Revascularization Enhances Walking Dynamics in Patients with Peripheral Artery Disease
Abstract
1. Introduction
2. Materials and Methods
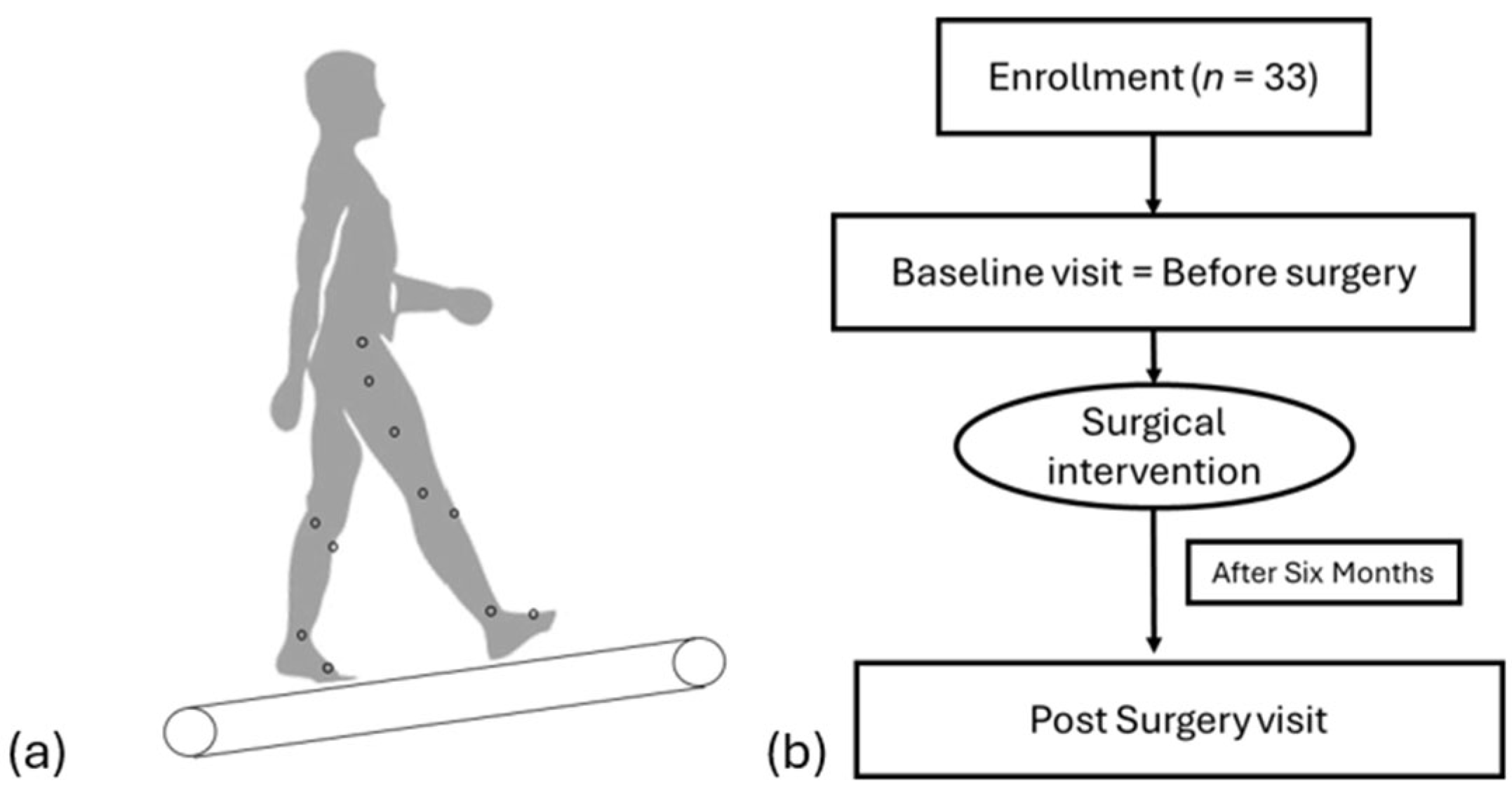
2.1. Participants
2.2. Data Collection
2.3. Data Analysis
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. Br. Med. J. 2018, 360, j5842. [Google Scholar] [CrossRef] [PubMed]
- Campia, U.; Gerhard-Herman, M.; Piazza, G.; Goldhaber, S.Z. Peripheral Artery Disease: Past, Present, and Future. Am. J. Med. 2019, 132, 1133–1141. [Google Scholar] [CrossRef]
- Hiramoto, J.S.; Teraa, M.; de Borst, G.J.; Conte, M.S. Interventions for lower extremity peripheral artery disease. Nat. Rev. Cardiol. 2018, 15, 332–350. [Google Scholar] [CrossRef] [PubMed]
- Firnhaber, J.M.; Powell, C.S. Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 362–369. [Google Scholar]
- Sykora, D.; Firth, C.; Girardo, M.; Bhatt, S.; Matti, L.; Tseng, A.; Shipman, J.; Liedl, D.; Wennberg, P.; Shamoun, F.E. Patient Age at Diagnosis of Peripheral Artery Disease and Its Impact on Cardiovascular and Limb Outcomes. Am. J. Cardiol. 2022, 177, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Montgomery, P.S. Impaired balance and higher prevalence of falls in subjects with intermittent claudication. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M454–M458. [Google Scholar] [CrossRef]
- Gardner, A.W.; Forrester, L.; Smith, G.V. Altered gait profile in subjects with peripheral arterial disease. Vasc. Med. 2001, 6, 31–34. [Google Scholar] [CrossRef]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35 (Suppl. S2), ii37–ii41. [Google Scholar] [CrossRef]
- Myers, S.A.; Johanning, J.M.; Stergiou, N.; Celis, R.I.; Robinson, L.; Pipinos, I.I. Gait variability is altered in patients with peripheral arterial disease. J. Vasc. Surg. 2009, 49, 924–931.E1. [Google Scholar] [CrossRef]
- Beckman, J.A.; Schneider, P.A.; Conte, M.S. Advances in Revascularization for Peripheral Artery Disease: Revascularization in PAD. Circ. Res. 2021, 128, 1885–1912. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109. [Google Scholar] [CrossRef]
- McDermott, M.M. Exercise Rehabilitation for Peripheral Artery Disease: A Review. J. Cardiopulm. Rehabil. Prev. 2018, 38, 63–69. [Google Scholar] [CrossRef]
- Mascarenhas, J.V.; Albayati, M.A.; Shearman, C.P.; Jude, E.B. Peripheral arterial disease. Endocrinol. Metab. Clin. N. Am. 2014, 43, 149–166. [Google Scholar] [CrossRef]
- Stevens, J.W.; Simpson, E.; Harnan, S.; Squires, H.; Meng, Y.; Thomas, S.; Michaels, J.; Stansby, G. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br. J. Surg. 2012, 99, 1630–1638. [Google Scholar] [CrossRef]
- Li, S.; Myers, S.A.; Thompson, J.; Kim, J.; Koutakis, P.; Williams, M.; Zhu, Z.; Schieber, M.; Lackner, T.; Willcockson, G.; et al. Different Outcomes after Revascularization or Standard Supervised Exercise Treadmill Training of Claudicating Patients with Peripheral Artery Disease. JVS Vasc. Sci. 2020, 1, 255. [Google Scholar] [CrossRef]
- Abry, L.; Weiss, S.; Makaloski, V.; Haynes, A.G.; Schmidli, J.; Wyss, T.R. Peripheral Artery Disease Leading to Major Amputation: Trends in Revascularization and Mortality Over 18 Years. Ann. Vasc. Surg. 2022, 78, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.; Montero-Odasso, M.; Bherer, L.; Almeida, Q.; Fraser, S.; Muir-Hunter, S.; Li, K.; Liu-Ambrose, T.; McGibbon, C.A.; McIlroy, W.; et al. Guidelines for Gait Assessments in the Canadian Consortium on Neurodegeneration in Aging (CCNA). Can. Geriatr. J. 2018, 21, 157–165. [Google Scholar] [CrossRef]
- Brach, J.S.; Studenski, S.A.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 983–988. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef]
- de Oliveira, E.A.; Andrade, A.O.; Vieira, M.F. Linear and nonlinear measures of gait variability after anterior cruciate ligament reconstruction. J. Electromyogr. Kinesiol. 2019, 46, 21–27. [Google Scholar] [CrossRef]
- Rahman, H.; Pipinos, I.I.; Johanning, J.M.; Myers, S.A. Gait variability is affected more by peripheral artery disease than by vascular occlusion. PLoS ONE 2021, 16, e0241727. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.A.; Pipinos, I.I.; Johanning, J.M.; Stergiou, N. Gait variability of patients with intermittent claudication is similar before and after the onset of claudication pain. Clin. Biomech. 2011, 26, 729–734. [Google Scholar] [CrossRef]
- Biswas, M.P.; Capell, W.H.; McDermott, M.M.; Jacobs, D.L.; Beckman, J.A.; Bonaca, M.P.; Hiatt, W.R. Exercise Training and Revascularization in the Management of Symptomatic Peripheral Artery Disease. JACC Basic. Transl. Sci. 2021, 6, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Nigg, B.M.; Cole, G.K.; Nachbauer, W. Effects of arch height of the foot on angular motion of the lower extremities in running. J. Biomech. 1993, 26, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh Gonabadi, A.; Cesar, G.M.; Buster, T.W.; Burnfield, J.M. Effect of gap-filling technique and gap location on linear and nonlinear calculations of motion during locomotor activities. Gait Posture 2022, 94, 85–92. [Google Scholar] [CrossRef]
- Mohammadzadeh Gonabadi, A.; Buster, T.W.; Cesar, G.M.; Burnfield, J.M. Effect of Data and Gap Characteristics on the Nonlinear Calculation of Motion During Locomotor Activities. J. Appl. Biomech. 2024, 40, 278–286. [Google Scholar] [CrossRef]
- McCamley, J.D.; Denton, W.; Arnold, A.; Raffalt, P.C.; Yentes, J.M. On the calculation of sample entropy using continuous and discrete human gait data. Entropy 2018, 20, 764. [Google Scholar] [CrossRef]
- Georgoulis, A.D.; Moraiti, C.; Ristanis, S.; Stergiou, N. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: The use of the approximate entropy in orthopaedics. J. Clin. Monit. Comput. 2006, 20, 11–18. [Google Scholar] [CrossRef]
- Busa, M.A.; Jones, S.L.; Hamill, J.; van Emmerik, R.E. Multiscale entropy identifies differences in complexity in postural control in women with multiple sclerosis. Gait Posture 2016, 45, 7–11. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Yentes, J.M.; Raffalt, P.C. Entropy Analysis in Gait Research: Methodological Considerations and Recommendations. Ann. Biomed. Eng. 2021, 49, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Fallahtafti, F.; Salamifar, Z.; Hassan, M.; Rahman, H.; Pipinos, I.; Myers, S.A. Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention. Entropy 2022, 24, 1422. [Google Scholar] [CrossRef]
- Raffalt, P.C.; Kent, J.A.; Wurdeman, S.R.; Stergiou, N. Selection Procedures for the Largest Lyapunov Exponent in Gait Biomechanics. Ann. Biomed. Eng. 2019, 47, 913–923. [Google Scholar] [CrossRef]
- Ma, K.F.; Levolger, S.; Vedder, I.R.; El Moumni, M.; de Vries, J.P.M.; Bokkers, R.P.H.; Viddeleer, A.R. The Impact of Lower Extremity Skeletal Muscle Atrophy and Myosteatosis on Revascularization Outcomes in Patients with Peripheral Arterial Disease. J. Clin. Med. 2021, 10, 3963. [Google Scholar] [CrossRef]
- Gan, W.S. Techniques in the Application of Chaos Theory in Signal and Image Processing. In Control and Dynamic Systems; Leondes, C.T., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 77, pp. 339–387. [Google Scholar]
- Vartanian, S.M.; Conte, M.S. Surgical Intervention for Peripheral Arterial Disease. Circ. Res. 2015, 116, 1614–1628. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Hadamus, A.; Białoszewski, D.; Błażkiewicz, M.; Kowalska, A.J.; Urbaniak, E.; Wydra, K.T.; Wiaderna, K.; Boratyński, R.; Kobza, A.; Marczyński, W. Assessment of the Effectiveness of Rehabilitation after Total Knee Replacement Surgery Using Sample Entropy and Classical Measures of Body Balance. Entropy 2021, 23, 164. [Google Scholar] [CrossRef]
- McDermott, M.M.; Ferrucci, L.; Gonzalez-Freire, M.; Kosmac, K.; Leeuwenburgh, C.; Peterson, C.A.; Saini, S.; Sufit, R. Skeletal Muscle Pathology in Peripheral Artery Disease: A Brief Review. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2577–2585. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- van Emmerik, R.E.A.; Ducharme, S.W.; Amado, A.C.; Hamill, J. Comparing dynamical systems concepts and techniques for biomechanical analysis. J. Sport Health Sci. 2016, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Nüesch, C.; Fiebig, O.; Stoll, T.M.; Köhler, M.; Barth, A.; Mündermann, A. Accelerometry-based physical activity, disability and quality of life before and after lumbar decompression surgery from a physiotherapeutic perspective: An observational cohort study. N. Am. Spine Soc. J. 2021, 8, 100087. [Google Scholar] [CrossRef] [PubMed]
- Smuck, M.; Muaremi, A.; Zheng, P.; Norden, J.; Sinha, A.; Hu, R.; Tomkins-Lane, C. Objective measurement of function following lumbar spinal stenosis decompression reveals improved functional capacity with stagnant real-life physical activity. Spine J. 2018, 18, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Celis, R.; Pipinos, I.I.; Scott-Pandorf, M.M.; Myers, S.A.; Stergiou, N.; Johanning, J.M. Peripheral arterial disease affects kinematics during walking. J. Vasc. Surg. 2009, 49, 127–132. [Google Scholar] [CrossRef]
- Hassabi, M.; Hassani, M.; Abedi-Yekta, A.H.; Esfahani, M.P.; Rabbani, A.; Salehi, S.; Khodabakhshian, S.; Aliabbar, S. The calf muscle strength correlation with the severity of ischemia and outcomes in patients with chronic lower limb ischemia. J. Bodyw. Mov. Ther. 2025, 42, 115–119. [Google Scholar] [CrossRef]
- Kim, K.; Thome, T.; Pass, C.; Stone, L.; Vugman, N.; Palzkill, V.; Yang, Q.; O’Malley, K.A.; Anderson, E.M.; Fazzone, B.; et al. Multiomic Analysis of Calf Muscle in Peripheral Artery Disease and Chronic Kidney Disease. Circ. Res. 2025, 136, 688–703. [Google Scholar] [CrossRef]
- Mohammadzadeh Gonabadi, A.; Fallahtafti, F.; Burnfield, J.M. How Gait Nonlinearities in Individuals Without Known Pathology Describe Metabolic Cost During Walking Using Artificial Neural Network and Multiple Linear Regression. Appl. Sci. 2024, 14, 11026. [Google Scholar] [CrossRef]

| Age (years) | Body Mass (kg) | Height (m) | BMI 1 (kg/m2) |
|---|---|---|---|
| 62.70 (5.31) | 85.76 (14.96) | 1.76 (0.07) | 27.46 (4.12) |
| Group | Ankle | Knee | Hip |
|---|---|---|---|
| ROM Mean (degrees) | |||
| Pre-surgical | 22.781 ± 3.841 | 45.203 ± 7.672 | 37.306 ± 4.583 |
| Post-surgical | 23.122 ± 3.775 | 47.524 ± 6.806 | 35.076 ± 4.539 |
| ROM Std (degrees) | |||
| Pre-surgical | 5.404 ± 1.940 | 14.296 ± 3.245 | 4.616 ± 3.526 |
| Post-surgical | 4.926 ± 1.932 | 14.030 ± 3.519 | 4.234 ± 3.547 |
| SampEn | |||
| Pre-surgical | 0.280 ± 0.068 | 0.175 ± 0.062 | 0.233 ± 0.039 |
| Post-surgical | 0.275 ± 0.061 | 0.154 ± 0.054 | 0.228 ± 0.043 |
| LyE | |||
| Pre-surgical | 1.218 ± 0.500 | 0.839 ± 0.286 | 0.773 ± 0.371 |
| Post-surgical | 1.304 ± 0.546 | 0.879 ± 0.348 | 0.800 ± 0.310 |
| Outcome | Mean Value Before Surgery | Model-Estimated Mean Change in Outcome | Bonferroni-Adjusted Confidence Interval for Mean Change | Bonferroni-Adjusted p-Value for Change Within Each Joint | p-Value for Main Effect of Joint (Differences in Change Observed Between Joints) | |
|---|---|---|---|---|---|---|
| SampEn | <0.001 | |||||
| Ankle | 0.28 | −0.005 | −0.023 | 0.014 | 1.00 k | |
| Hip | 0.23 | −0.003 | −0.022 | 0.015 | 1.00 k | |
| Knee | 0.17 | −0.018 | −0.037 | 0.000 | 0.05 a,h | |
| LyE | 0.003 | |||||
| Ankle | 1.22 | 0.084 | −0.152 | 0.321 | 1.00 h,k | |
| Hip | 0.77 | 0.029 | −0.111 | 0.170 | 1.00 a | |
| Knee | 0.84 | 0.039 | −0.112 | 0.189 | 1.00 a | |
| ROMMean (degree) | <0.001 | |||||
| Ankle | 22.78 | 0.341 | −1.233 | 22.78 | 1.00 k | |
| Hip | 37.31 | −2.232 | −3.806 | 37.31 | 0.003 k | |
| Knee | 45.20 | 2.322 | 0.748 | 45.20 | 0.002 a,h | |
| ROM Std (degree) | <0.001 | |||||
| Ankle | 5.40 | −0.476 | −1.486 | 0.535 | 0.75 k | |
| Hip | 4.62 | −0.384 | −1.394 | 0.627 | 1.00 k | |
| Knee | 14.30 | −0.268 | −1.278 | 0.743 | 1.00 a,h | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallahtafti, F.; Mohammadzadeh Gonabadi, A.; Samson, K.; Woods, M.; Pipinos, I.; Myers, S. Revascularization Enhances Walking Dynamics in Patients with Peripheral Artery Disease. Appl. Mech. 2025, 6, 40. https://doi.org/10.3390/applmech6020040
Fallahtafti F, Mohammadzadeh Gonabadi A, Samson K, Woods M, Pipinos I, Myers S. Revascularization Enhances Walking Dynamics in Patients with Peripheral Artery Disease. Applied Mechanics. 2025; 6(2):40. https://doi.org/10.3390/applmech6020040
Chicago/Turabian StyleFallahtafti, Farahnaz, Arash Mohammadzadeh Gonabadi, Kaeli Samson, Megan Woods, Iraklis Pipinos, and Sara Myers. 2025. "Revascularization Enhances Walking Dynamics in Patients with Peripheral Artery Disease" Applied Mechanics 6, no. 2: 40. https://doi.org/10.3390/applmech6020040
APA StyleFallahtafti, F., Mohammadzadeh Gonabadi, A., Samson, K., Woods, M., Pipinos, I., & Myers, S. (2025). Revascularization Enhances Walking Dynamics in Patients with Peripheral Artery Disease. Applied Mechanics, 6(2), 40. https://doi.org/10.3390/applmech6020040







