experience considerable stress, indicating a broader range of force application. This phenomenon is closely related to temperature distribution characteristics; simulation analyses reveal distinct temperature field distributions for single-crystal diamond and NCD under laser exposure, which subsequently leads to differences in thermal stress distribution. The disparity in thermal stresses serves as a critical factor contributing to the notable differences in graphite phase transformation between these two materials when subjected to laser treatment. Consequently, phase transformation in SCD is predominantly restricted to areas near the ablation pit, whereas for NCD, this transformation extends into the GBs of the material under the laser influence.
3.2. Phase Transition Characteristics of SCD and NCD Under Nanosecond Pulsed Laser Irradiation
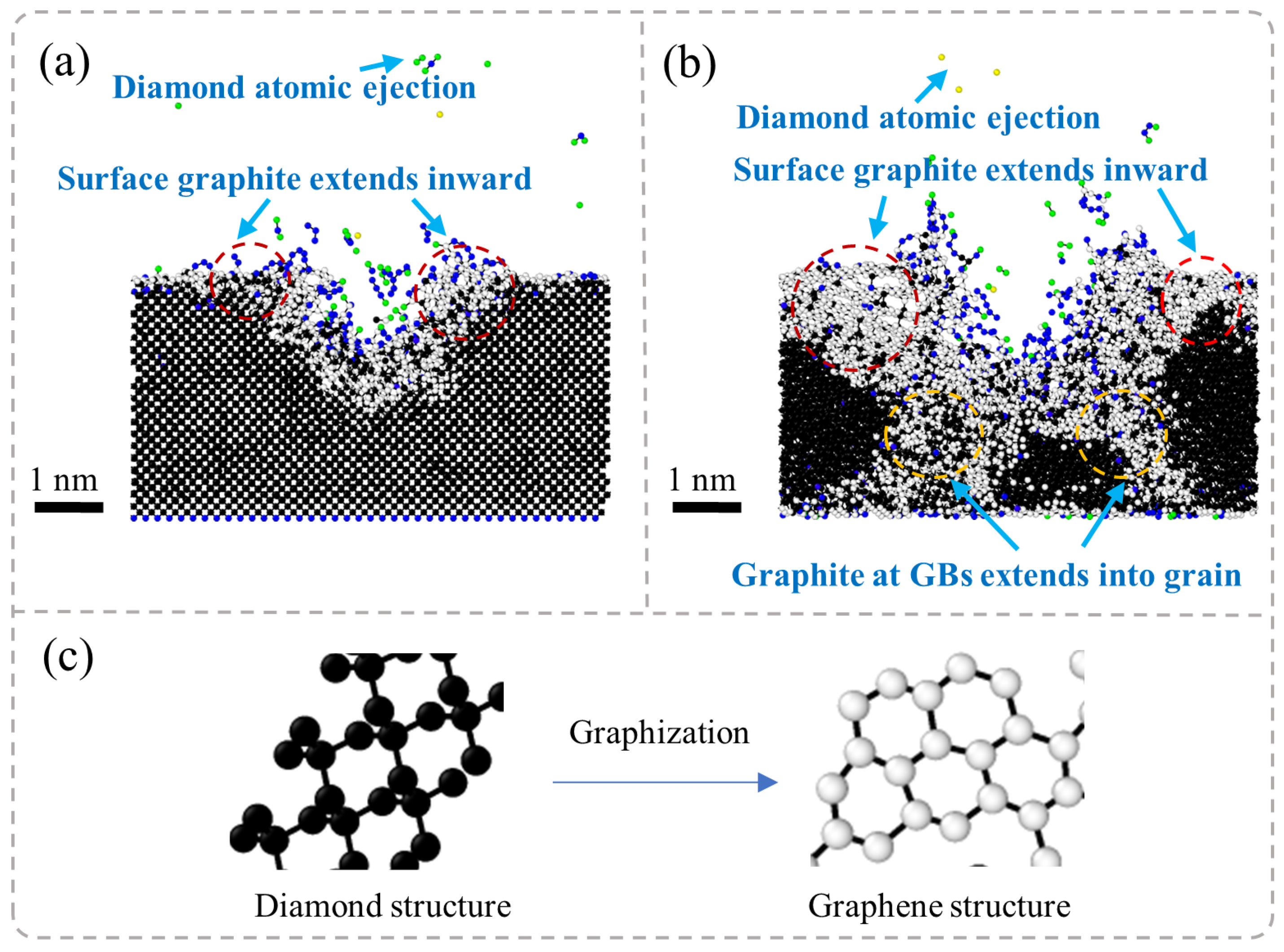
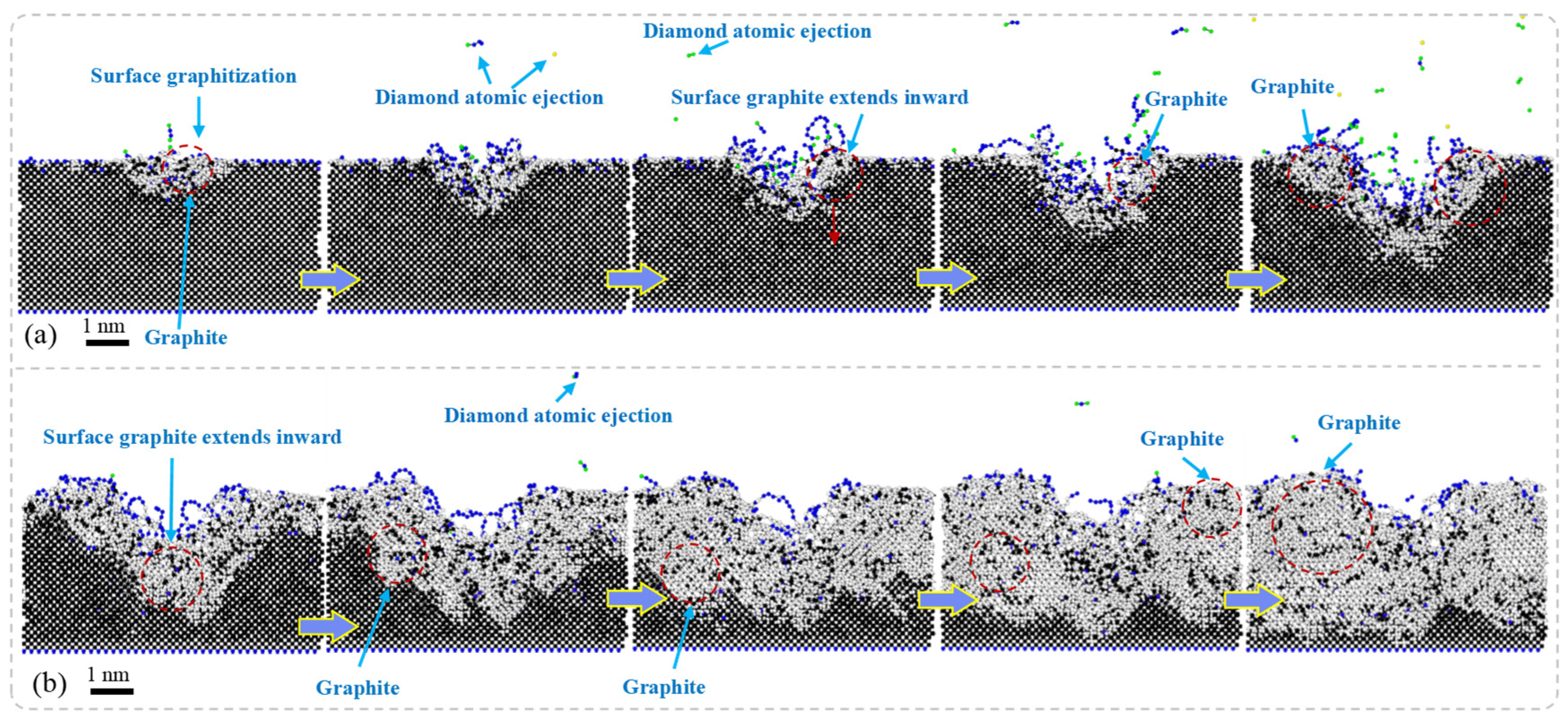
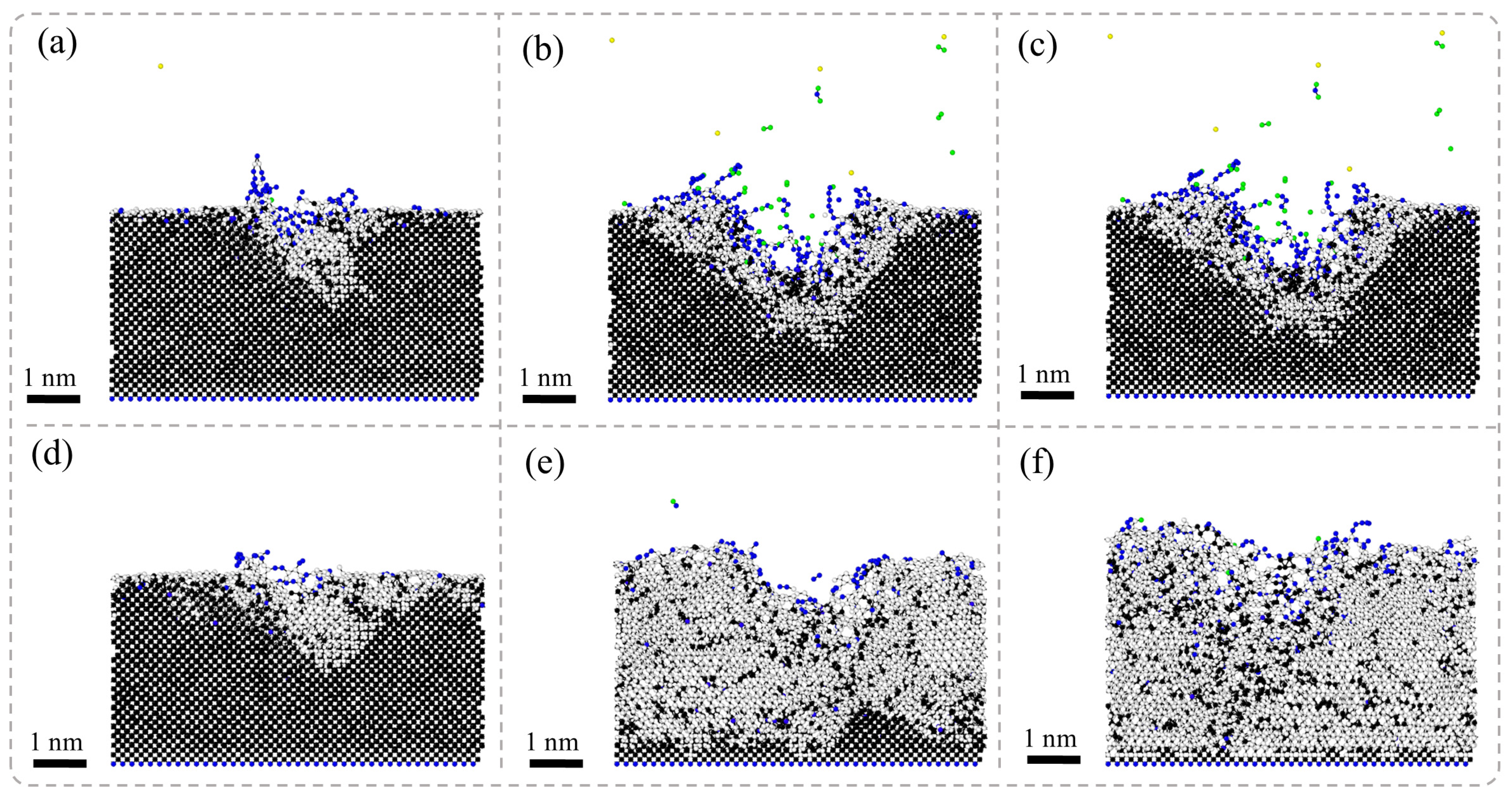
Figure 5 presents the results of MD simulations depicting the cross-sectional morphology of SCD subjected to nanosecond pulsed laser irradiation at different stages: the laser irradiation stage and the energy decay stage. In the laser irradiation stage, due to the deposition of laser energy, the temperature of the diamond surface rises rapidly, leading to non-equilibrium heating in localized regions. This phenomenon promotes a phase transition in the diamond, resulting in the initial formation of graphite phases. Under high-energy-density conditions, an atomic sputtering effect occurs rapidly within the region where the laser interacts with the diamond surface; some diamond atoms are directly ejected. Accompanying this process, graphite phases gradually expand both within and around the ablation pit and spread outward along its boundaries, even penetrating into the interior of the diamond. Ultimately, this leads to a significant accumulation of graphite near the ablation pit.
In the laser energy decay stage, the post-laser heating (referring to the sustained thermal effects maintained by thermal inertia and residual energy after laser pulse termination) maintains the system under high-temperature and high-pressure conditions, where the gradual energy release drives further structural transformations in diamonds. The entire system tends toward thermodynamic stability as the diamonds progressively convert into graphite. Due to diamonds having a much higher density than that of graphite, volumetric expansion occurs during this phase transition process. Consequently, this results in a transformation from dense diamond structures to layered graphite forms.
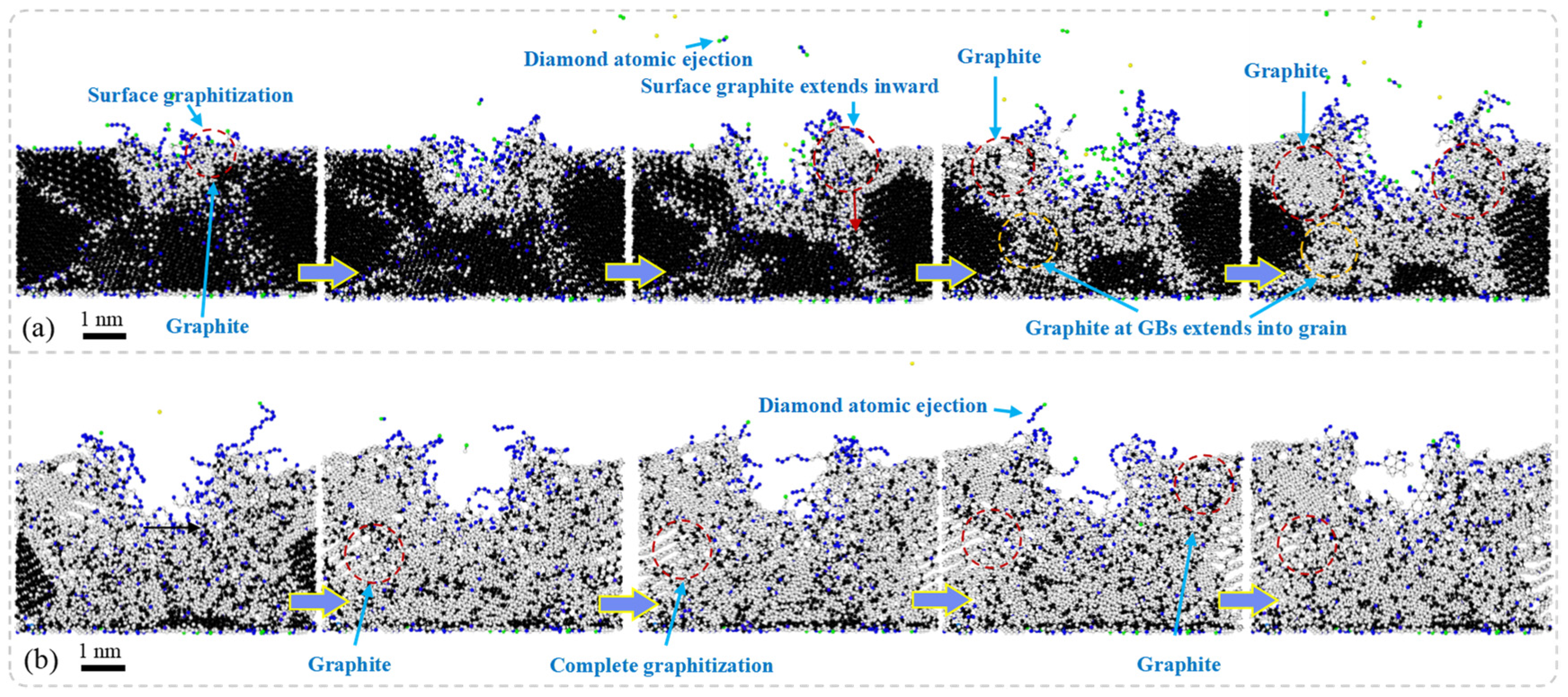
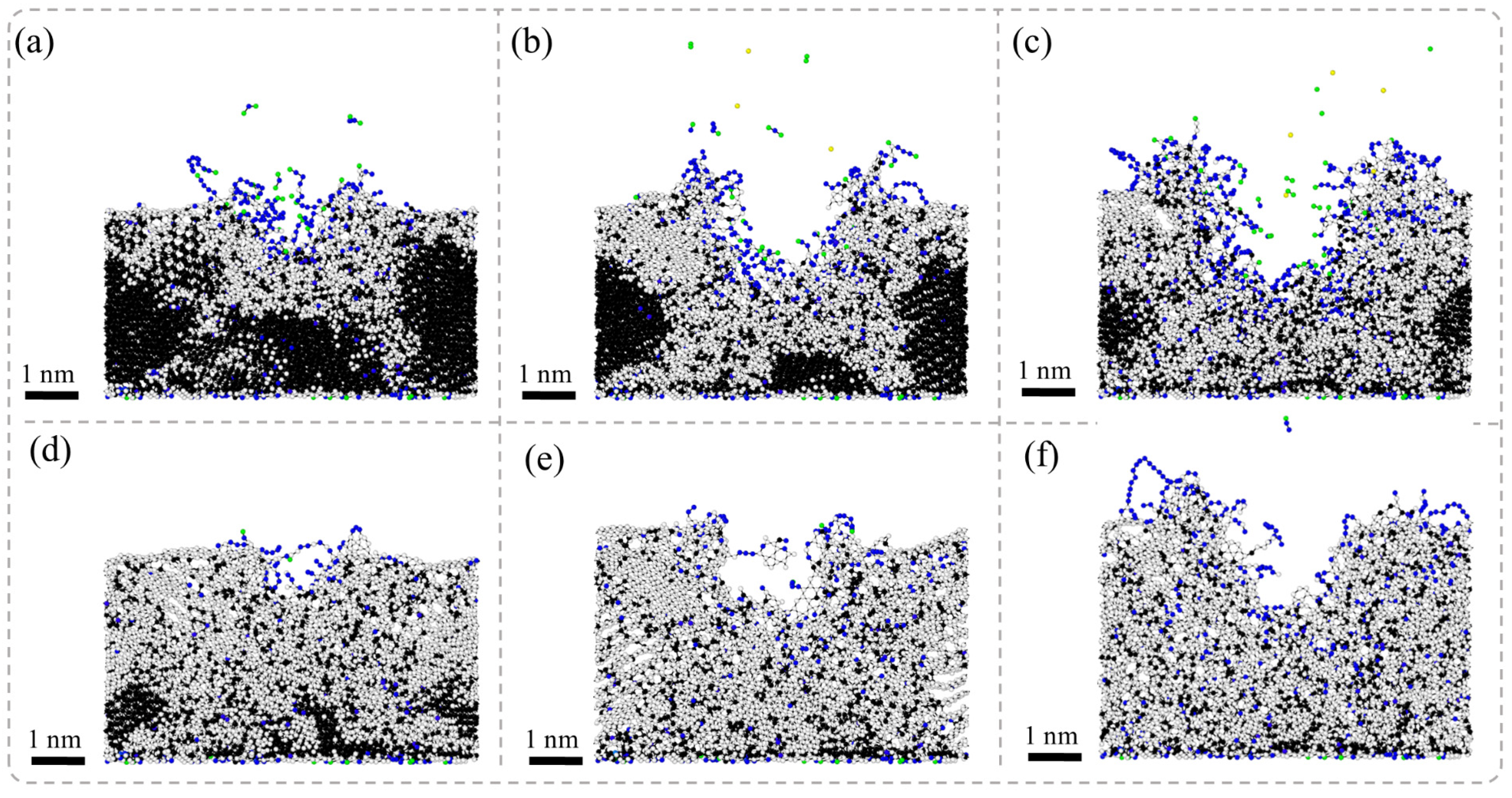
Figure 6 presents the results of MD simulations depicting the cross-sectional morphology of NCD subjected to nanosecond pulsed laser irradiation at different stages: the laser irradiation stage and the energy decay stage. In the laser irradiation stage, the surface of NCD experiences a rapid increase in temperature, leading to phase transitions in localized regions and resulting in surface graphitization. As laser energy continues to be inputted, the graphitization phenomenon extends beyond the surface and gradually propagates towards the GB regions. Significant ablation occurs on the material’s surface, forming ablation pits accompanied by the ejection of diamond atoms, which results in the localized mass loss of the material. Subsequently, graphite phases begin to expand from these ablation pits into surrounding areas, while graphitized atoms at GBs further infiltrate into the interior of grains, progressively creating larger zones of graphitization. During the energy decay stage, graphitization continues to spread, ultimately causing the volumetric expansion of the material and forming layered graphite structures around the ablation pits as well as throughout the entire model. Compared to SCD, NCD exhibits a more pronounced response to graphitization under laser exposure.
The NCD is composed of numerous grains and their GBs. Compared to SCD, the atomic arrangement in the boundary regions is more disordered, resulting in lower bonding energy. Consequently, structural transformations are more likely to occur under high-temperature conditions. Particularly at the GBs, where bonding energies are weaker, the graphitization process occurs more rapidly, leading to the expansion of graphite into the interior along these boundaries. Moreover, the formation of laser ablation pits is accompanied by localized melting and evaporation of materials, which alters the surrounding stress state and subsequently affects phase transition behavior. This promotes a broader expansion of graphite. During the energy dissipation stage, heated materials gradually cool down; however, since graphitization is an irreversible process, the further development of graphite within the material occurs alongside volumetric expansion phenomena. The formation of this layered graphite structure is closely related to rapid thermal expansion and cooling rates during laser heating processes. Additionally, smaller grain sizes and higher GB densities exacerbate this phenomenon, making NCD more susceptible to graphitization than SCD.
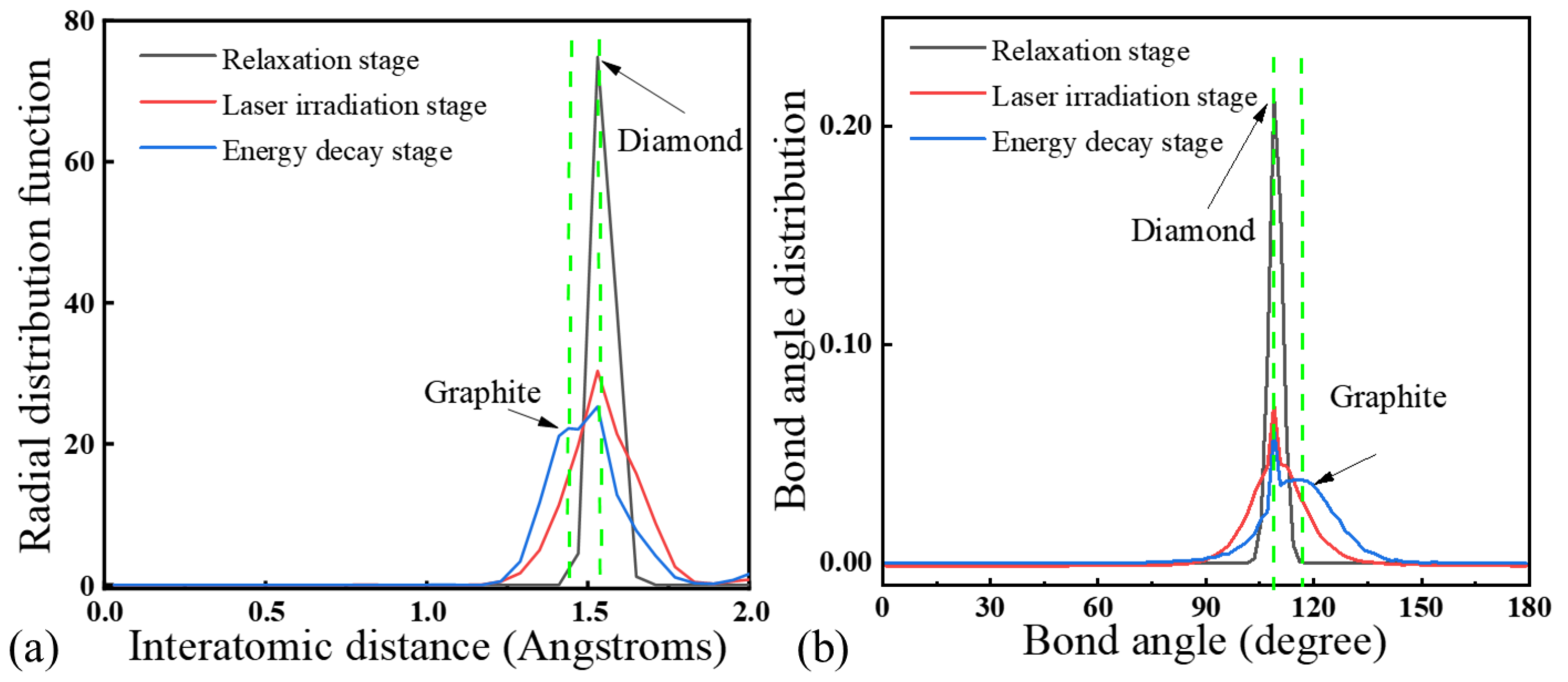
Figure 7 presents the RDF analysis of an SCD sample at different stages of laser irradiation. Following the laser irradiation phase, the characteristic peaks of the diamond significantly weaken, accompanied by a faint emergence of graphite peaks. This indicates that during this process, the carbon atoms in the localized regions undergo rearrangement, leading to a partial transformation of the diamond structure into graphite phases. After the energy dissipation phase concludes, the diamond peaks further diminish while the graphite peaks are markedly enhanced. This suggests that the system progressively evolves towards graphitization, ultimately resulting in a state where both diamond and graphite coexist. Under nanosecond laser irradiation, the SCD lattice rapidly absorbs energy, resulting in a sudden increase in local temperature. This leads to enhanced atomic thermal vibrations, causing the rearrangement of sp
3-hybridized carbon bonds within the diamond structure. Due to the decreased stability of diamond at elevated temperatures, its local structure may undergo carbon–carbon bond breakage and reorganization. Consequently, certain regions transition from sp
3-hybridized carbon to sp
2-hybridized carbon, thereby forming graphite phases. During the energy dissipation stage, as the system gradually cools down, some carbon atoms remain in a graphitized state while other areas retain their diamond structure. This results in a coexistence of the diamond and graphite phases.
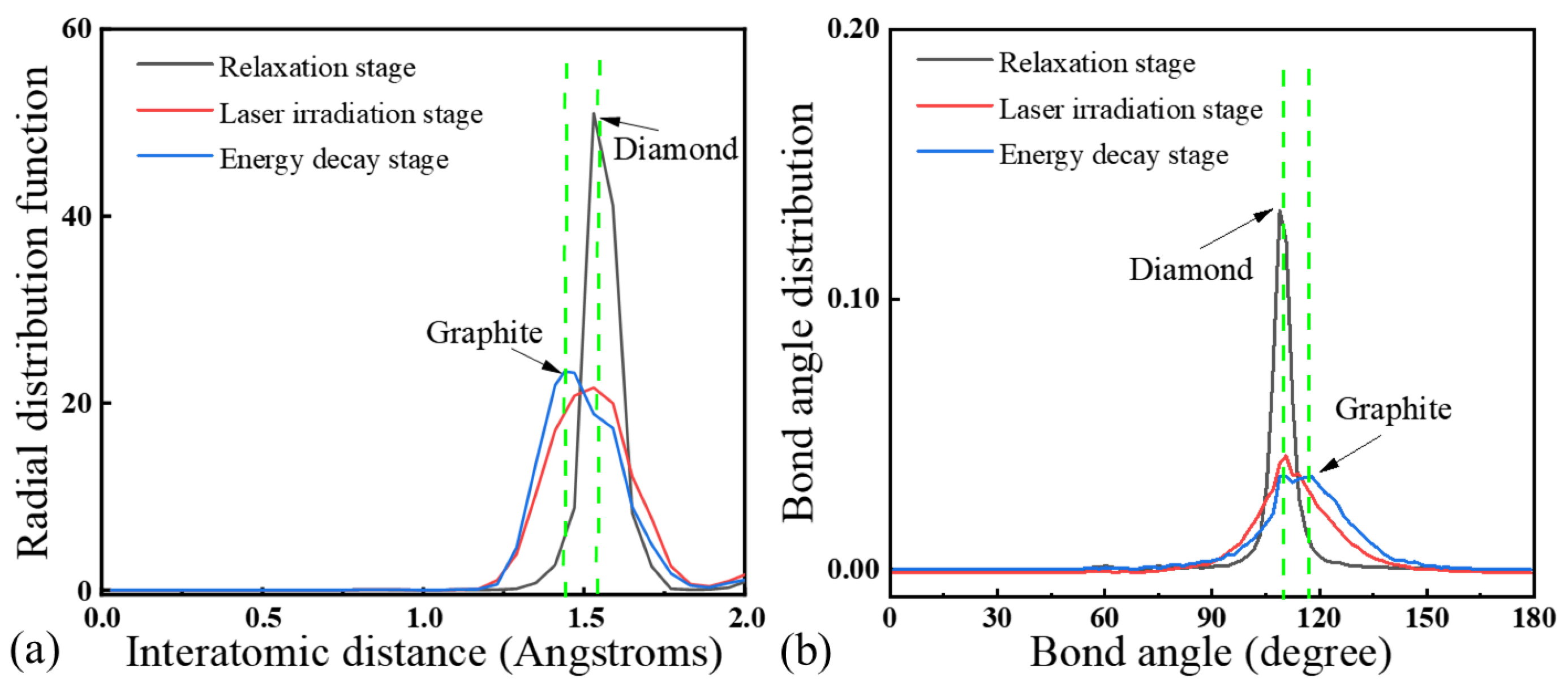
Figure 8 presents the RDF analysis of an NCD sample at different stages of laser irradiation. During the process of the laser ablation of NCD, it was observed that in the laser irradiation stage, as the laser irradiation progressed, the characteristic peaks of diamond gradually diminished while the peaks associated with graphite significantly intensified. This indicates a structural transformation from diamond to graphite. Such structural changes became particularly evident after the conclusion of the energy decay stage. In the final spectrum, only the graphite peaks were detectable, and all the diamond peaks had completely vanished, indicating that NCD had undergone complete surface graphitization.
MD simulations reveal that the diamond lattice undergoes a direct solid-state phase transition to a highly oriented graphite layer during nanosecond pulsed laser ablation. Within the laser irradiation stage, sp3 bonds break and reconstruct into sp2 hybridization, with no intermediate molten carbon phase. Radial distribution function RDF and BAD analyses confirm the sp2 bond length and angles matching those of graphite, while the sp3 signatures vanish. The sharp graphite–diamond interface without molten or disordered regions further indicates a solid-state diffusion-dominated transition.
Figure 9 illustrates von Mises stress distribution during the laser irradiation stage of the nanosecond pulsed laser irradiation of SCD and NCD. The stress evolution in SCD and NCD exhibits significant differences. In the laser ablation region of SCD (
Figure 9a), an initial increase in stress levels is observed, which then propagates radially outward. This indicates that the thermally induced stress from the laser accumulates rapidly in a localized area and transmits through the lattice structure to surrounding regions. In contrast, for NCD (
Figure 9b), the stress resulting from laser exposure also experiences a notable increase initially within the affected zone and similarly expands outward. However, compared to SCD, the overall stress levels in NCD are higher, and the evolution of this stress is more uniform, gradually spreading throughout the entire material over time. The differing patterns of stress evolution directly influence their respective graphitization phase transition mechanisms.
The microstructural characteristics of materials play a decisive role in the evolution of laser-induced stress and its phase transition mechanisms. SCD possesses a complete long-range-ordered lattice structure, where thermal expansion and stress conduction are primarily influenced by crystallographic anisotropy. Under laser irradiation, thermal expansion induces high thermal stresses in localized regions; however, due to the integrity of the single-crystal structure, stress is predominantly transmitted through the lattice, resulting in a more concentrated stress gradient that diffuses along specific directions. In contrast, NCD consists of nanoscale diamond particles with a high density of GBs and small grain sizes. This complexity leads to more intricate heat conduction pathways, causing the distribution of laser energy within the material to be more uniform. Furthermore, the presence of GBs restricts directional stress propagation, promoting the uniform accumulation of stress over a larger area and ultimately resulting in higher overall stress levels. This enhanced uniformity in stress distribution amplifies local thermal accumulation effects within the material and facilitates homogeneous graphitization transitions. Consequently, the graphitization phase transitions in SCD are often confined to localized areas, whereas NCD exhibits an increased propensity for phase transitions across broader regions.
3.3. Effect of Laser Energy Density on SCD and NCD Laser-Induced Graphitization
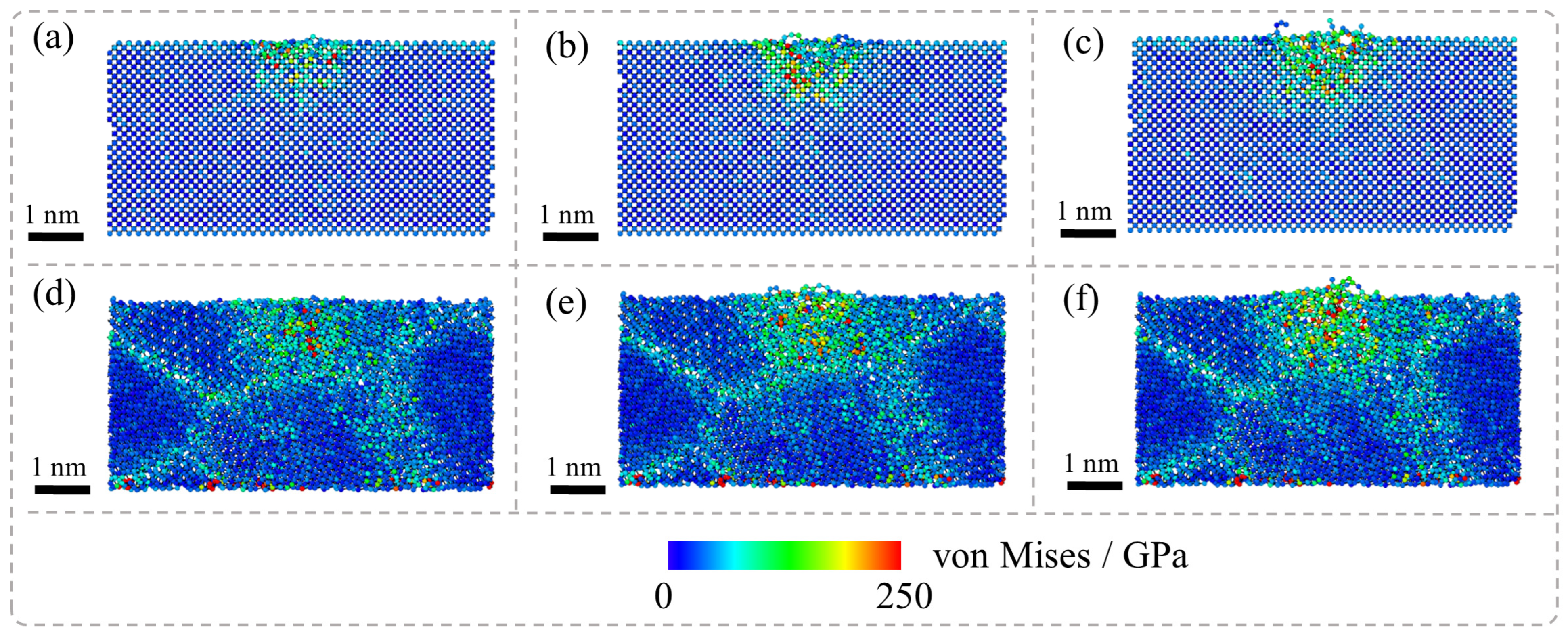
Figure 10 illustrates the cross-sectional morphology of SCD subjected to nanosecond pulsed laser irradiation at varying stages and laser energy densities. As the laser energy density increases, the size of the ablation pit significantly expands at the end of the laser irradiation phase. Concurrently, the degree of graphitization surrounding the ablation pit intensifies. During the energy dissipation phase, a higher laser energy density results in an increased overall expansion of the material, leading to a notable increase in volume and a further exacerbation of graphitization. This process substantially enhances the quantity of the layered graphite formed.
Figure 11 illustrates the cross-sectional morphology of NCD subjected to nanosecond pulsed laser irradiation at varying stages and laser energy densities. In the laser irradiation stage, as the energy density of the laser increases, the ablation pit area on the material’s surface significantly expands, and the graphite formation at GBs becomes increasingly pronounced. Specifically, under conditions of low energy density, ablation is primarily confined to localized areas with a relatively low degree of graphitization; limited graphitization only occurs at the surface and certain GB regions. However, when laser energy density is elevated, not only does the ablation area expand but so too does the region exhibiting the graphitization at GBs, displaying more pronounced phase transition characteristics. During the energy dissipation stage, further observations reveal that under higher-energy-density conditions, there is a substantial increase in the graphitization levels. Additionally, the formation of layered graphite structures becomes more evident as layered graphitized areas gradually develop within the material.
Figure 12 illustrates the von Mises stress distribution of NCD and SCD subjected to nanosecond pulsed laser irradiation with different diamond and laser energy densities, which reveal that the von Mises stress field during the laser irradiation stage dynamically correlates with bond breaking and graphitization. At the same time point, there are significant differences in the thermal stress distribution between SCD and nanocrystalline diamond under varying laser energy density conditions. For SCD, as the laser energy density increases, the range of high-stress distribution concentrated near the area affected by the laser gradually expands. In contrast, for nanocrystalline diamond, in addition to the expansion of high-stress areas directly influenced by the laser as the energy density increases, a notable rise in stress levels can also be observed at GB regions. Furthermore, the extent of the affected GBs broadens with increasing laser energy density. This phenomenon aligns with previous analyses regarding thermal stress during the graphite phase transition occurring under the laser treatment on diamonds and indicates that the characteristics of thermal stress distribution play a crucial role during phase transitions.
In SCD, due to the continuity of the crystal structure and the limited presence of defects, laser-induced thermal stress is primarily influenced by localized thermal expansion, resulting in a relatively uniform stress distribution within the material. In contrast, NCD comprises numerous grains and GBs; the atomic arrangement at these boundaries is disordered with lower binding energy. Consequently, under laser irradiation, stress concentration is more readily generated in this form of diamond. Additionally, the thermal conductivity at GBs typically falls below that within individual grains, leading to heat accumulation in boundary regions which results in elevated local temperature gradients that further intensify thermal stress levels. Furthermore, increased thermal stress facilitates a reduction in the activation energy for graphitization transitions, thereby making it easier for graphitization to occur at GBs and enabling its expansion under conditions of higher energy density.
4. Summary
In summary, we computationally explored the mechanism of graphitization on the surfaces of SCD and NCD under laser irradiation. The results indicate that stress in SCD is concentrated in localized areas with a relatively limited range, leading to a more localized graphitization process. In contrast, NCD exhibits a broader stress distribution, particularly around ablation pits and GBs where stress is higher, suggesting a wider area of influence and greater susceptibility to structural transformation at elevated temperatures. Notably, due to weaker atomic bonding at GBs, the graphitization process occurs rapidly there, causing graphite to expand inward along these boundaries.
The microstructure of the material plays a crucial role in the evolution of laser-induced stress and phase transition mechanisms. The presence of GBs restricts directional stress propagation while promoting uniform accumulation over larger areas, resulting in an overall higher stress level that further affects phase transition behavior. Under varying laser energy densities, differences in microstructures between SCD and NCD lead to distinct thermal stress distributions at their GB and surface regions, significantly influencing their respective graphitization behaviors under laser exposure. These findings provide important theoretical insights for understanding and controlling the surface modification processes of diamond materials during laser irradiation.