Analysis of Performance of Bone-Anchored Implants for Amputation Limb Prostheses
Abstract
1. Introduction
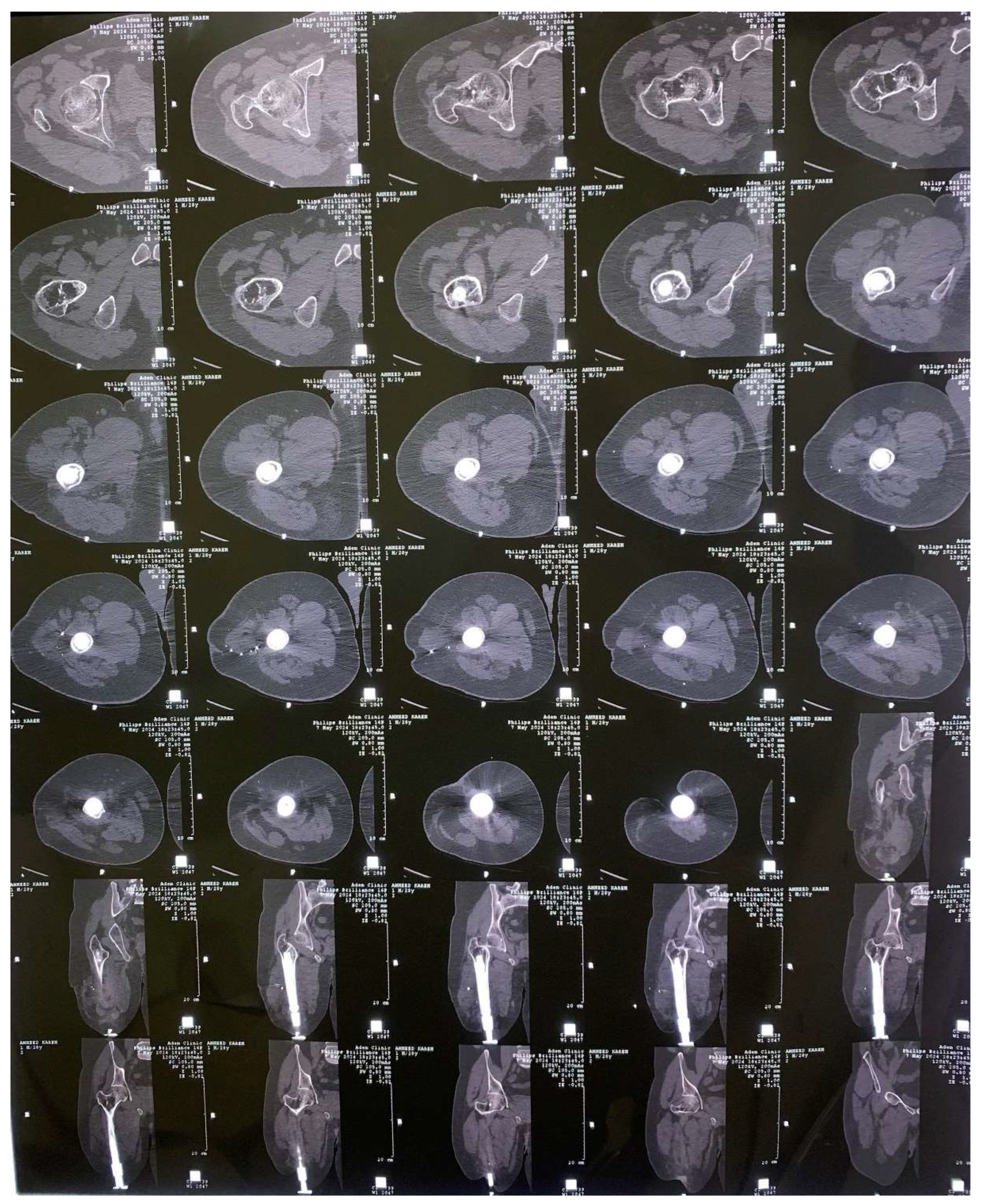
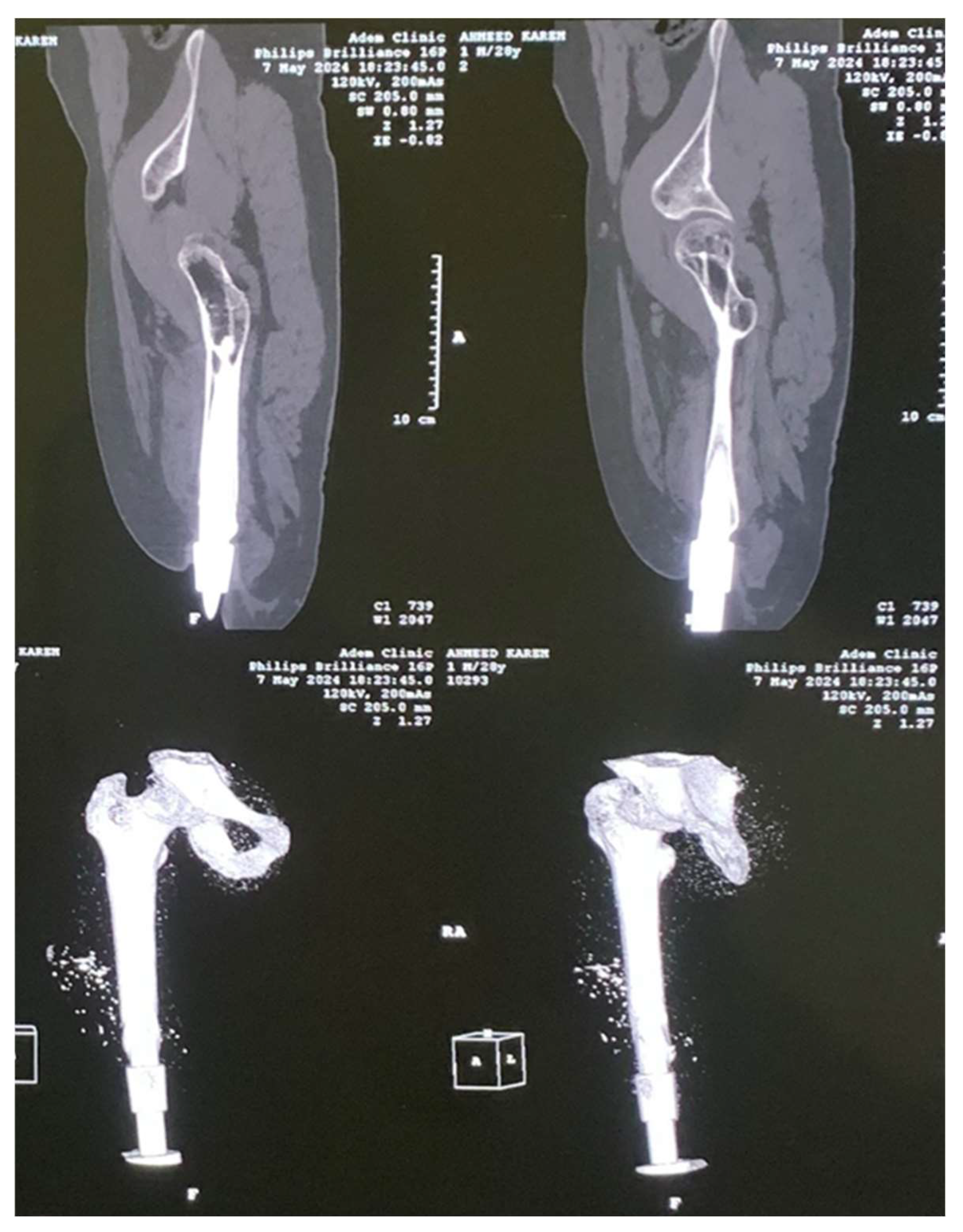
2. Experimental Works
3. Numerical Simulations
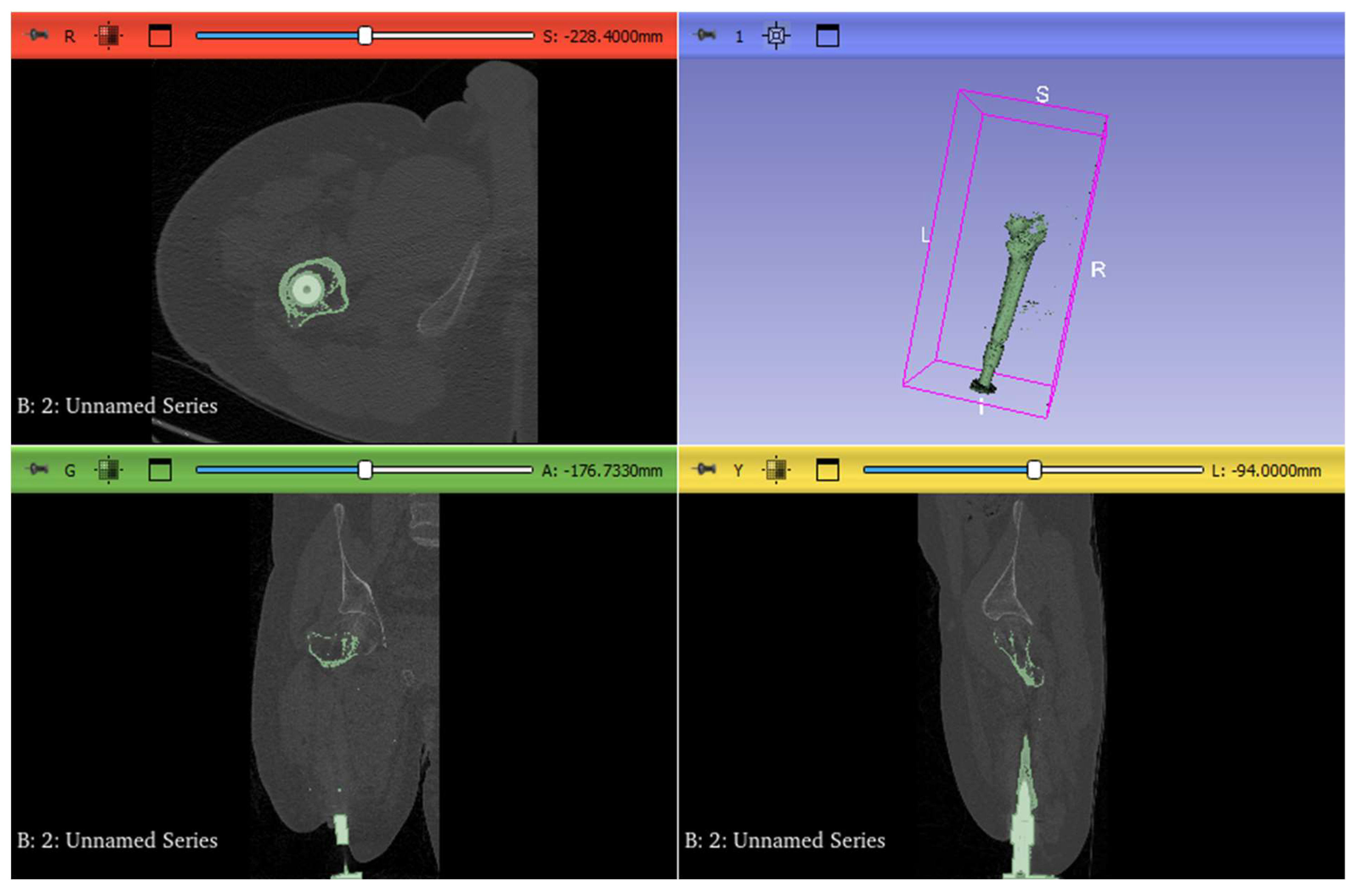
3.1. CAD Model
3.2. Material Selection
3.3. Mesh Generation
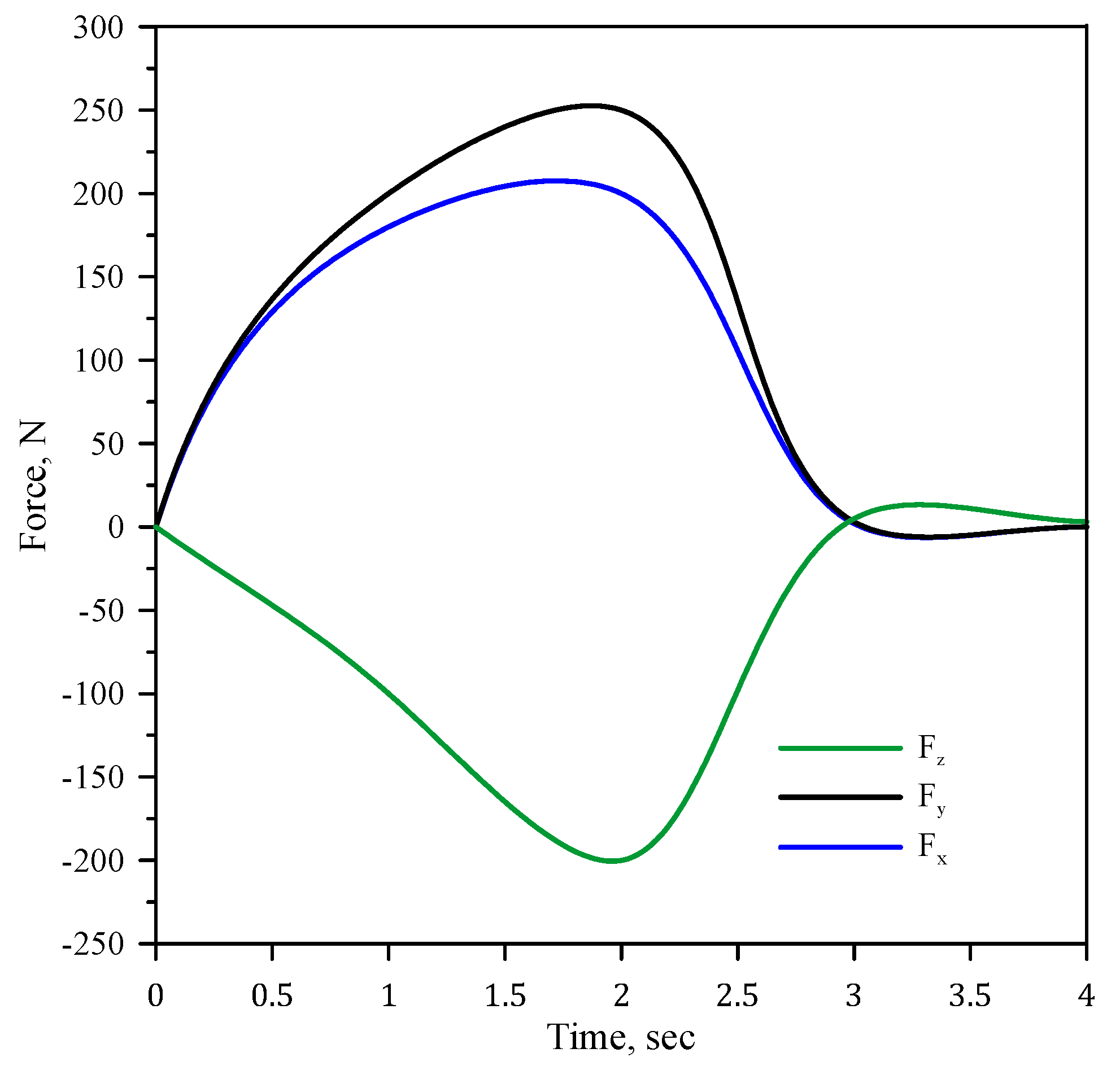
3.4. FEM Procedure
4. Results and Discussion
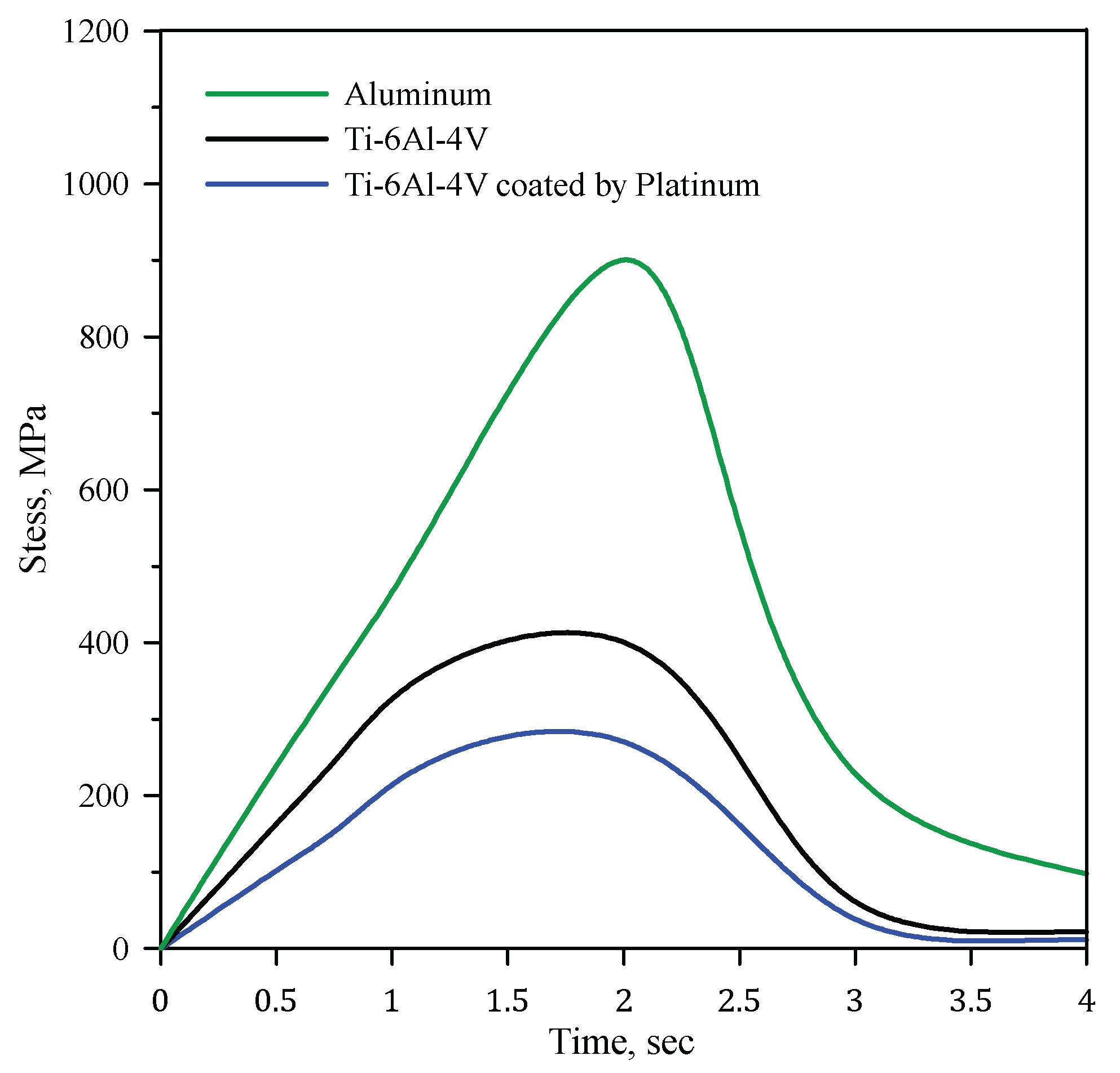
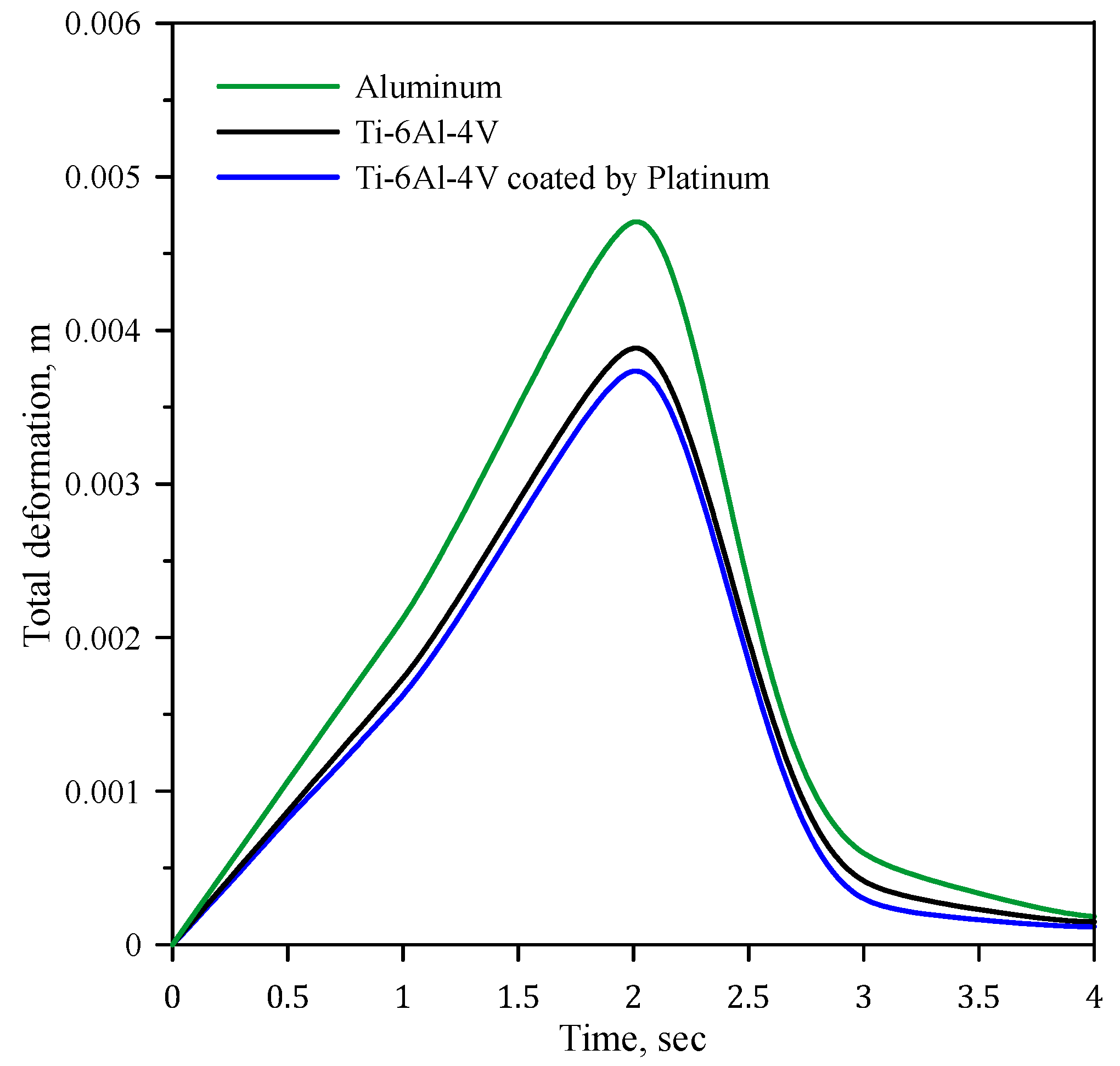
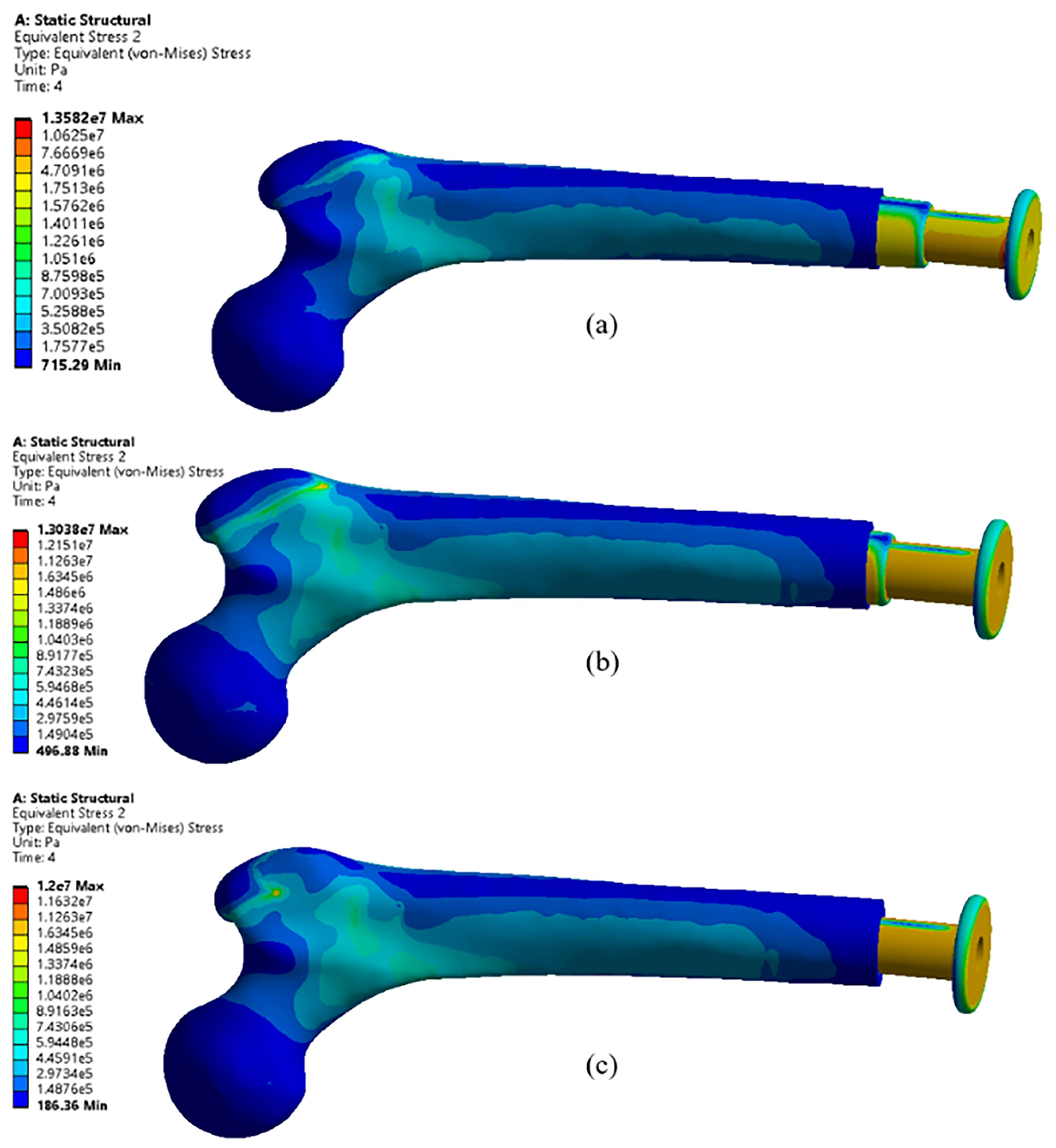
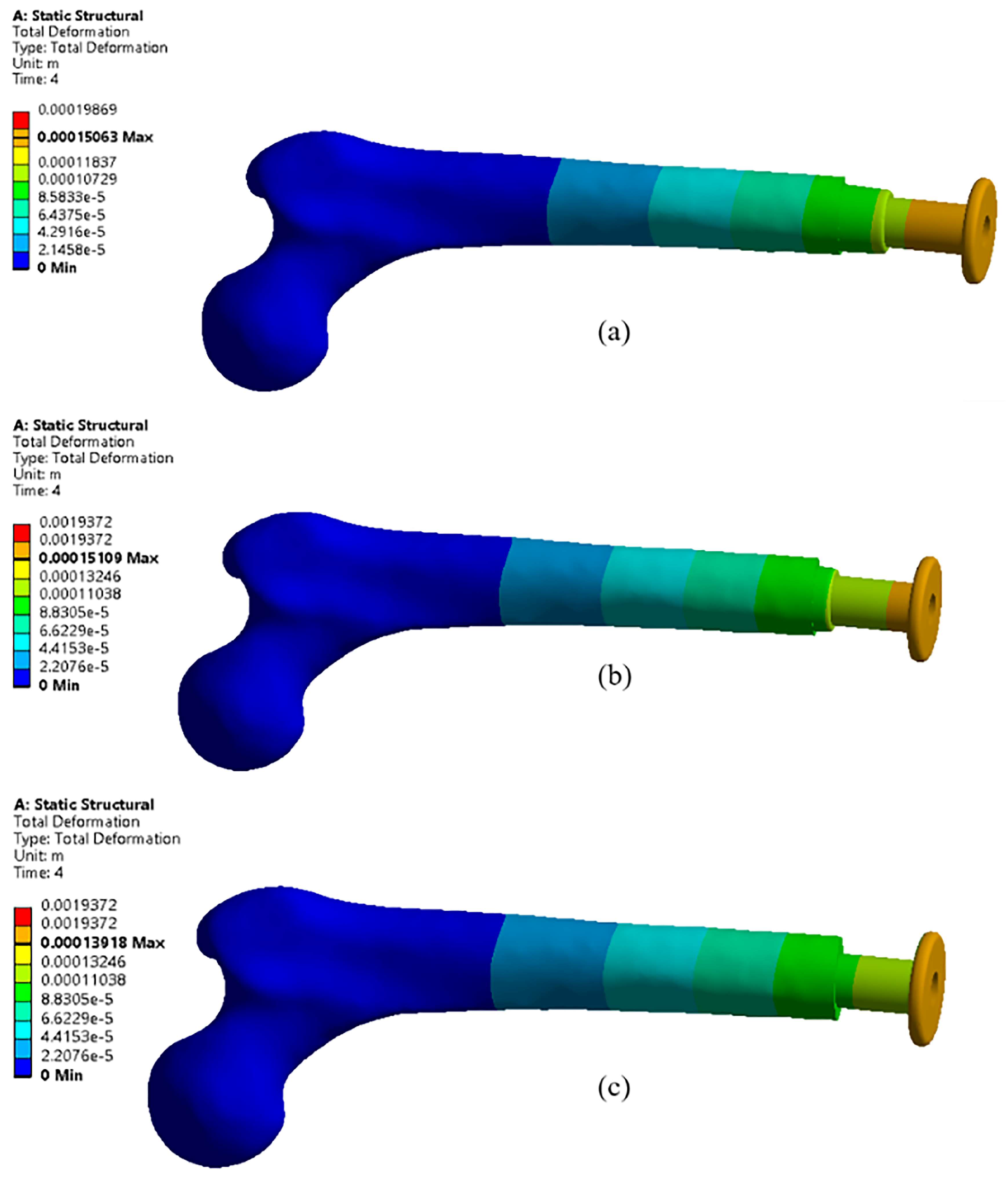
4.1. Effects of Different Materials
4.2. Effects of Different Lengths
5. Conclusions
- Using Ti-6Al-4V and Ti-6Al-4V coated with 10 µm platinum reduces the stress by 46% and 65%, respectively.
- Ti-6Al-4V coated with 10 µm platinum achieves the minimum equivalent stress, indicating the importance of platinum coating.
- The Ti-6Al-4V coated with a 10 µm layer of platinum exhibits the lowest deformation, measuring 22.92% less than aluminum and 5.13% less than the uncoated Ti-6Al-4V.
- Shorter implant lengths result in reduced deformation due to minimized bending effects and increased stiffness, while longer implants, like the 217 mm length, exhibit higher deformation due to increased flexibility and a pronounced lever arm effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FEA | Finite element analysis |
| CT | Computed tomography |
| CAD | Computer-aided design |
| OPL | Osseointegration prosthetic limb |
References
- Robinson, D.L.; Safai, L.; Harandi, V.J.; Graf, M.; Lizama, L.E.C.; Lee, P.; Galea, M.P.; Khan, F.; Tse, K.M.; Ackland, D.C. Load response of an osseointegrated implant used in the treatment of unilateral transfemoral amputation: An early implant loosening case study. Clin. Biomech. 2020, 73, 201–212. [Google Scholar] [CrossRef]
- Gil, S.; Fernandez-Pineda, I.; Rao, B.; Neel, M.D.; Baker, J.N.; Wu, H.; Wu, J.; Anghelescu, D.L. Role of amputation in improving mobility, pain outcomes, and emotional and psychological well-being in children with metastatic osteosarcoma. Am. J. Hosp. Palliat. Med. 2019, 36, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Valente, F.; Marrocco, A.; Falcinelli, C. Impact of physiological and non-physiological loading scenarios and crown material on periimplant bone stress distribution: A 3D finite element study. J. Mech. Behav. Biomed. Mater. 2025, 163, 106894. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Hirota, M.; Shibata, R.; Matsuura, T.; Komatsu, K.; Saruta, J.; Att, W. The 3D theory of osseointegration: Material, topography, and time as interdependent determinants of bone–implant integration. Int. J. Implant Dent. 2025, 11, 49. [Google Scholar] [CrossRef]
- Taylor, T.D.; Agar, J.R. Twenty years of progress in implant prosthodontics. J. Prosthet. Dent. 2002, 88, 89–95. [Google Scholar] [CrossRef]
- Stenlund, P.; Trobos, M.; Lausmaa, J.; Branemark, R.; Thomsen, P.; Palmquist, A. Effect of load on the bone around bone-anchored amputation prostheses. J. Orthop. Res. 2017, 35, 1113–1122. [Google Scholar] [CrossRef]
- Koehler, S.R.; Dhaher, Y.Y.; Hansen, A.H. Cross-validation of a portable, six-degree-of-freedom load cell for use in lower-limb prosthetics research. J. Biomech. 2014, 47, 1542–1547. [Google Scholar] [CrossRef]
- Prochor, P.; Sajewicz, E. The Influence of Geometry of Implants for Direct Skeletal Attachment of Limb Prosthesis on Rehabilitation Program and Stress-Shielding Intensity. BioMed Res. Int. 2019, 2019, 6067952. [Google Scholar] [CrossRef]
- Al-Maliky, F.T.; Chiad, J.S. Study and evaluation of four bar polycentric knee used in the prosthetic limb for transfemoral amputee during the gait cycle. Mater. Today Proc. 2021, 42, 2706–2712. [Google Scholar] [CrossRef]
- Frossard, L.; Laux, S.; Geada, M.; Heym, P.P.; Lechler, K. Load applied on osseointegrated implant by transfemoral bone-anchored prostheses fitted with state-of-the-art prosthetic components. Clin. Biomech. 2021, 89, 105457. [Google Scholar] [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021, 124, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Heimke, G.; Leyen, S.; Willmann, G. Knee arthoplasty: Recently developed ceramics offer new solutions. Biomaterials 2002, 23, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Ricci, S.; Pancanti, A.; Cappello, A. Numerical model to predict the long-term mechanical stability of cementless orthopaedic implants. Med. Biol. Eng. Comput. 2004, 42, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Helgason, B.; Palsson, H.; Runarsson, T.P.; Frossard, L.; Viceconti, M. Risk of failure during gait for direct skeletal attachment of a femoral prosthesis: A finite element study. Med. Eng. Phys. 2009, 31, 595–600. [Google Scholar] [CrossRef]
- Tillander, J.; Hagberg, K.; Hagberg, L.; Branemark, R. Osseointegrated titanium implants for limb prostheses attachments: Infectious complications. Clin. Orthop. Relat. Res. 2010, 468, 2781–2788. [Google Scholar] [CrossRef]
- Branemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomedicine 2011, 7, 220–227. [Google Scholar] [CrossRef]
- Tomaszewski, P.K.; Verdonschot, N.; Bulstra, S.K.; Rietman, J.S.; Verkerke, G.J. Simulated bone remodeling around two types of osseointegrated implants for direct fixation of upper-leg prostheses. J. Mech. Behav. Biomed. Mater. 2012, 15, 167–175. [Google Scholar] [CrossRef]
- Frossard, L.; Langton, C.; Perevoshchikova, N.; Feih, S.; Powrie, R.; Barrett, R.; Lloyd, D. Next-generation devices to diagnose residuum health of individuals suffering from limb loss: A narrative review of trends, opportunities, and challenges. J. Sci. Med. Sport 2023, 26 (Suppl. 1), S22–S29. [Google Scholar] [CrossRef]
- Pitkin, M. Design features of implants for direct skeletal attachment of limb prostheses. J. Biomed. Mater. Res. A 2013, 101, 3339–3348. [Google Scholar] [CrossRef]
- Tsikandylakis, G.; Berlin, O.; Branemark, R. Implant survival, adverse events, and bone remodeling of osseointegrated percutaneous implants for transhumeral amputees. Clin. Orthop. Relat. Res. 2014, 472, 2947–2956. [Google Scholar] [CrossRef]
- van Eck, C.F.; McGough, R.L. Clinical outcome of osseointegrated prostheses for lower extremity amputations. Curr. Orthop. Pract. 2015, 26, 349–357. [Google Scholar] [CrossRef]
- Muderis, M.A.; Aschoff, H.H.; Bosley, B.; Raz, G.; Gerdesmeyer, L.; Burkett, B. Direct skeletal attachment prosthesis for the amputee athlete: The unknown potential. Sports Eng. 2016, 19, 141–145. [Google Scholar] [CrossRef]
- Li, Y.; Lindeque, B. Percutaneous Osseointegrated Prostheses for Transfemoral Amputations. Orthopedics 2018, 41, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Prochor, P.; Sajewicz, E. A Comparative Analysis of Standardised Threads for Use in Implants for Direct Skeletal Attachment of Limb Prosthesis: A Finite Element Analysis. Appl. Bionics Biomech. 2019, 2019, 8027064. [Google Scholar] [CrossRef] [PubMed]
- Branemark, R.P.; Hagberg, K.; Kulbacka-Ortiz, K.; Berlin, O.; Rydevik, B. Osseointegrated Percutaneous Prosthetic System for the Treatment of Patients with Transfemoral Amputation: A Prospective Five-year Follow-up of Patient-reported Outcomes and Complications. J. Am. Acad. Orthop. Surg. 2019, 27, e743–e751. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lynch, J.P. Quantitative assessment of compress-type osseointegrated prosthetic implants in human bone using electromechanical impedance spectroscopic methods. Biomed. Eng. Lett. 2020, 10, 129–147. [Google Scholar] [CrossRef]
- Prochor, P.; Frossard, L.; Sajewicz, E. Effect of the material’s stiffness on stress-shielding in osseointegrated implants for bone-anchored prostheses: A numerical analysis and initial benchmark data. Acta Bioeng. Biomech. 2020, 22, 69–81. [Google Scholar] [CrossRef]
- Thesleff, A.; Ortiz-Catalan, M.; Branemark, R. The effect of cortical thickness and thread profile dimensions on stress and strain in bone-anchored implants for amputation prostheses. J. Mech. Behav. Biomed. Mater. 2022, 129, 105148. [Google Scholar] [CrossRef]
- Hagberg, K.; Ghasemi Jahani, S.A.; Omar, O.; Thomsen, P. Osseointegrated prostheses for the rehabilitation of patients with transfemoral amputations: A prospective ten-year cohort study of patient-reported outcomes and complications. J. Orthop. Transl. 2023, 38, 56–64. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Li, J.; Zhou, X.; Xie, J.; Wang, A. Selective Laser Melting Molding of Individualized Femur Implant: Design, Process, Optimization. J. Bionic Eng. 2021, 18, 128–137. [Google Scholar] [CrossRef]
- van Hoogstraten, S.W.G.; van Agtmaal, J.L.; Samijo, S.K.; Peeters, L.C.W.; van Maris, M.P.F.H.L.; Arts, J.J.C. Antimicrobial and biocompatibility assessment of platinum-based metal alloy coatings for total joint arthroplasty. Surf. Coat. Technol. 2025, 511, 132281. [Google Scholar] [CrossRef]
- Faadhila, A.; Taufiqurrakhman, M.; Katili, P.A.; Rahman, S.F.; Lestari, D.C.; Whulanza, Y. Optimizing PEEK implant surfaces for improved stability and biocompatibility through sandblasting and the platinum coating approach. Front. Mech. Eng. 2024, 10, 1360743. [Google Scholar] [CrossRef]
- Jayasree, A.; Cartmell, S.; Ivanovski, S.; Gulati, K. Electrically stimulated dental implants triggers soft-tissue integration and bactericidal functions. Adv. Funct. Mater. 2024, 34, 2311027. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Li, B.; Cong, X.; Chen, Q. Mechanical and electrochemical properties of platinum coating by double glow plasma on titanium alloy substrate. Russ. J. Electrochem. 2013, 49, 76–80. [Google Scholar] [CrossRef]
- Akobundu, U.U.; Ifijen, I.H.; Duru, P.; Igboanugo, J.C.; Ekanem, I.; Fagbolade, M.; Ajayi, A.S.; George, M.; Atoe, B.; Matthews, J.T. Exploring the role of strontium-based nanoparticles in modulating bone regeneration and antimicrobial resistance: A public health perspective. RSC Adv. 2025, 15, 10902–10957. [Google Scholar] [CrossRef]
- Alshimaysawee, S.; Fadhel Obaid, R.; Al-Gazally, M.E.; Alexis Ramírez-Coronel, A.; Bathaei, M.S. Recent advancements in metallic drug-eluting implants. Pharmaceutics 2023, 15, 223. [Google Scholar] [CrossRef]
- Woodward, B.K. 6—Platinum group metals (PGMs) for permanent implantable electronic devices. In Precious Metals for Biomedical Applications; Baltzer, N., Copponnex, T., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 130–147. [Google Scholar]
- Mirulla, A.I.; Di Paolo, S.; Di Simone, F.; Ingrassia, T.; Nigrelli, V.; Zaffagnini, S.; Bragonzoni, L. Biomechanical analysis of two types of osseointegrated transfemoral prosthesis. Appl. Sci. 2020, 10, 8263. [Google Scholar] [CrossRef]







| Property | Symbol | Al | Ti-6Al-4V | Platinum |
|---|---|---|---|---|
| Density [kg/m3] | ρ | 2770 | 4430 | 21,100 |
| Young’s Modulus [GPa] | E | 71 | 115 | 172 |
| Poisson’s Ratio [-] | υ | 0.33 | 0.22 | 0.38 |
| Bulk Modulus [GPa] | B | 69.6 | 114.3 | 233 |
| Shear Modulus [GPa] | G | 26.7 | 35.3 | 68 |
| Tensile yield strength [MPa] | σy | 280 | 93 | 93 |
| Comprehensive Yield Strength [MPa] | σc | 280 | 93 | 82 |
| Tensile ultimate strength [MPa] | σu | 310 | 1070 | 1250 |
| Property | Symbol | Value |
|---|---|---|
| Density [kg/m3] | ρ | 1900 |
| Young’s Modulus [GPa] | E | 6 |
| Poisson’s Ratio [-] | υ | 0.49 |
| Bulk Modulus [GPa] | B | 100 |
| Shear Modulus [GPa] | G | 2.013 |
| Tensile yield strength [MPa] | σy | 135 |
| Comprehensive Yield Strength [MPa] | σc | 130 |
| Tensile ultimate strength [MPa] | σu | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tameemi, R.B.; Mazaheri, H.; Chiad, J.S.; Shaban, M. Analysis of Performance of Bone-Anchored Implants for Amputation Limb Prostheses. Appl. Mech. 2025, 6, 77. https://doi.org/10.3390/applmech6040077
Al-Tameemi RB, Mazaheri H, Chiad JS, Shaban M. Analysis of Performance of Bone-Anchored Implants for Amputation Limb Prostheses. Applied Mechanics. 2025; 6(4):77. https://doi.org/10.3390/applmech6040077
Chicago/Turabian StyleAl-Tameemi, Riyam Basim, Hashem Mazaheri, Jumaa Salman Chiad, and Mahdi Shaban. 2025. "Analysis of Performance of Bone-Anchored Implants for Amputation Limb Prostheses" Applied Mechanics 6, no. 4: 77. https://doi.org/10.3390/applmech6040077
APA StyleAl-Tameemi, R. B., Mazaheri, H., Chiad, J. S., & Shaban, M. (2025). Analysis of Performance of Bone-Anchored Implants for Amputation Limb Prostheses. Applied Mechanics, 6(4), 77. https://doi.org/10.3390/applmech6040077





