Skin Imaging Using Optical Coherence Tomography and Photoacoustic Imaging: A Mini-Review
Abstract
1. Introduction
2. Application of OCT in Skin Imaging
3. Application of Photoacoustic Imaging (PAI) for Skin Imaging
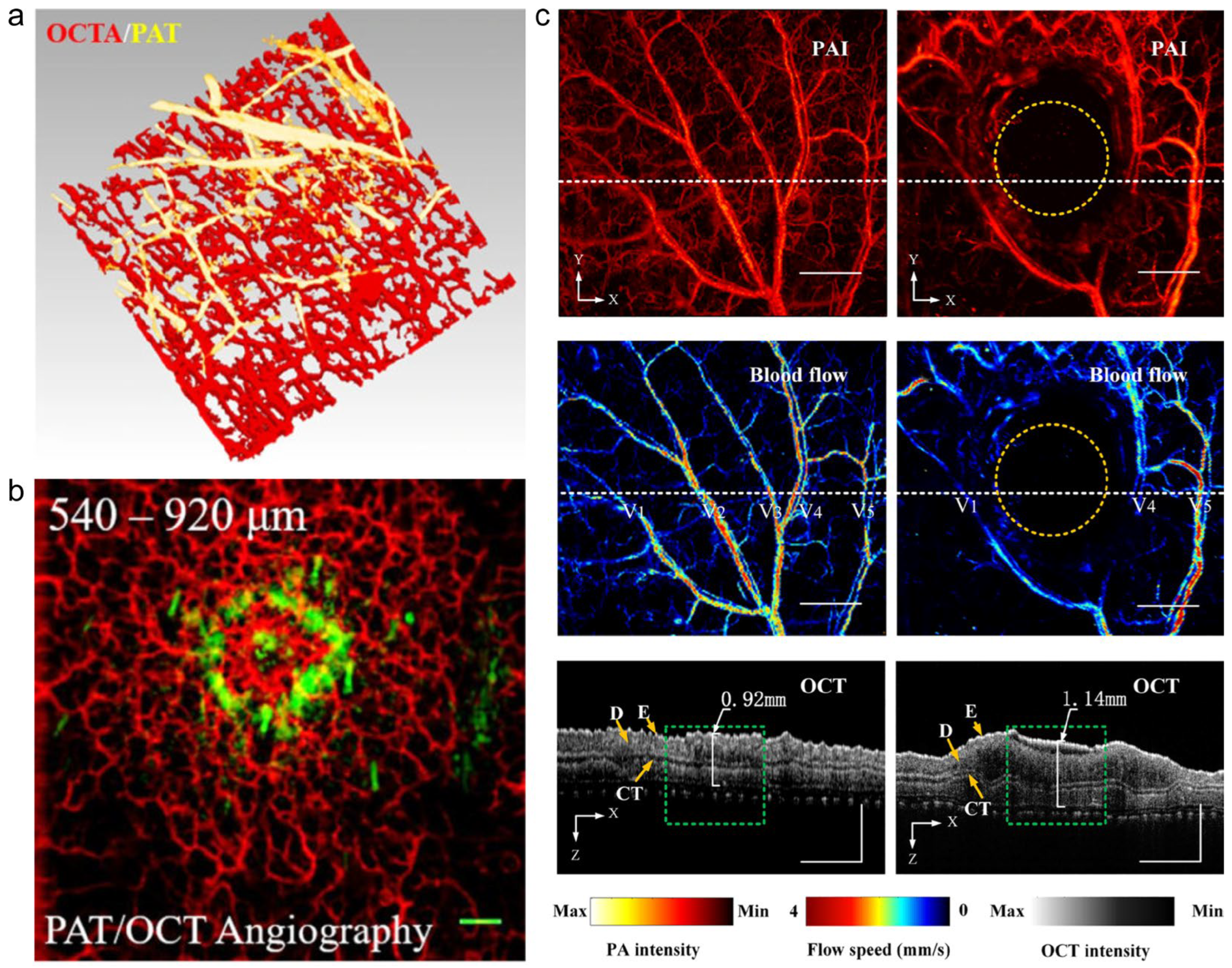
4. Application of Multimodal Skin Imaging Systems
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jablonski, N.G. The evolution of human skin and skin color. Annu. Rev. Anthropol. 2004, 33, 585–623. [Google Scholar] [CrossRef]
- Liu, M.; Drexler, W. Optical coherence tomography angiography and photoacoustic imaging in dermatology. Photochem. Photobiol. Sci. 2019, 18, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Z.; Zabihian, B.; Sinz, C.; Zhang, E.; Beard, P.C.; Ginner, L.; Hoover, E.; Minneman, M.P.; Leitgeb, R.A. Combined multi-modal photoacoustic tomography, optical coherence tomography (OCT) and OCT angiography system with an articulated probe for in vivo human skin structure and vasculature imaging. Biomed. Opt. Express 2016, 7, 3390–3402. [Google Scholar] [CrossRef] [PubMed]
- Diotallevi, F.; Offidani, A. Skin, Autoimmunity and Inflammation: A Comprehensive Exploration through Scientific Research. Int. J. Mol. Sci. 2023, 24, 15857. [Google Scholar] [CrossRef] [PubMed]
- Wassef, C.; Rao, B.K. Uses of non-invasive imaging in the diagnosis of skin cancer: An overview of the currently available modalities. Int. J. Dermatol. 2013, 52, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.; Shah, K.; Wysong, A.; Wortsman, X.; Humphreys, T.R. The role of imaging in the management of patients with nonmelanoma skin cancer: Diagnostic modalities and applications. J. Am. Acad. Dermatol. 2017, 76, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Nelson, J.S.; Jung, B. Multimodal facial color imaging modality for objective analysis of skin lesions. J. Biomed. Opt. 2008, 13, 064007–064008. [Google Scholar] [CrossRef]
- Burkes, S.; Adams, D.; Hammill, A.; Chute, C.; Eaton, K.; Welge, J.; Wickett, R.; Visscher, M. Skin imaging modalities quantify progression and stage of infantile haemangiomas. Br. J. Dermatol. 2015, 173, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Halani, S.; Foster, F.S.; Breslavets, M.; Shear, N.H. Ultrasound and infrared-based imaging modalities for diagnosis and management of cutaneous diseases. Front. Med. 2018, 5, 115. [Google Scholar] [CrossRef]
- Wilhelm, K.-P.; Elsner, P.; Berardesca, E.; Maibach, H.I. Bioengineering of the Skin: Skin Imaging & Analysis; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-field confocal optical coherence tomography: A new tool for the differentiation between nevi and melanomas? Cancers 2022, 14, 1140. [Google Scholar] [CrossRef]
- Larin, K.V.; Sampson, D.D. Optical coherence elastography–OCT at work in tissue biomechanics. Biomed. Opt. Express 2017, 8, 1172–1202. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Boppart, S.A. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans. Biomed. Eng. 2010, 57, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Kirby, M.A.; Le, N.; Li, Y.; Zeinstra, N.; Lu, G.N.; Murry, C.E.; Zheng, Y.; Wang, R.K. Polarization sensitive optical coherence tomography with single input for imaging depth-resolved collagen organizations. Light: Sci. Appl. 2021, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Zeppieri, M.; Marsili, S.; Enaholo, E.S.; Shuaibu, A.O.; Uwagboe, N.; Salati, C.; Spadea, L.; Musa, M. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina 2023, 59, 2114. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, S.; Men, S.; Wang, R.K. Long ranging swept-source optical coherence tomography-based angiography outperforms its spectral-domain counterpart in imaging human skin microcirculations. J. Biomed. Opt. 2017, 22, 116007. [Google Scholar] [CrossRef] [PubMed]
- Eybposh, M.H.; Turani, Z.; Mehregan, D.; Nasiriavanaki, M. Cluster-based filtering framework for speckle reduction in OCT images. Biomed. Opt. Express 2018, 9, 6359–6373. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, M.; Laissue, P.P.; Hojjatoleslami, A. De-noising speckled optical coherence tomography images using an algorithm based on artificial neural network. J. Neurosci. Neuroeng. 2013, 2, 347–352. [Google Scholar] [CrossRef]
- Rahaman, J.; Lukas, B.; May, J.; Puyana, C.; Tsoukas, M.; Avanaki, K. A Fast Normalization and Despeckled Method for Skin Optical Coherence Tomography Image via Deep Learning; SPIE: Bellingham, WA, USA, 2023; Volume 12352. [Google Scholar]
- Cheng, Y.; Chu, Z.; Wang, R.K. Robust three-dimensional registration on optical coherence tomography angiography for speckle reduction and visualization. Quant. Imaging Med. Surg. 2021, 11, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; May, J.; Smith, J.; Fakhoury, J.; Daveluy, S.; Chen, W.; Avanaki, K. Study of variation of attenuation coefficient in different skin color and age groups using optical coherence tomography. In Proceedings of the Photonics in Dermatology and Plastic Surgery 2022, San Francisco, CA, USA, 20–24 February 2022; p. PC119340P. [Google Scholar]
- Lu, J.; Deegan, A.J.; Cheng, Y.; Liu, T.; Zheng, Y.; Mandell, S.P.; Wang, R.K. Application of OCT-derived attenuation coefficient in acute burn-damaged skin. Lasers Surg. Med. 2021, 53, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Turani, Z.; Fatemizadeh, E.; Blumetti, T.; Daveluy, S.; Moraes, A.F.; Chen, W.; Mehregan, D.; Andersen, P.E.; Nasiriavanaki, M. Optical Radiomic Signatures Derived from Optical Coherence Tomography Images Improve Identification of Melanoma. Cancer Res. 2019, 79, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Turani, Z.; Fatemizadeh, E.; Blumetti, T.; Daveluy, S.; Moraes, A.F.; Chen, W.; Mehregan, D.; Andersen, P.E.; Avanaki, K. Melanoma detection using quantitative analysis of optical coherence tomography images. In Proceedings of the Optical Interactions with Tissue and Cells XXXII, Online, 6–11 March 2021; p. 116400T. [Google Scholar]
- Lin, C.-H.; Lukas, B.E.; Rajabi-Estarabadi, A.; May, J.R.; Pang, Y.; Puyana, C.; Tsoukas, M.; Avanaki, K. Rapid measurement of epidermal thickness in OCT images of skin. Sci. Rep. 2024, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.G.; Schmitt, J.M. Principles of OCT. In Optical Coherence Tomography in Cardiovascular Research; CRC Press: Boca Raton, FL, USA, 2007; pp. 35–50. [Google Scholar]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical coherence tomography (OCT): Principle and technical realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 59–85. [Google Scholar]
- Li, D.; Humayun, L.; Vienneau, E.; Vu, T.; Yao, J. Seeing through the skin: Photoacoustic tomography of skin vasculature and beyond. JID Innov. 2021, 1, 100039. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q. The Advanced Applications for Optical Coherence Tomography in Skin Imaging; Wayne State University: Detroit, MI, USA, 2021. [Google Scholar]
- Fayyaz, Z.; Mohammadian, N.; Reza Rahimi Tabar, M.; Manwar, R.; Avanaki, K. A comparative study of optimization algorithms for wavefront shaping. J. Innov. Opt. Health Sci. 2019, 12, 1942002. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Rajabi-Estarabadi, A.; Williams, N.M.; Avanaki, M.R.; Nouri, K. The efficacy and morphological effects of hydrogen peroxide 40% topical solution for the treatment of seborrheic keratoses, evaluated by dynamic optical coherence tomography. Ski. Res. Technol. 2020, 26, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, E.; Xu, Q.; Horton, L.; Fotouhi, A.; Reddy, S.; Manwar, R.; Daveluy, S.; Mehregan, D.; Gelovani, J.; Avanaki, K. Contrast-enhanced optical coherence tomography for melanoma detection: An in vitro study. J. Biophotonics 2020, 13, e201960097. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, M.R.; Podoleanu, A. En-face time-domain optical coherence tomography with dynamic focus for high-resolution imaging. J. Biomed. Opt. 2017, 22, 056009. [Google Scholar] [CrossRef] [PubMed]
- Tes, D.; Aber, A.; Zafar, M.; Horton, L.; Fotouhi, A.; Xu, Q.; Moiin, A.; Thompson, A.D.; Moraes Pinto Blumetti, T.C.; Daveluy, S. Granular cell tumor imaging using optical coherence tomography. Biomed. Eng. Comput. Biol. 2018, 9, 1179597218790250. [Google Scholar] [CrossRef]
- Avanaki, M.R.; Hojjatoleslami, A.; Sira, M.; Schofield, J.B.; Jones, C.; Podoleanu, A.G. Investigation of basal cell carcinoma using dynamic focus optical coherence tomography. Appl. Opt. 2013, 52, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, M.R.; Hojjat, A.; Podoleanu, A.G. Investigation of computer-based skin cancer detection using optical coherence tomography. J. Mod. Opt. 2009, 56, 1536–1544. [Google Scholar] [CrossRef]
- Xu, Q.; Jalilian, E.; Fakhoury, J.W.; Manwar, R.; Michniak-Kohn, B.; Elkin, K.B.; Avanaki, K. Monitoring the topical delivery of ultrasmall gold nanoparticles using optical coherence tomography. Ski. Res. Technol. 2020, 26, 263–268. [Google Scholar] [CrossRef]
- Lee, J.; Benavides, J.; Manwar, R.; Puyana, C.; May, J.; Tsoukas, M.; Avanaki, K. Noninvasive imaging exploration of phacomatosis pigmentokeratotica using high-frequency ultrasound and optical coherence tomography: Can biopsy of PPK patients be avoided? Ski. Res. Technol. 2023, 29, e13279. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, K.; Andersen, P. Oct Radiomic Features for Differentiation of Early Malignant Melanoma from Benign Nevus. Google Patents: 2020 Patent no. US20200359887, 19 November 2020. [Google Scholar]
- Avanaki, K.; Andersen, P.E. Optical Coherence Tomography for Melanoma Detection. In New Technologies in Dermatological Science and Practice; CRC Press: Boca Raton, FL, USA, 2021; pp. 47–58. [Google Scholar]
- Turani, Z.; Fatemizadeh, E.; Blumetti, T.; Daveluy, S.; Moraes, A.F.; Chen, W.; Mehregan, D.; Andersen, P.E.; Nasiriavanaki, M. Optical radiomic signatures derived from OCT images to improve identification of melanoma. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 20–24 June 2021; p. 11078_23. [Google Scholar]
- Lukas, B.; May, J.R.; Tsoukas, M.; Avanaki, K. Skin cancer detection using optical coherence tomography. In Optical Spectroscopy And Imaging For Cancer Diagnostics: Fundamentals, Progress, and Challenges; World Scientific: Singapore, 2023; pp. 473–495. [Google Scholar]
- Xu, Q.; Adabi, S.; Clayton, A.; Daveluy, S.; Mehregan, D.; Nasiriavanaki, M. Swept-Source Optical Coherence Tomography–Supervised Biopsy. Dermatol. Surg. 2018, 44, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Adabi, S.; Hosseinzadeh, M.; Noei, S.; Conforto, S.; Daveluy, S.; Clayton, A.; Mehregan, D.; Nasiriavanaki, M. Universal in vivo textural model for human skin based on optical coherence tomograms. Sci. Rep. 2017, 7, 17912. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Boms, S.; Stücker, M.; Kreuter, A.; Moussa, G.; Sand, M.; Altmeyer, P.; Hoffmann, K. Epidermal thickness assessed by optical coherence tomography and routine histology: Preliminary results of method comparison. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Matip, R.; Moussa, G.; Altmeyer, P.; Hoffmann, K. In vivo data of epidermal thickness evaluated by optical coherence tomography: Effects of age, gender, skin type, and anatomic site. J. Dermatol. Sci. 2006, 44, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Morsy, H.A.; Thrane, L.; Jemec, G.B.E. Morphology and Epidermal Thickness of Normal Skin Imaged by Optical Coherence Tomography. Dermatology 2008, 217, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Josse, G.; George, J.; Black, D. Automatic measurement of epidermal thickness from optical coherence tomography images using a new algorithm. Ski. Res. Technol. 2011, 17, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Weissman, J.; Hancewicz, T.; Kaplan, P. Optical coherence tomography of skin for measurement of epidermal thickness by shapelet-based image analysis. Opt. Express 2004, 12, 5760–5769. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Gaetti, G.; Grandahl, K.; Jemec, G.B.E. Optical coherence tomography quantifying photo aging: Skin microvasculature depth, epidermal thickness and UV exposure. Arch. Dermatol. Res. 2022, 314, 469–476. [Google Scholar] [CrossRef]
- Welzel, J.; Bruhns, M.; Wolff, H.H. Optical coherence tomography in contact dermatitis and psoriasis. Arch. Dermatol. Res. 2003, 295, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Ash, Z.; Del Galdo, F.; Marzo-Ortega, H.; Wakefield, R.J.; Emery, P.; McGonagle, D. Optical Coherence Tomography: A New Tool to Assess Nail Disease in Psoriasis? Dermatology 2011, 222, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Morsy, H.; Kamp, S.; Thrane, L.; Behrendt, N.; Saunder, B.; Zayan, H.; Elmagid, E.A.; Jemec, G.B.E. Optical coherence tomography imaging of psoriasis vulgaris: Correlation with histology and disease severity. Arch. Dermatol. Res. 2010, 302, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Aldahan, A.S.; Chen, L.L.; Fertig, R.M.; Holmes, J.; Shah, V.V.; Mlacker, S.; Hsu, V.M.; Nouri, K.; Tosti, A. Vascular features of nail psoriasis using dynamic optical coherence tomography. Ski. Appendage Disord. 2016, 2, 102–108. [Google Scholar] [CrossRef]
- Deegan, A.J.; Talebi-Liasi, F.; Song, S.; Li, Y.; Xu, J.; Men, S.; Shinohara, M.M.; Flowers, M.E.; Lee, S.J.; Wang, R.K. Optical coherence tomography angiography of normal skin and inflammatory dermatologic conditions. Lasers Surg. Med. 2018, 50, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Themstrup, L.; de Carvalho, N.; Manfredi, M.; Grana, C.; Ciardo, S.; Kästle, R.; Holmes, J.; Whitehead, R.; Jemec, G.B.E.; et al. Dynamic Optical Coherence Tomography in Dermatology. Dermatology 2016, 232, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Orlikov, A.; Vasa, R.; Moussa, G.; Hoffmann, K.; Stücker, M.; Altmeyer, P.; Bechara, F.G. In vivo optical coherence tomography of basal cell carcinoma. J. Dermatol. Sci. 2007, 45, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Saleah, S.A.; Gu, Y.; Wijesinghe, R.E.; Seong, D.; Cho, H.; Jeon, M.; Kim, J. Comparative quantifications and morphological monitoring of the topical treatment approach for onychomycosis-affected in vivo toenail using optical coherence tomography: A case study. Biomed. Signal Process. Control 2024, 88, 105648. [Google Scholar] [CrossRef]
- Byers, R.A.; Maiti, R.; Danby, S.G.; Pang, E.J.; Mitchell, B.; Carré, M.J.; Lewis, R.; Cork, M.J.; Matcher, S.J. Sub-clinical assessment of atopic dermatitis severity using angiographic optical coherence tomography. Biomed. Opt. Express 2018, 9, 2001–2017. [Google Scholar] [CrossRef] [PubMed]
- Byers, R.A.; Maiti, R.; Danby, S.G.; Pang, E.J.; Mitchell, B.; Carré, M.J.; Lewis, R.; Cork, M.J.; Matcher, S.J. Characterizing the microcirculation of atopic dermatitis using angiographic optical coherence tomography. In Photonics in Dermatology and Plastic Surgery; SPIE: Bellingham, WA, USA, 2017; Volume 10037, pp. 114–122. [Google Scholar]
- Manfredini, M.; Liberati, S.; Ciardo, S.; Bonzano, L.; Guanti, M.; Chester, J.; Kaleci, S.; Pellacani, G. Microscopic and functional changes observed with dynamic optical coherence tomography for severe refractory atopic dermatitis treated with dupilumab. Ski. Res. Technol. 2020, 26, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Ha-Wissel, L.; Yasak, H.; Huber, R.; Zillikens, D.; Ludwig, R.J.; Thaçi, D.; Hundt, J.E. Case report: Optical coherence tomography for monitoring biologic therapy in psoriasis and atopic dermatitis. Front. Med. 2022, 9, 995883. [Google Scholar] [CrossRef]
- Boone, M.A.L.M.; Jemec, G.B.E.; Del Marmol, V. Differentiating allergic and irritant contact dermatitis by high-definition optical coherence tomography: A pilot study. Arch. Dermatol. Res. 2015, 307, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, M.E.; Tearney, G.J.; Boppart, S.A.; Swanson, E.A.; Southern, J.F.; Fujimoto, J.G. Optical Biopsy with Optical Coherence Tomography: Feasibility for Surgical Diagnostics. J. Surg. Res. 1997, 71, 32–40. [Google Scholar] [CrossRef]
- Kuo, W.-C.; Kim, J.; Shemonski, N.D.; Chaney, E.J.; Spillman, D.R.; Boppart, S.A. Real-time three-dimensional optical coherence tomography image-guided core-needle biopsy system. Biomed. Opt. Express 2012, 3, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Shostak, E.; Hariri, L.P.; Cheng, G.Z.; Adams, D.C.; Suter, M.J. Needle-based Optical Coherence Tomography to Guide Transbronchial Lymph Node Biopsy. J. Bronchol. Interv. Pulmonol. 2018, 25, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hariri, L.P.; Mino-Kenudson, M.; Applegate, M.B.; Mark, E.J.; Tearney, G.J.; Lanuti, M.; Channick, C.L.; Chee, A.; Suter, M.J. Toward the Guidance of Transbronchial Biopsy: Identifying Pulmonary Nodules with Optical Coherence Tomography. Chest 2013, 144, 1261–1268. [Google Scholar] [CrossRef]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of Atorvastatin Therapy on Fibrous Cap Thickness in Coronary Atherosclerotic Plaque as Assessed by Optical Coherence Tomography. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Takarada, S.; Imanishi, T.; Kubo, T.; Tanimoto, T.; Kitabata, H.; Nakamura, N.; Tanaka, A.; Mizukoshi, M.; Akasaka, T. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: Assessment by optical coherence tomography study. Atherosclerosis 2009, 202, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Pieper, M.; Schulz-Hildebrandt, H.; Mall, M.A.; Hüttmann, G.; König, P. Intravital microscopic optical coherence tomography imaging to assess mucus-mobilizing interventions for muco-obstructive lung disease in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L518–L524. [Google Scholar] [CrossRef] [PubMed]
- Giuseppina, A.; Sibel Zehra, A.; Concepción, C.-G.; Vasiliki, L.; Daniel, W.; Adam, M.; Richard, J.W.; Dennis, G.M.; Paul, E.; Francesco Del, G. Virtual skin biopsy by optical coherence tomography: The first quantitative imaging biomarker for scleroderma. Ann. Rheum. Dis. 2013, 72, 1845. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Liu, Y.; Zhou, Q. Combining optical coherence tomography with magnetic resonance angiography and Doppler ultrasonography for clinical detection of scleroderma. Anat. Rec. 2020, 303, 3108–3116. [Google Scholar] [CrossRef]
- Sahin-Atik, S.; Koc, F.; Akin-Sari, S.; Ozmen, M. Retinal Nerve Fiber and Optic Disc Morphology Using Spectral-Domain Optical Coherence Tomography in Scleroderma Patients. Eur. J. Ophthalmol. 2017, 27, 281–284. [Google Scholar] [CrossRef]
- Levine, A.; Wang, K.; Markowitz, O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol. Clin. 2017, 35, 465–488. [Google Scholar] [CrossRef] [PubMed]
- di Ruffano, L.F.; Dinnes, J.; Deeks, J.J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12. [Google Scholar]
- Rajabi-Estarabadi, A.; Bittar, J.M.; Zheng, C.; Nascimento, V.; Camacho, I.; Feun, L.G.; Nasiriavanaki, M.; Kunz, M.; Nouri, K. Optical coherence tomography imaging of melanoma skin cancer. Lasers Med. Sci. 2019, 34, 411–420. [Google Scholar] [CrossRef]
- Mogensen, M.; Jørgensen, T.M.; Nurnberg, B.M.; Morsy, H.A.; Thomsen, J.B.; Thrane, L.; Jemec, G.B. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: Observer-blinded evaluation by dermatologists and pathologists. Dermatol. Surg. 2009, 35, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Alawi, S.A.; Kuck, M.; Wahrlich, C.; Batz, S.; McKenzie, G.; Fluhr, J.W.; Lademann, J.; Ulrich, M. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—A practical approach. Exp. Dermatol. 2013, 22, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-Q.; Mo, Y.; Wen, Y.-Q.; Cheng, M.-J.; Huo, S.-T.; Chen, X.-J.; Chen, Q. Optical coherence tomography for the diagnosis of malignant skin tumors: A meta-analysis. J. Biomed. Opt. 2018, 23, 020902. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.M.; Tycho, A.; Mogensen, M.; Bjerring, P.; Jemec, G.B.E. Machine-learning classification of non-melanoma skin cancers from image features obtained by optical coherence tomography. Ski. Res. Technol. 2008, 14, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; Suppa, M.; Dhaenens, F.; Miyamoto, M.; Marneffe, A.; Jemec, G.; Del Marmol, V.; Nebosis, R.J.A.o.d.r. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch. Dermatol. Res. 2016, 308, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Forsea, A.M.; Carstea, E.M.; Ghervase, L.; Giurcaneanu, C.; Pavelescu, G. Clinical application of optical coherence tomography for the imaging of non-melanocytic cutaneous tumors: A pilot multi-modal study. J. Med. Life 2010, 3, 381–389. [Google Scholar] [PubMed]
- Wang, Y.-J.; Wang, J.-Y.; Wu, Y.-H. Application of cellular resolution full-field optical coherence tomography in vivo for the diagnosis of skin tumours and inflammatory skin diseases: A pilot study. Dermatology 2022, 238, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.A.; Parasca, S.V.; Savastru, R.; Calin, M.R.; Dontu, S. Optical techniques for the noninvasive diagnosis of skin cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1083–1104. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.M.; Patalay, R.; Lynch, M.D. Applications and future directions for optical coherence tomography in dermatology. Br. J. Dermatol. 2021, 184, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Themstrup, L.; Nürnberg, B.M.; Jemec, G.B.E. Adjunct use of optical coherence tomography increases the detection of recurrent basal cell carcinoma over clinical and dermoscopic examination alone. Photodiagnosis Photodyn. Ther. 2016, 14, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.A.; Kirshnamoorthi, H.; Ellis, D.L.; van Leeuwen, T.G.; Mahadevan-Jansen, A. A clinical instrument for combined raman spectroscopy-optical coherence tomography of skin cancers. Lasers Surg. Med. 2011, 43, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Brunetti, T.; Cartocci, A.; Tognetti, L.; Suppa, M.; Malvehy, J.; Perez-Anker, J.; Puig, S.; Perrot, J.L.; Rubegni, P. Diagnostic Accuracy of Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas. Diagnostics 2023, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; Suppa, M.; Miyamoto, M.; Marneffe, A.; Jemec, G.; Del Marmol, V. In vivo assessment of optical properties of basal cell carcinoma and differentiation of BCC subtypes by high-definition optical coherence tomography. Biomed. Opt. Express 2016, 7, 2269–2284. [Google Scholar] [CrossRef]
- Patil, C.; Krishnamoorthi, H.; Ellis, D.; van Leeuwen, T.; Mahadevan-Jansen, A. A Clinical Probe for Combined Raman Spectroscopy-Optical Coherence Tomography (RS-OCT) of the Skin Cancers; SPIE: Bellingham, WA, USA, 2010; Volume 7548. [Google Scholar]
- Hamdoon, Z.; Jerjes, W.; Upile, T.; McKenzie, G.; Jay, A.; Hopper, C. Optical coherence tomography in the assessment of suspicious oral lesions: An immediate ex vivo study. Photodiagnosis Photodyn. Ther. 2013, 10, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Saboowala, H.K. Understanding the Principles of Skin Optical Coherence Tomography (OCT), Technology & Clinical Applications: An Overview. 2022. Available online: https://books.google.co.jp/books?id=7EBnEAAAQBAJ&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 6 February 2024).
- Panzarella, V.; Buttacavoli, F.; Gambino, A.; Capocasale, G.; Di Fede, O.; Mauceri, R.; Rodolico, V.; Campisi, G. Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study. Cancers 2022, 14, 5916. [Google Scholar] [CrossRef] [PubMed]
- Mesa, K.J.; Selmic, L.E.; Pande, P.; Monroy, G.L.; Reagan, J.; Samuelson, J.; Driskell, E.; Li, J.; Marjanovic, M.; Chaney, E.J.; et al. Intraoperative optical coherence tomography for soft tissue sarcoma differentiation and margin identification. Lasers Surg. Med. 2017, 49, 240–248. [Google Scholar] [CrossRef]
- Ha, R.; Friedlander, L.C.; Hibshoosh, H.; Hendon, C.; Feldman, S.; Ahn, S.; Schmidt, H.; Akens, M.K.; Fitzmaurice, M.; Wilson, B.C.; et al. Optical Coherence Tomography: A Novel Imaging Method for Post-lumpectomy Breast Margin Assessment—A Multi-reader Study. Acad. Radiol. 2018, 25, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Abignano, G.; Laws, P.; Del Galdo, F.; Marzo-Ortega, H.; McGonagle, D. Three-dimensional nail imaging by optical coherence tomography: A novel biomarker of response to therapy for nail disease in psoriasis and psoriatic arthritis. Clin. Exp. Dermatol. 2019, 44, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Castillo-Gallego, C.; Ash, Z.R.; Abignano, G.; Marzo-Ortega, H.; Wittmann, M.; Del Galdo, F.; McGonagle, D. Potential Use of Optical Coherence Tomography and High-Frequency Ultrasound for the Assessment of Nail Disease in Psoriasis and Psoriatic Arthritis. Dermatology 2013, 227, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sattler, E.; Kaestle, R.; Rothmund, G.; Welzel, J. Confocal laser scanning microscopy, optical coherence tomography and transonychial water loss for in vivo investigation of nails. Br. J. Dermatol. 2012, 166, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Saleah, S.A.; Kim, P.; Seong, D.; Wijesinghe, R.E.; Jeon, M.; Kim, J. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci. Rep. 2021, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.S.; Holmes, J.; Rudnick, A.; Woods, D.; Kelly, K.M. Angiographic optical coherence tomography imaging of hemangiomas and port wine birthmarks. Lasers Surg. Med. 2018, 50, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Glinos, G.D.; Verne, S.H.; Aldahan, A.S.; Liang, L.; Nouri, K.; Elliot, S.; Glassberg, M.; Cabrera DeBuc, D.; Koru-Sengul, T.; Tomic-Canic, M. Optical coherence tomography for assessment of epithelialization in a human ex vivo wound model. Wound Repair Regen. 2017, 25, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Kislevitz, M.; Akgul, Y.; Wamsley, C.; Hoopman, J.; Kenkel, J. Use of optical coherence tomography (OCT) in aesthetic skin assessment—A short review. Lasers Surg. Med. 2020, 52, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography—Practical applications in dermatology and comparison with established imaging methods. Ski. Res. Technol. 2021, 27, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.A.; Tang, P.; Liou, H.-C.; Kuriakose, M.; Pitre, J.J.; Pham, T.N.; Ettinger, R.E.; Wang, R.K.; O’Donnell, M.; Pelivanov, I. Probing elastic anisotropy of human skin in vivo with light using non-contact acoustic micro-tapping OCE and polarization sensitive OCT. Sci. Rep. 2022, 12, 3963. [Google Scholar] [CrossRef] [PubMed]
- Makita, S.; Yasuno, Y. In vivo photothermal optical coherence tomography for non-invasive imaging of endogenous absorption agents. Biomed. Opt. Express 2015, 6, 1707–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mo, L.; Chen, H.; Chen, C.; Wu, J.; Tang, Z.; Guo, Z.; Hu, C.; Liu, Z. Carbon Dots with Intrinsic Bioactivities for Photothermal Optical Coherence Tomography, Tumor-Specific Therapy and Postoperative Wound Management. Adv. Healthc. Mater. 2022, 11, 2101448. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Levine, A.; Markowitz, O. Optical coherence tomography in dermatology. Cutis 2017, 100, 163–166. [Google Scholar] [PubMed]
- Welzel, J. Optical coherence tomography in dermatology: A review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Moussa, G.; Sand, M.; Sand, D.; Altmeyer, P.; Hoffmann, K. Applications of optical coherence tomography in dermatology. J. Dermatol. Sci. 2005, 40, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Holmes, J.; Jemec, G.B. Advances in optical coherence tomography in dermatology—A review. J. Biomed. Opt. 2018, 23, 040901. [Google Scholar] [CrossRef] [PubMed]
- Hojjatoleslami, S.; Avanaki, M.; Podoleanu, A.G. Image quality improvement in optical coherence tomography using Lucy–Richardson deconvolution algorithm. Appl. Opt. 2013, 52, 5663–5670. [Google Scholar] [CrossRef]
- Adabi, S.; Rashedi, E.; Clayton, A.; Mohebbi-Kalkhoran, H.; Chen, X.-w.; Conforto, S.; Avanaki, M.N. Learnable despeckling framework for optical coherence tomography images. J. Biomed. Opt. 2018, 23, 016013. [Google Scholar] [CrossRef] [PubMed]
- Turani, Z.; Fatemizadeh, E.; Adabi, S.; Mehregan, D.; Daveluy, S.; Nasiriavanaki, M. Noise Reduction in OCT Skin Images; SPIE: Bellingham, WA, USA, 2017; Volume 10137. [Google Scholar]
- O’leary, S.; Fotouhi, A.; Turk, D.; Sriranga, P.; Rajabi-Estarabadi, A.; Nouri, K.; Daveluy, S.; Mehregan, D.; Nasiriavanaki, M. OCT image atlas of healthy skin on sun-exposed areas. Ski. Res. Technol. 2018, 24, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Hojjatoleslami, A.; Avanaki, M.R. OCT skin image enhancement through attenuation compensation. Appl. Opt. 2012, 51, 4927–4935. [Google Scholar] [CrossRef]
- Nasiri-Avanaki, M.-R.; Hojjatoleslami, S.; Paun, H.; Tuohy, S.; Meadway, A.; Dobre, G.; Podoleanu, A. Optical coherence tomography system optimization using simulated annealing algorithm. Proc. Math. Methods Appl. Comput. 2009, 669, 552–557. [Google Scholar]
- Adabi, S.; Turani, Z.; Fatemizadeh, E.; Clayton, A.; Nasiriavanaki, M. Optical coherence tomography technology and quality improvement methods for optical coherence tomography images of skin: A short review. Biomed. Eng. Comput. Biol. 2017, 8, 1179597217713475. [Google Scholar] [CrossRef] [PubMed]
- Adabi, S.; Fotouhi, A.; Xu, Q.; Daveluy, S.; Mehregan, D.; Podoleanu, A.; Nasiriavanaki, M. An overview of methods to mitigate artifacts in optical coherence tomography imaging of the skin. Ski. Res. Technol. 2018, 24, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Turani, Z.; Fatemizadeh, E.; Xu, Q.; Daveluy, S.; Mehregan, D.; Nasiriavanaki, M. Refractive index correction in optical coherence tomography images of multilayer tissues. J. Biomed. Opt. 2018, 23, 070501. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, M.R.; Hojjatoleslami, A. Skin layer detection of optical coherence tomography images. Optik 2013, 124, 5665–5668. [Google Scholar] [CrossRef]
- Avanaki, M.R.; Cernat, R.; Tadrous, P.J.; Tatla, T.; Podoleanu, A.G.; Hojjatoleslami, S.A. Spatial compounding algorithm for speckle reduction of dynamic focus OCT images. IEEE Photonics Technol. Lett. 2013, 25, 1439–1442. [Google Scholar] [CrossRef]
- Avanaki, M.R.; Laissue, P.P.; Eom, T.J.; Podoleanu, A.G.; Hojjatoleslami, A. Speckle reduction using an artificial neural network algorithm. Appl. Opt. 2013, 52, 5050–5057. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Lee, J.; Puyana, C.; Tsoukas, M.; Avanaki, K. 40638 Comprehensive Atlas of Healthy Skin Using Optical Coherence Tomography. J. Am. Acad. Dermatol. 2023, 89, AB140. [Google Scholar] [CrossRef]
- Taghavikhalilbad, A.; Adabi, S.; Clayton, A.; Soltanizadeh, H.; Mehregan, D.; Avanaki, M.R. Semi-automated localization of dermal epidermal junction in optical coherence tomography images of skin. Appl. Opt. 2017, 56, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, M.R.; Podoleanu, A.G.; Price, M.C.; Corr, S.A.; Hojjatoleslami, S. Two applications of solid phantoms in performance assessment of optical coherence tomography systems. Appl. Opt. 2013, 52, 7054–7061. [Google Scholar] [CrossRef] [PubMed]
- Herman, C. Emerging technologies for the detection of melanoma: Achieving better outcomes. Clin. Cosmet. Investig. Dermatol. 2012, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Manwar, R.; Li, X.; Mahmoodkalayeh, S.; Asano, E.; Zhu, D.; Avanaki, K. Deep learning protocol for improved photoacoustic brain imaging. J. Biophotonics 2020, 13, e202000212. [Google Scholar] [CrossRef] [PubMed]
- Kratkiewicz, K.; Manwar, R.; Zhou, Y.; Mozaffarzadeh, M.; Avanaki, K. Technical considerations in the Verasonics research ultrasound platform for developing a photoacoustic imaging system. Biomed. Opt. Express 2021, 12, 1050–1084. [Google Scholar] [CrossRef] [PubMed]
- Kratkiewicz, K.; Manwar, R.; Rajabi-Estarabadi, A.; Fakhoury, J.; Meiliute, J.; Daveluy, S.; Mehregan, D.; Avanaki, K.M. Photoacoustic/Ultrasound/Optical Coherence Tomography Evaluation of Melanoma Lesion and Healthy Skin in a Swine Model. Sensors 2019, 19, 2815. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Fatima, A.; Mohammadian, N.; Bely, N.; Nasiriavanaki, M. Towards low cost photoacoustic microscopy system for evaluation of skin health. In Proceedings of the Imaging Spectrometry XXI, San Diego, CA, USA, 19 September 2016; p. 99760X. [Google Scholar]
- Siegel, A.P.; Avanaki, K. The power of light and sound: Optoacoustic skin imaging for diabetes progression monitoring. Light: Sci. Appl. 2023, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Sinnamon, A.J.; Neuwirth, M.G.; Song, Y.; Schultz, S.M.; Liu, S.; Xu, X.; Sehgal, C.M.; Karakousis, G.C. Multispectral photoacoustic imaging for the detection of subclinical melanoma. J. Surg. Oncol. 2019, 119, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.H.; Park, B.; Seo, H.M.; Bang, C.H.; Park, G.S.; Park, Y.M.; Rhie, J.W.; Lee, J.H.; Kim, C. Multispectral ex vivo photoacoustic imaging of cutaneous melanoma for better selection of the excision margin. Br. J. Dermatol. 2018, 179, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.; Concannon, E.; Dorairaj, J.J.; Shaharan, S.; McGrath, J.; Jose, J.; Kelly, J.L.; Leahy, M.J. Preoperative measurement of cutaneous melanoma and nevi thickness with photoacoustic imaging. J. Med. Imaging 2018, 5, 015004. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, L.; Wang, G.; Ye, T.; Wang, B.; Xiao, J.; Liu, X.J.A.O. In-vivo imaging of melanoma with simultaneous dual-wavelength acoustic-resolution-based photoacoustic/ultrasound microscopy. Appl. Opt. 2021, 60, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.; Concannon, L.; Aalto, L.; Dorairaj, J.; Subhash, H.M.; Kelly, J.; Leahy, M.J. Assessment of cutaneous melanoma and pigmented skin lesions with photoacoustic imaging. In Proceedings of the Photonic Therapeutics and Diagnostics XI, San Francisco, CA, USA, 2–7 February 2013; pp. 19–23. [Google Scholar]
- Oh, J.-T.; Li, M.-L.; Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Three-dimensional imaging of skin melanoma in vivo by dual-wavelength photoacoustic microscopy. J. Biomed. Opt. 2006, 11, 034032–034034. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Bang, C.H.; Lee, C.; Han, J.H.; Choi, W.; Kim, J.; Park, G.S.; Rhie, J.W.; Lee, J.H.; Kim, C. 3D wide-field multispectral photoacoustic imaging of human melanomas in vivo: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Lockau, H.; Ntziachristos, V.; Grimm, J.; Kircher, M.F. Lymph Node Micrometastases and In-Transit Metastases from Melanoma: In Vivo Detection with Multispectral Optoacoustic Imaging in a Mouse Model. Radiology 2016, 280, 137–150. [Google Scholar] [CrossRef]
- He, H.; Schönmann, C.; Schwarz, M.; Hindelang, B.; Berezhnoi, A.; Steimle-Grauer, S.A.; Darsow, U.; Aguirre, J.; Ntziachristos, V. Fast raster-scan optoacoustic mesoscopy enables assessment of human melanoma microvasculature in vivo. Nat. Commun. 2022, 13, 2803. [Google Scholar] [CrossRef] [PubMed]
- Visscher, M.O.; Burkes, S.A.; Randall Wickett, R.; Eaton, K.P. Chapter 38—From Image to Information: Image Processing in Dermatology and Cutaneous Biology. In Imaging in Dermatology; Hamblin, M.R., Avci, P., Gupta, G.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 519–535. [Google Scholar] [CrossRef]
- Lao, Y.; Xing, D.; Yang, S. Noninvasive Mapping of Subcutaneous Vasculature with High Resolution Photoacoustic Imaging; SPIE: Bellingham, WA, USA, 2008; Volume 6826. [Google Scholar]
- He, H.; Fasoula, N.-A.; Karlas, A.; Omar, M.; Aguirre, J.; Lutz, J.; Kallmayer, M.; Füchtenbusch, M.; Eckstein, H.-H.; Ziegler, A. Opening a window to skin biomarkers for diabetes stage with optoacoustic mesoscopy. Light Sci. Appl. 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, D.; Kim, S.; Kim, H.H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Lutzweiler, C.; Meier, R.; Rummeny, E.; Ntziachristos, V.; Razansky, D. Real-time optoacoustic tomography of indocyanine green perfusion and oxygenation parameters in human finger vasculature. Opt. Lett. 2014, 39, 4061–4064. [Google Scholar] [CrossRef] [PubMed]
- Zeitouni, N.C.; Rohrbach, D.J.; Aksahin, M.; Sunar, U. Preoperative Ultrasound and Photoacoustic Imaging of Nonmelanoma Skin Cancers. Dermatol. Surg. 2015, 41, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Li, J.; Zhu, T.; Pang, W.; Chow, L.; Nie, L.; Sun, L.; Lai, P. Small Animal In Situ Drug Delivery Effects via Transdermal Microneedles Array versus Intravenous Injection: A Pilot Observation Based on Photoacoustic Tomography. Pharmaceutics 2022, 14, 2689. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to drug delivery and responses via photoacoustic imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, K.; Mathiyazhakan, M.; Wiraja, C.; Upputuri, P.K.; Xu, C.; Pramanik, M. Near-infrared light-responsive liposomal contrast agent for photoacoustic imaging and drug release applications. J. Biomed. Opt. 2017, 22, 041007. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Chen, F.; Moore, C.; Jokerst, J.V. Noninvasive staging of pressure ulcers using photoacoustic imaging. Wound Repair Regen. 2019, 27, 488–496. [Google Scholar] [CrossRef]
- Ida, T.; Iwazaki, H.; Kawaguchi, Y.; Kawauchi, S.; Ohkura, T.; Iwaya, K.; Tsuda, H.; Saitoh, D.; Sato, S.; Iwai, T. Burn depth assessments by photoacoustic imaging and laser Doppler imaging. Wound Repair Regen. 2016, 24, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Kawaguchi, Y.; Kawauchi, S.; Iwaya, K.; Tsuda, H.; Saitoh, D.; Sato, S.; Iwai, T. Real-time photoacoustic imaging system for burn diagnosis. J. Biomed. Opt. 2014, 19, 086013. [Google Scholar] [CrossRef]
- Nam, S.Y.; Chung, E.; Suggs, L.J.; Emelianov, S.Y. Combined Ultrasound and Photoacoustic Imaging to Noninvasively Assess Burn Injury and Selectively Monitor a Regenerative Tissue-Engineered Construct. Tissue Eng. Part C Methods 2015, 21, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Mantri, Y.; Tsujimoto, J.; Donovan, B.; Fernandes, C.C.; Garimella, P.S.; Penny, W.F.; Anderson, C.A.; Jokerst, J.V. Photoacoustic monitoring of angiogenesis predicts response to therapy in healing wounds. Wound Repair Regen. 2022, 30, 258–267. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, Y.; Harris, L.M.; Khan, S.; Xia, J. A portable three-dimensional photoacoustic tomography system for imaging of chronic foot ulcers. Quant. Imaging Med. Surg. 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Moore, C.; Mantri, Y.; Jokerst, J.V. Photoacoustic Imaging as a Tool for Assessing Hair Follicular Organization. Sensors 2020, 20, 5848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yuan, Y.; Zhang, H.; Yi, X.; Xiao, W.; Yu, A. Photoacoustic Microscopy Provides Early Prediction of Tissue Necrosis in Skin Avulsion Injuries. Clin. Cosmet. Investig. Dermatol. 2021, 14, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, F.; Zhang, W.; Yang, S. Quantitative and anatomical imaging of human skin by noninvasive photoacoustic dermoscopy. Bio-Protoc. 2022, 12, e4372. [Google Scholar] [CrossRef] [PubMed]
- Hindelang, B.; Aguirre, J.; Schwarz, M.; Berezhnoi, A.; Eyerich, K.; Ntziachristos, V.; Biedermann, T.; Darsow, U. Non-invasive imaging in dermatology and the unique potential of raster-scan optoacoustic mesoscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1051–1061. [Google Scholar] [CrossRef]
- Aguirre, J.; Schwarz, M.; Garzorz, N.; Omar, M.; Buehler, A.; Eyerich, K.; Ntziachristos, V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017, 1, 0068. [Google Scholar] [CrossRef]
- Geisler, E.L.; Brannen, A.; Pressler, M.; Perez, J.; Kane, A.A.; Hallac, R.R. 3D imaging of vascular anomalies using raster-scanning optoacoustic mesoscopy. Lasers Surg. Med. 2022, 54, 1269–1277. [Google Scholar] [CrossRef]
- He, H.; Paetzold, J.C.; Börner, N.; Riedel, E.; Gerl, S.; Schneider, S.; Fisher, C.; Ezhov, I.; Shit, S.; Li, H. Machine learning analysis of human skin by optoacoustic mesoscopy for automated extraction of psoriasis and aging biomarkers. IEEE Trans. Med. Imaging 2024. [Google Scholar] [CrossRef]
- Nitkunanantharajah, S.; Haedicke, K.; Moore, T.B.; Manning, J.B.; Dinsdale, G.; Berks, M.; Taylor, C.; Dickinson, M.R.; Jüstel, D.; Ntziachristos, V.; et al. Three-dimensional optoacoustic imaging of nailfold capillaries in systemic sclerosis and its potential for disease differentiation using deep learning. Sci. Rep. 2020, 10, 16444. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Duan, F.; Zhang, J.; Li, S.; Ma, H.; Nie, L. In vivo dual-scale photoacoustic surveillance and assessment of burn healing. Biomed. Opt. Express 2019, 10, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E. Skin type classification systems old and new. Dermatol. Clin. 2009, 27, 529–533. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Razansky, D. Optoacoustic imaging of the skin. Exp. Dermatol. 2021, 30, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Kukk, A.F.; Scheling, F.; Panzer, R.; Emmert, S.; Roth, B. Combined ultrasound and photoacoustic C-mode imaging system for skin lesion assessment. Sci. Rep. 2023, 13, 17947. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, K.; Kersten, B.E.; van den Ende, C.H.; van den Hoogen, F.H.; Vonk, M.C.; de Korte, C.L. Photoacoustic and high-frequency ultrasound imaging of systemic sclerosis patients. Arthritis Res. Ther. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Huang, Y.; Huang, L.; Wu, D.; Zhong, R.; Jiang, S.; Zhang, Y.; Liu, T.; Liu, X.; Jiang, H. Photoacoustic/Ultrasound Dual-Modal Imaging of Human Nails: A pilot study. J. Innov. Opt. Health Sci. 2004, 18, 010502. [Google Scholar] [CrossRef]
- Seeger, M.; Dehner, C.; Jüstel, D.; Ntziachristos, V. Label-free concurrent 5-modal microscopy (Co5M) resolves unknown spatio-temporal processes in wound healing. Commun. Biol. 2021, 4, 1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, Z.; Xing, D. Integrated photoacoustic and hyperspectral dual-modality microscopy for co-imaging of melanoma and cutaneous squamous cell carcinoma in vivo. J. Biophotonics 2020, 13, e202000105. [Google Scholar] [CrossRef] [PubMed]
- Dontu, S.; Miclos, S.; Savastru, D.; Tautan, M. Combined spectral-domain optical coherence tomography and hyperspectral imaging applied for tissue analysis: Preliminary results. Appl. Surf. Sci. 2017, 417, 119–123. [Google Scholar] [CrossRef]
- Chen, Z.; Rank, E.; Meiburger, K.M.; Sinz, C.; Hodul, A.; Zhang, E.; Hoover, E.; Minneman, M.; Ensher, J.; Beard, P.C. Non-invasive multimodal optical coherence and photoacoustic tomography for human skin imaging. Sci. Rep. 2017, 7, 17975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.Z.; Povazay, B.; Laufer, J.; Alex, A.; Hofer, B.; Pedley, B.; Glittenberg, C.; Treeby, B.; Cox, B.; Beard, P. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed. Opt. Express 2011, 2, 2202–2215. [Google Scholar] [CrossRef] [PubMed]
- Kukk, A.F.; Wu, D.; Gaffal, E.; Panzer, R.; Emmert, S.; Roth, B. Multimodal imaging system with ultrasound, photoacoustics, and optical coherence tomography for optical biopsy of melanoma. In Proceedings of the Multimodal Biomedical Imaging XVIII, San Francisco, CA, USA, 28 January 2023; pp. 16–22. [Google Scholar]
- Varkentin, A.; Mazurenka, M.; Blumenröther, E.; Behrendt, L.; Emmert, S.; Morgner, U.; Meinhardt-Wollweber, M.; Rahlves, M.; Roth, B. Trimodal system for in vivo skin cancer screening with combined optical coherence tomography-Raman and colocalized optoacoustic measurements. J. Biophotonics 2018, 11, e201700288. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Z.; Zhou, W.; Xing, D. Towards quantitative assessment of burn based on photoacoustic and optical coherence tomography. J. Biophotonics 2020, 13, e202000126. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Pljakic, A.; Schmitz, L. Recent advances in clinical application of optical coherence tomography of human skin. Clin. Cosmet. Investig. Dermatol. 2015, 8, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Otto, T.; Caujolle, S.; Kübler, J.; Aumann, S.; Fischer, J.; Reisman, C.; Spahr, H.; Lessmann, A. Lateral resolution of a commercial optical coherence tomography instrument. Transl. Vis. Sci. Technol. 2022, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Felice, S.; Bressler, M.Y.; Karim, R.; Markowitz, O. Transforming the treatment of psoriasis to the 21st century: Detecting subclinical therapeutic response to secukinumab using optical coherence tomography as a prognostic indicator. Lasers Surg. Med. 2022, 54, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Hindelang, B.; Nau, T.; Englert, L.; Berezhnoi, A.; Lauffer, F.; Darsow, U.; Biedermann, T.; Eyerich, K.; Aguirre, J.; Ntziachristos, V. Enabling precision monitoring of psoriasis treatment by optoacoustic mesoscopy. Sci. Transl. Med. 2022, 14, eabm8059. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Byers, R.A.; Danby, S.G.; Sahib, S.; Cha, A.; Zang, C.; Werth, J.; Adiri, R.; Taylor, R.N.; Cork, M.J. Potential application of PS-OCT in the safety assessment of non-steroidal topical creams for atopic dermatitis treatment. Biomed. Opt. Express 2023, 14, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.W.; Amma, D.U.K.S.; Kuan, A.H.Y.; Li, X.; Dev, K.; Attia, A.B.E.; Bi, R.; Moothanchery, M.; Balasundaram, G.; Aguirre, J. Raster-scanning optoacoustic mesoscopy imaging as an objective disease severity tool in atopic dermatitis patients. J. Am. Acad. Dermatol. 2021, 84, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Moothanchery, M.; Attia, A.B.E.; Xiuting, L.; Weng, Y.Y.; Guan, S.T.T.; Dinish, U.; Olivo, M. Assessment of oxygen saturation in microvasculature of atopic dermatitis patients using multispectral optoacoustic mesoscopy. In Proceedings of the Photonic Diagnosis, Monitoring, Prevention, and Treatment of Infections and Inflammatory Diseases 2022, San Francisco, CA, USA, 3–4 February 2020; p. 1193902. [Google Scholar]
- Attia, A.B.E.; Chuah, S.Y.; Razansky, D.; Ho, C.J.H.; Malempati, P.; Dinish, U.; Bi, R.; Fu, C.Y.; Ford, S.J.; Lee, J.S.-S.J.P. Noninvasive real-time characterization of non-melanoma skin cancers with handheld optoacoustic probes. Photoacoustics 2017, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hult, J.; Dahlstrand, U.; Merdasa, A.; Wickerström, K.; Chakari, R.; Persson, B.; Cinthio, M.; Erlöv, T.; Albinsson, J.; Gesslein, B. Unique spectral signature of human cutaneous squamous cell carcinoma by photoacoustic imaging. J. Biophotonics 2020, 13, e201960212. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, E.; Sharma, V.; Smith, L.; Pinder, C.; Moore, T.; Manning, J.; Dinsdale, G.; Berks, M.; Newton, V.; Wilkinson, S. Polarisation-sensitive optical coherence tomography measurement of retardance in fibrosis, a non-invasive biomarker in patients with systemic sclerosis. Sci. Rep. 2022, 12, 2893. [Google Scholar] [CrossRef] [PubMed]
- Abignano, G.; Karadağ, D.T.; Gundogdu, O.; Lettieri, G.; Padula, M.; Padula, A.; Emery, P.; D’angelo, S.; Del Galdo, F. FRI0226 Optical Coherence Tomography of the Skin Detects Scleroderma Changes in Clinically Unaffected Skin: An Opportunity for Early Detection of Systemic Sclerosis; BMJ Publishing Group Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Lee, J.; Beirami, M.J.; Ebrahimpour, R.; Puyana, C.; Tsoukas, M.; Avanaki, K. Optical coherence tomography confirms non-malignant pigmented lesions in phacomatosis pigmentokeratotica using a support vector machine learning algorithm. Ski. Res. Technol. 2023, 29, e13377. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Mandal, M.; Mitra, P.; Chatterjee, J. Attenuation corrected-optical coherence tomography for quantitative assessment of skin wound healing and scar morphology. J. Biophotonics 2021, 14, e202000357. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.J.; Mandell, S.P.; Wang, R.K. Optical coherence tomography correlates multiple measures of tissue damage following acute burn injury. Quant. Imaging Med. Surg. 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Jokerst, J.V. Strategies for Image-Guided Therapy, Surgery, and Drug Delivery Using Photoacoustic Imaging. Theranostics 2019, 9, 1550–1571. [Google Scholar] [CrossRef] [PubMed]


| Condition | OCT | PAI |
|---|---|---|
| Psoriasis, eczema, and other inflammatory conditions | Changes in epidermal thickness, structural anomalies, alterations in dermal vascular network [45,46,47,48,49,50,55,185]. | Changes in vascularity [143,144,186]. |
| Atopic dermatitis treatment efficacy | Changes in skin architecture and dermal vascular network over time [51,59,60,61,62,63,187]. | Changes in vascularity [188,189]. |
| Hyperkeratosis and acanthosis of the epidermis | Changes in skin architecture [51,52,53,54]. | |
| Non-melanoma skin cancers | Detection and identification of tumor margins [76,79,81,82,83,84,85,86,87,88,89,90,91,92,93]. | Changes in vascularity, oxygenation, melanin [190,191]. |
| Nail disorders | Difficult-to-biopsy information on nail plate and bed [52,54,96,97,98,99]. | Changes in blood oxygenation and collagen content [174]. |
| Hemangiomas and port-wine stains | Disordered vasculature [100]. | Changes in vascularity [143,144]. |
| Scleroderma and skin involvement in systemic sclerosis | Changes in skin architecture, skin fibrosis [71,72,73,192,193]. | |
| Burn assessment | Tissue architecture, dermal vascularity [22]. | Vascularity and angiogenesis [156,157,169]. |
| Characterization of pigmented lesions and melanoma | Using machine learning/deep learning analysis [23,194]. | Describes depth and distribution of pigmentation [132,133,134,135,136,137,138,139,140,141,142]. |
| Wound depth assessment, healing, and peripheral vascular diseases | Skin architecture, mechanical properties [195,196]. | Assessment of oxygen saturation levels in blood vessels [155,156,157,158,159,160,166]. |
| Staging diabetes progression and potential for complications | Changes in dermal microvasculature [145]. | |
| Preparation for skin biopsies and guiding dermatological procedures | Detection of regions of interest through structural changes [64,65,66,67]. | Use vascularity, melanin location for guidance [135,151,152,153,154]. |
| Guiding delivery of therapeutic interventions and real-time tissue response | Observation of molecular changes associated with skin disease, including with the use of dyes [146,147,148,149,150,152,153,154,197]. | |
| Guiding cosmetic procedures | Assessment of skin architecture can evaluate the efficacy of many skin aesthetic procedures [102]. | Assess collagen and elastin content [161,162,163]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.; Siegel, A.P.; Avanaki, K.; Manwar, R. Skin Imaging Using Optical Coherence Tomography and Photoacoustic Imaging: A Mini-Review. Optics 2024, 5, 248-266. https://doi.org/10.3390/opt5020018
Zafar M, Siegel AP, Avanaki K, Manwar R. Skin Imaging Using Optical Coherence Tomography and Photoacoustic Imaging: A Mini-Review. Optics. 2024; 5(2):248-266. https://doi.org/10.3390/opt5020018
Chicago/Turabian StyleZafar, Mohsin, Amanda P. Siegel, Kamran Avanaki, and Rayyan Manwar. 2024. "Skin Imaging Using Optical Coherence Tomography and Photoacoustic Imaging: A Mini-Review" Optics 5, no. 2: 248-266. https://doi.org/10.3390/opt5020018
APA StyleZafar, M., Siegel, A. P., Avanaki, K., & Manwar, R. (2024). Skin Imaging Using Optical Coherence Tomography and Photoacoustic Imaging: A Mini-Review. Optics, 5(2), 248-266. https://doi.org/10.3390/opt5020018





