Growth and Properties of (Yb-Er) Co-Doped ZnO Thin Films Deposited via Spray Pyrolysis Technique
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
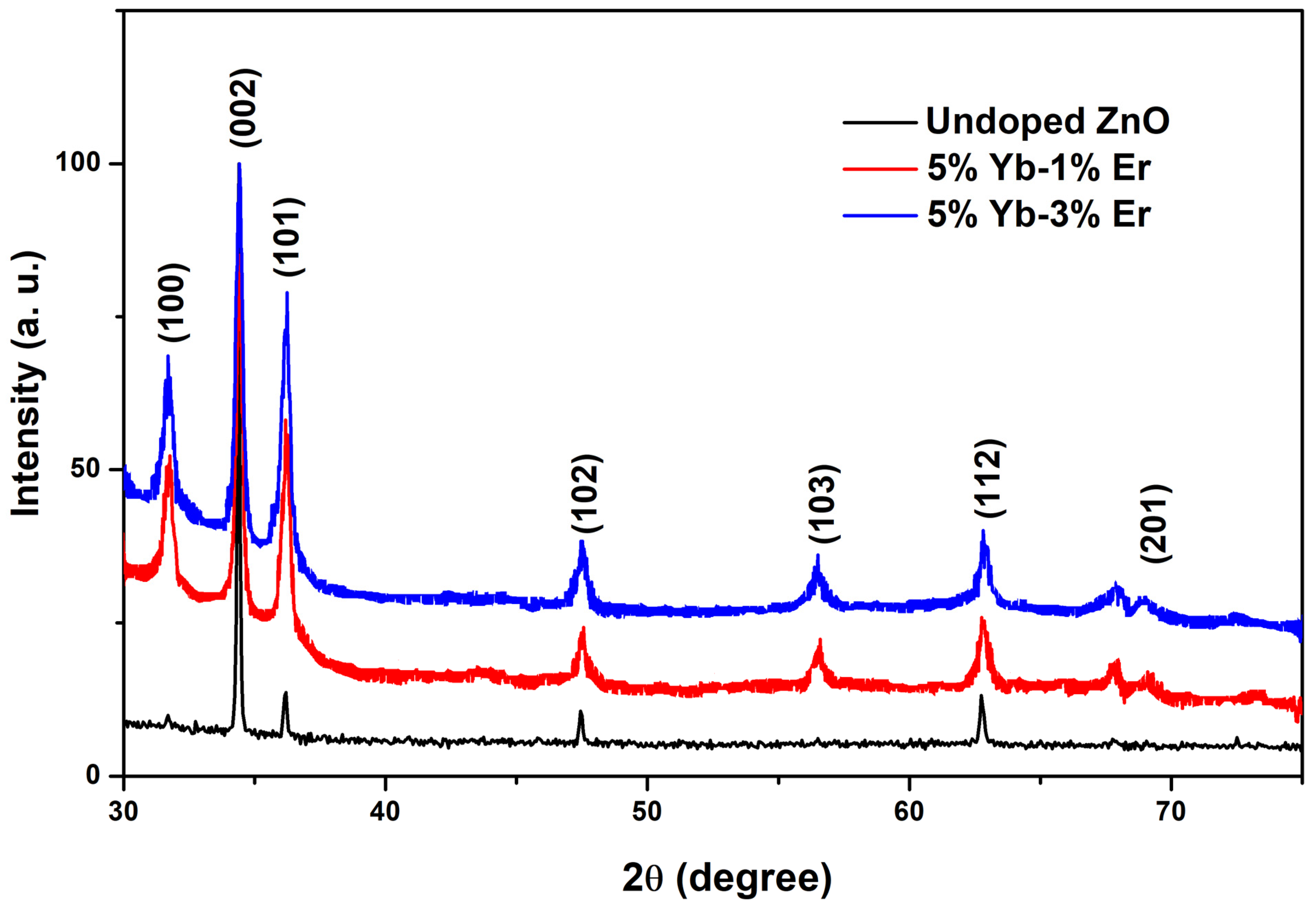
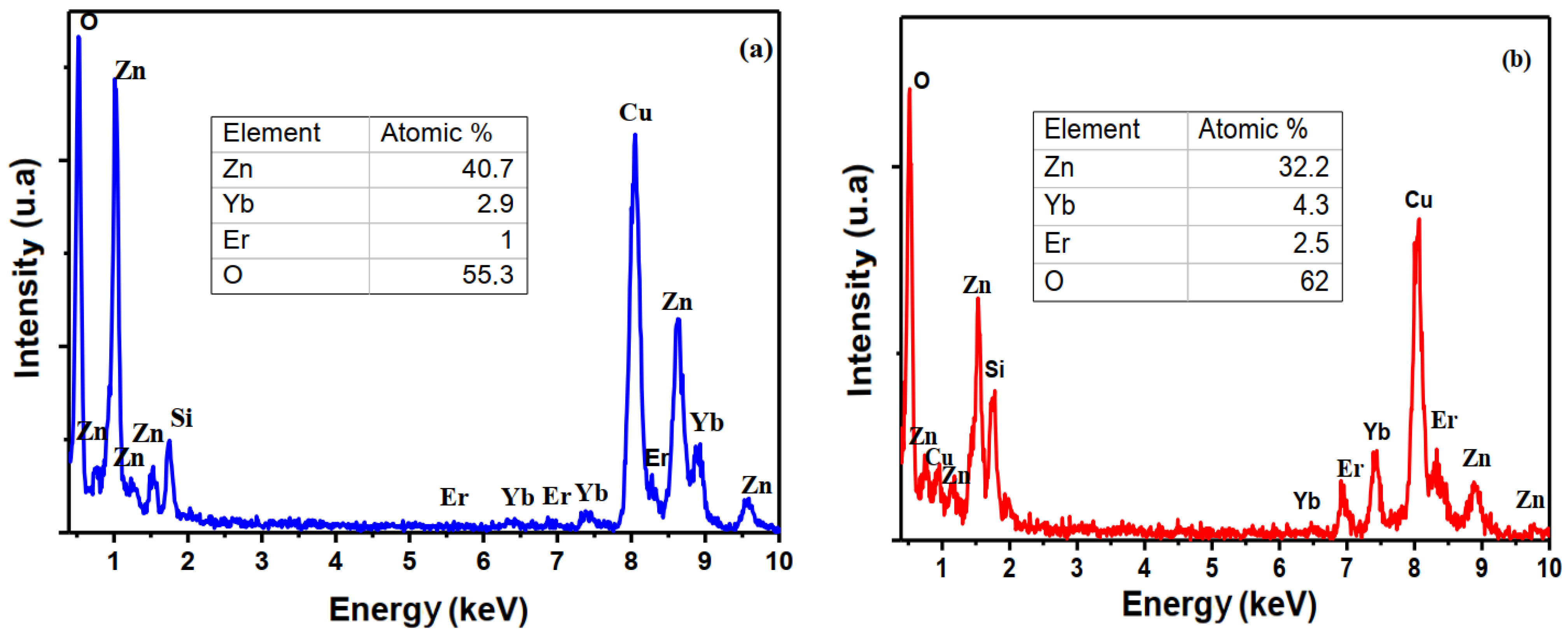
3.1. Micro-Structural and Morphological Properties
| Samples | D (nm) | Tc (002) | δ (Lines·µm−2) | a (nm) | c (nm) | Ref |
|---|---|---|---|---|---|---|
| Undoped ZnO | 76 | 2.7 | 173 | 0.3262 | 0.5201 | This work |
| 5% Yb | 83 | 3.5 | – | 0.3260 | 0.5202 | [23] |
| 1% Er | 27 | – | 1370 | 0.3255 | 0.5265 | [24] |
| 5% Yb_1% Er | 28 | 1.6 | 1270 | 0.3187 | 0.5204 | This work |
| 5% Yb_3% Er | 28 | 1.6 | 1270 | 0.3188 | 0.5207 | This work |
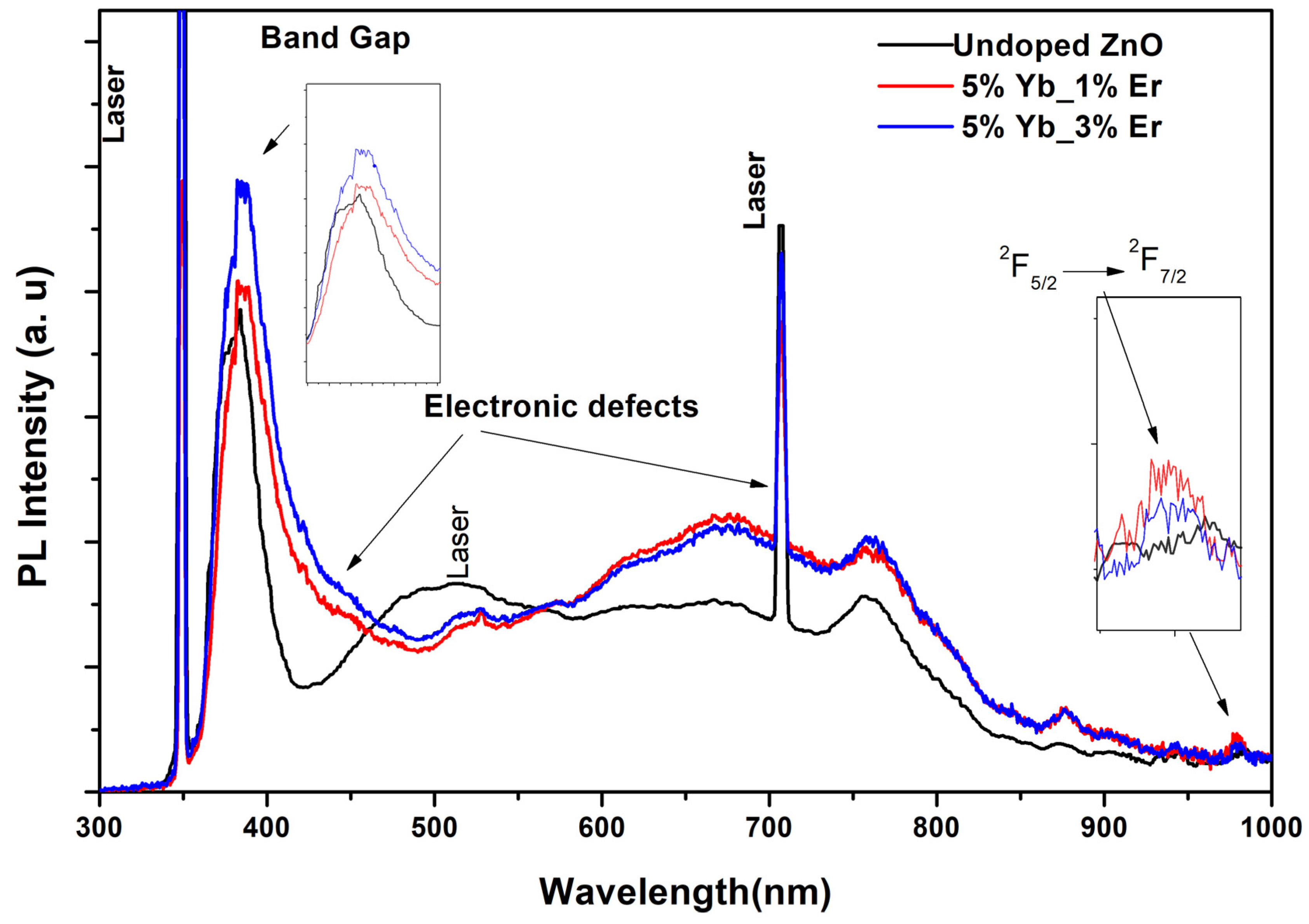
3.2. Optical Properties and Photoluminescence
3.3. Electrical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.; Kamruzzaman, M.; Zapien, J.A.; Afrose, R.; Anam, T.K.; Liton, M.N.H.; Helal, M.A.; Khan, M.K.R. Conversion of n-type to p-type conductivity in ZnO by incorporation of Ag and Ag-Li. Mater. Today Commun. 2022, 33, 104278. [Google Scholar] [CrossRef]
- Suman, A.; Chahal, S.; Kumar, S.; Kumar, A.; Duhan, S.; Kumar, P. Magnetism in Zinc Oxide (ZnO). In Defect-Induced Magnetism in Oxide Semiconductors; Woodhead Publishing: Sawston, UK, 2023; pp. 547–561. [Google Scholar]
- Li, X.; Yan, J.; Yu, T.; Zhang, B. Versatile nonfluorinated superhydrophobic coating with self-cleaning, anti-fouling, anti-corrosion and mechanical stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128701. [Google Scholar] [CrossRef]
- Le, A.T.; Ahmadipour, M.; Pung, S.-Y. A review on ZnO-based piezoelectric nanogenerators: Synthesis, characterization techniques, performance enhancement and applications. J. Alloys Compd. 2020, 844, 156172. [Google Scholar] [CrossRef]
- Fujii, S.; Adachi, Y.; Uchino, T. Excitonic stimulated emission from MgxZn1− x O films due to enhanced exciton binding energy. Phys. Rev. B 2020, 102, 075204. [Google Scholar] [CrossRef]
- Gherendi, F.; Dobrin, D.; Nistor, M. Transparent Structures for ZnO Thin Film Paper Transistors Fabricated by Pulsed Electron Beam Deposition. Micromachines 2024, 15, 265. [Google Scholar] [CrossRef]
- Badgujar, A.C.; Yadav, B.S.; Jha, G.K.; Dhage, S.R. Room temperature sputtered aluminum-doped ZnO thin film transparent electrode for application in solar cells and for low-band-gap optoelectronic devices. ACS Omega 2022, 7, 14203–14210. [Google Scholar] [CrossRef]
- Saadi, H.; Khaldi, O.; Pina, J.; Costa, T.; Seixas de Melo, J.S.; Vilarinho, P.; Benzarti, Z. Effect of Co doping on the physical properties and organic pollutant photodegradation efficiency of ZnO nanoparticles for environmental applications. Nanomaterials 2024, 14, 122. [Google Scholar] [CrossRef]
- Malyutina-Bronskaya, V.; Zalesski, V.; Zhyhulin, D.; Mudryi, A. Structural, optical and photoelectric properties of Tb doped ZnO thin films for device applications. Opt. Mater. 2022, 127, 112305. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Ahmad, A.A.; Qattan, I.A.; Al-Bataineh, Q.M.; Albataineh, Z. Structural, optoelectrical, linear, and nonlinear optical characterizations of dip-synthesized undoped ZnO and group III elements (B, Al, Ga, and In)-doped ZnO thin films. Crystals 2020, 10, 252. [Google Scholar] [CrossRef]
- Abdel-Galil, A.; Hussien, M.S.; Yahia, I.S. Synthesis and optical analysis of nanostructured F-doped ZnO thin films by spray pyrolysis: Transparent electrode for photocatalytic applications. Opt. Mater. 2021, 114, 110894. [Google Scholar] [CrossRef]
- Kumawat, A.; Chattopadhyay, S.; Kumar Verma, R.; Prakash Misra, K. Eu doped ZnO nanoparticles with strong potential of thermal sensing and bioimaging. Mater. Lett. 2022, 308, 131221. [Google Scholar] [CrossRef]
- Gong, X.; Tang, L.; Wang, R.; Guo, Z.; Huang, P.; Zhou, L.; He, R. Achieving efficient photocatalytic uranium extraction within a record short period of 3 min by Up-conversion erbium doped ZnO nanosheets. Chem. Eng. J. 2022, 450, 138044. [Google Scholar] [CrossRef]
- Cerrato, E.; Zickler, G.A.; Paganini, M.C. The role of Yb doped ZnO in the charge transfer process and stabilization. J. Alloys Compd. 2020, 816, 152555. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Yang, K.; Zhang, X.; Song, Y.; Wang, C.H. Enhanced upconverted photoluminescence in Er3+ and Yb3+ codoped ZnO nanocrystals with and without Li+ ions. Opt. Commun. 2008, 281, 5448. [Google Scholar] [CrossRef]
- Lluscà, M.; López-Vidrier, J.; Antony, A.; Hernández, S.; Garrido, B.; Bertomeu, J. Up-conversion effect of Er-and Yb-doped ZnO thin films. Thin Solid Film 2014, 562, 456. [Google Scholar] [CrossRef][Green Version]
- Shinde, S.; Shinde, V.; Wadkar, P. Rapid Response and Quick Recovery LPG Sensor Fabricated Using Aqueous Sol–Gel Synthesized ZnO/Zn (OH)2 Hexagonal Nanoparticles. J. Electron. Mater. 2024, 53, 5222–5237. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, W.; Zhang, L. Crystalline size effects on texture coefficient, electrical and optical properties of sputter-deposited Ga-doped ZnO thin films. J. Mater. Sci. Technol. 2015, 31, 175–181. [Google Scholar] [CrossRef]
- Sadatgol, M.; Bihari, N.; Joshua, M.P.; Durdu, O.G. calable honeycomb top contact to increase the light absorption and reduce the series resistance of thin film solar cells. Opt. Mater. Express 2019, 9, 256–268. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Correia, F.C.; Salvador, P.B.; Rebouta, L.; Alves, L.C.; Alves, E.; Barradas, N.P.; Mendes, A.; Tavares, C.J. Compositional analysis by RBS, XPS and EDX of ZnO: Al, Bi and ZnO: Ga, Bi thin films deposited by dc magnetron sputtering. Vacuum 2019, 161, 268–275. [Google Scholar] [CrossRef]
- Scherrer, P. Gott. Nachrichten 1918, 2, 98. [Google Scholar]
- Prabhu, Y.T.; Sreedhar, B.; Pal, U. Achieving enhanced photocatalytic activity of ZnO supported on MWCNTs towards degradation of pollutants under visible light. Mater. Today Proc. 2019, 8, 419–426. [Google Scholar] [CrossRef]
- Soumahoro, I.; Schmerber, G.; Douayar, A.; Colis, S.; Abd-Lefdil, M.; Hassanain, N.; Berrada, A.; Muller, D.; Slaoui, A.; Rinnert, H.; et al. Structural, optical, and electrical properties of Yb-doped ZnO thin films prepared by spray pyrolysis method. J. Appl. Phys. 2011, 109, 033708. [Google Scholar] [CrossRef]
- Kumar, K.D.A.; Valanarasu, S.; Ponraj, J.S.; Fernandes, B.J.; Shkir, M.; AlFaify, S.; Murahari, P.; Ramesh, K. Effect of Er doping on the ammonia sensing properties of ZnO thin films prepared by a nebulizer spray technique. J. Phys. Chem. Solids 2020, 144, 109513. [Google Scholar] [CrossRef]
- Makoed, I.I.; Liedienov, N.A.; Pashchenko, A.V.; Levchenko, G.G.; Tatarchuk, D.D.; Didenko, Y.V.; Amirov, A.A.; Rimski, G.S.; Yanushkevich, K.I. Influence of rare-earth doping on the structural and dielectric properties of orthoferrite La0.50R0.50FeO3 ceramics synthesized under high pressure. J. Alloys Compd. 2020, 842, 155859. [Google Scholar] [CrossRef]
- Xu, P.; Sun, Y.; Shi, C.; Xu, F.; Pan, H. Electronic structure of ZnO and its defects. Sci. China Ser. A Math. 2001, 44, 1174–1181. [Google Scholar] [CrossRef]
- Lin, T.H.; Lan, W.H.; Shih, M.C.; Wang, M.C.; Chang, K.J.; Lin, J.C.; Lee, S.Y.; Lin, W.J.; Huang, C.J. Resistance Study of Er-doped Zinc Oxide Diode by Spray Pyrolysis. Sens. Mater. 2018, 30, 939–946. [Google Scholar]
- Kretzschmar, B.S.M.; Wendler, E.; Heft, A.; Köcher, R.; Voigt, C.; Ronning, C.; Grünler, B.; Rädlein, E. Comprehensive porosity determination of combustion-deposited SiOx thin films and correlation with FTIR signal. Surf. Coat. Technol. 2019, 375, 256–265. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, M.-W.; Kim, Y.; Swihart, M.T.; Yoon, S.S. Hierarchical zeolitic imidazolate framework-derived manganese-doped zinc oxide decorated carbon nanofiber electrodes for high performance flexible supercapacitors. Chem. Eng. J. 2019, 371, 657–665. [Google Scholar] [CrossRef]
- Rathgeber, C.; Schmit, H.; Hiebler, S.; Voigt, W. Application of the modified BET model to concentrated salt solutions with relatively high water activities: Predicting solubility phase diagrams of NaCl+ H2O, NaCl+ LiCl+ H2O, and NaCl+ CaCl2+ H2O. Calphad 2019, 66, 101633. [Google Scholar] [CrossRef]
- El Hat, A.; Hadri, A.; Chafi, F.Z.; Fares, B.; Hassanain, N.; Mzerd, A. Effect of cu on the physical properties of ZnO synthesized by spray pyrolysis technique. Rom. J. Mater./Rev. Romana Mater. 2017, 47, 71–77. [Google Scholar]
- El Hat, A.; Chaki, I.; Essajai, R.; Mzerd, A.; Schmerber, G.; Regragui, M.; Belayachi, A.; Sekkat, Z.; Dinia, A.; Slaoui, A.; et al. Growth and characterization of (Tb, Yb) Co-doping sprayed ZnO thin films. Crystals 2020, 10, 169. [Google Scholar] [CrossRef]
- Qin, R.; Meng, F.; Khan, M.W.; Yu, B.; Li, H.; Fan, Z.; Gong, J. Fabrication and enhanced photocatalytic property of TiO2-ZnO composite photocatalysts. Mater. Lett. 2019, 240, 84–87. [Google Scholar] [CrossRef]
- Behera, D.; Acharya, B.S. Nano-star formation in Al-doped ZnO thin film deposited by dip-dry method and its characterization using atomic force microscopy, electron probe microscopy, photoluminescence and laser Raman spectroscopy. J. Lumin. 2008, 128, 1577. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Collins, T.C.; Harsch, W.; Cantwell, G. Neutral-donor–bound-exciton complexes in ZnO crystals. Phys. Rev. B Condens. Matter 1998, 57, 12151. [Google Scholar] [CrossRef]
- Thonke, K.; Gruber, T.; Teofilov, N.; Schonfelder, R.; Waag, A.; Sauer, R. Donor–acceptor pair transitions in ZnO substrate material. Phys. B Condens. Matter 2001, 945, 308. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407. [Google Scholar] [CrossRef]
- Teng, X.M.; Fan, H.T.; Pan, S.S.; Ye, C.; Li, G.H. Photoluminescence of ZnO thin films on Si substrate with and without ITO buffer layer. J. Phys. D Appl. Phys. 2006, 39, 471. [Google Scholar] [CrossRef]
- Xu, P.S.; Sun, Y.M.; Shi, C.S.; Xu, F.Q.; Pan, H.B. The electronic structure and spectral properties of ZnO and its defects. Nucl. Instrum. Methods Phys. Res. B 2003, 199, 286. [Google Scholar] [CrossRef]
- Mahamuni, S.; Borgohain, K.; Bendre, B.S.; Leppert, V.J.; Risbud, S.H. Spectroscopic and structural characterization of electrochemically grown ZnO quantum dots. J. Appl. Phys. 1999, 85, 2861. [Google Scholar] [CrossRef]
- Srinet, G.; Varshneya, P.; Kumar, R.; Sajal, V.; Kulriya, P.K.; Knobel, M.; Sharma, S.K. Structural, optical and magnetic properties of Zn1− xCoxO prepared by the sol–gel route. Ceram. Int. 2013, 39, 6077. [Google Scholar] [CrossRef]
- Zeferino, R.S.; Flores, M.B.; Pal, U. Photoluminescence and Raman scattering in Ag-doped ZnO nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Elfakir, A.; Douayar, A.; Diaz, R.; Chaki, I.; Prieto, P.; Loghmarti, M.; Belayachi, A.; Abd-Lefdil, M. Elaboration and characterization of sprayed Tb-doped ZnO thin films. Sens. Transducers 2014, 27, 161. [Google Scholar]
- Balestrieria, M.; Ferblantier, G.; Colis, S.; Schmerber, G.; Ulhaq-Bouillet, C.; Muller, D.; Dinia, A. Structural and optical properties of Yb-doped ZnO films deposited by magnetron reactive sputtering for photon conversion. Sol. Energy Mater. Sol. Cells 2013, 117, 363. [Google Scholar] [CrossRef]
- Shestakov, M.V.; Baranov, A.N.; Tikhomirov, V.K.; Zubavichus, Y.V.; Kuznetsov, A.S.; Veligzhanin, A.A.; Kharin, A.Y.; Rösslhuber, R.; Timoshenko, V.Y.; Moshchalkov, V.V. Energy-transfer luminescence of a zinc oxide/ytterbium oxide nanocomposite. R. Soc. Chem. Adv. 2012, 2, 8783. [Google Scholar] [CrossRef]
- Luo, Q.; Qiao, X.; Fan, X.; Zhang, X. Near-infrared emission of Yb3+ through energy transfer from ZnO to Yb3+ in glass ceramic containing ZnO nanocrystals. Opt. Lett. 2011, 36, 2767. [Google Scholar] [CrossRef]
- Okada, R.; Miao, W.; Terai, Y.; Tsuji, T.; Fujiwara, Y. Sputtering-assisted metal-organic chemical vapor deposition of Yb-doped ZnO for photonic conversion in Si solar cells. Phys. Status Solidi C 2014, 11, 1292. [Google Scholar] [CrossRef]
- Zheng, J.H.; Song, J.L.; Zhao, Z.; Jiang, Q.; Lian, J.S. Optical and magnetic properties of Nd-doped ZnO nanoparticles. Cryst. Res. Technol. 2012, 47, 713. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Lin, W.; Ma, R.; Shao, W.; Liu, B. Structural, electrical and optical properties of Gd doped and undoped ZnO: Al (ZAO) thin films prepared by RF magnetron sputtering. Appl. Surf. Sci. 2007, 253, 5179. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Caglar, Y.; Ilican, S.; Caglar, M. The effects of fluorine on the structural, surface morphology and optical properties of ZnO thin films. Phys. B 2007, 394, 86. [Google Scholar] [CrossRef]
- Tomakin, M.; Altunbas, M.; Bacaksız, E.; Polat, I. Preparation and characterization of new window material CdS thin films at low substrate temperature (< 300 K) with vacuum deposition. Mater. Sci. Semicond. Process. 2011, 14, 120. [Google Scholar]
- Freund, L.B.; Suresh, S. Thin Film Materials; Cambridge University Press: Cambridge, UK, 2003; p. 192. [Google Scholar]
- Lee, Y.H.; Lee, W.J.; Kwon, Y.S.; Yeom, G.Y.; Yoon, J.K. Effects of CdS substrates on the physical properties of polycrystalline CdTe Films. Thin Solid Films 1999, 341, 172. [Google Scholar] [CrossRef]
- Zhou, Z.; Lan, C.; Wei, R.; Ho, J.C. Transparent metal-oxide nanowires and their applications in harsh electronics. J. Mater. Chem. C 2019, 7, 202–217. [Google Scholar] [CrossRef]
- Huang, Z.; Ruan, H.; Zhang, H.; Shi, D.; Li, W.; Qin, G.; Wu, F.; Fang, L.; Kong, C. Conversion mechanism of conductivity and properties of nitrogen implanted ZnO single crystals induced by post-annealing. J. Mater. Sci. Mater. Electron. 2019, 30, 4555–4561. [Google Scholar] [CrossRef]
- Pan, L.L.; Li, G.Y.; Lian, J.S. Structural, optical and electrical properties of cerium and gadolinium doped CdO thin films. Appl. Surf. Sci. 2013, 274, 365. [Google Scholar] [CrossRef]
- Oshima, M.; Yoshino, K. Electron scattering mechanism of FTO films grown by spray pyrolysis method. J. Electron. Mater. 2010, 39, 819. [Google Scholar] [CrossRef]

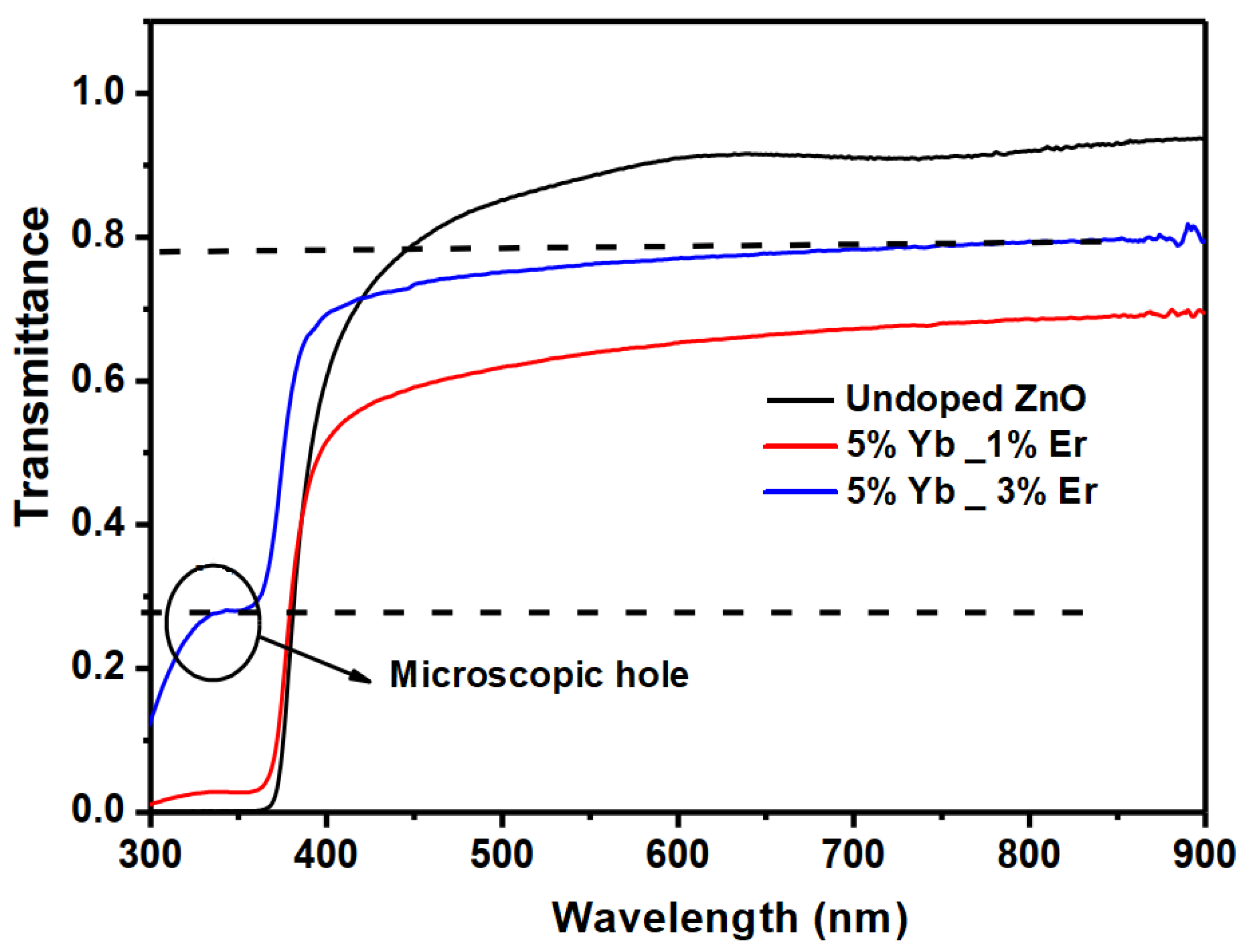
| Samples | Thickness (nm) | Eg (eV) | T% (600 nm) | T% (800 nm) | Ref |
|---|---|---|---|---|---|
| Undoped ZnO | 430 | 3.27 | 91 | 92 | This work |
| 5% Yb3+ | 455 | 3.33 | – | 88 | [23] |
| 1% Er | 388 | 3.26 | – | 75 | [24] |
| 5% Yb_1% Er | 455 | 3.24 | 65 | 68 | This work |
| 5% Yb_3% Er | 465 | 3.21 | 77 | 79 | This work |
| Samples | ne (1020 cm−3) | ρ (10−2 Ω cm) | µ (10−1 cm2/V·s) | l (nm) | Ref |
|---|---|---|---|---|---|
| Undoped ZnO | 0.13 | 64.5 | 7.3 | 4.8 | This work |
| 5% Yb | 92 | 8.00 | 0.12 | 0.42 | [23] |
| 5% Yb_1% Er | 10 | 7.45 | 0.30 | 0.203 | This work |
| 5% Yb_3% Er | 16 | 6.00 | 0.66 | 0.024 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hat, A.; Chaki, I.; Essajai, R.; Fakhim Lamrani, A.; Fares, B.; Regragui, M.; Dinia, A.; Abd-Lefdil, M. Growth and Properties of (Yb-Er) Co-Doped ZnO Thin Films Deposited via Spray Pyrolysis Technique. Optics 2025, 6, 14. https://doi.org/10.3390/opt6020014
El Hat A, Chaki I, Essajai R, Fakhim Lamrani A, Fares B, Regragui M, Dinia A, Abd-Lefdil M. Growth and Properties of (Yb-Er) Co-Doped ZnO Thin Films Deposited via Spray Pyrolysis Technique. Optics. 2025; 6(2):14. https://doi.org/10.3390/opt6020014
Chicago/Turabian StyleEl Hat, Abderrahim, Imane Chaki, Rida Essajai, Abdelmajid Fakhim Lamrani, Boubker Fares, Mohammed Regragui, Aziz Dinia, and Mohammed Abd-Lefdil. 2025. "Growth and Properties of (Yb-Er) Co-Doped ZnO Thin Films Deposited via Spray Pyrolysis Technique" Optics 6, no. 2: 14. https://doi.org/10.3390/opt6020014
APA StyleEl Hat, A., Chaki, I., Essajai, R., Fakhim Lamrani, A., Fares, B., Regragui, M., Dinia, A., & Abd-Lefdil, M. (2025). Growth and Properties of (Yb-Er) Co-Doped ZnO Thin Films Deposited via Spray Pyrolysis Technique. Optics, 6(2), 14. https://doi.org/10.3390/opt6020014








