Effectiveness and Safety of Topically Applied Tranexamic Acid with Epinephrine in Surgical Procedures: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
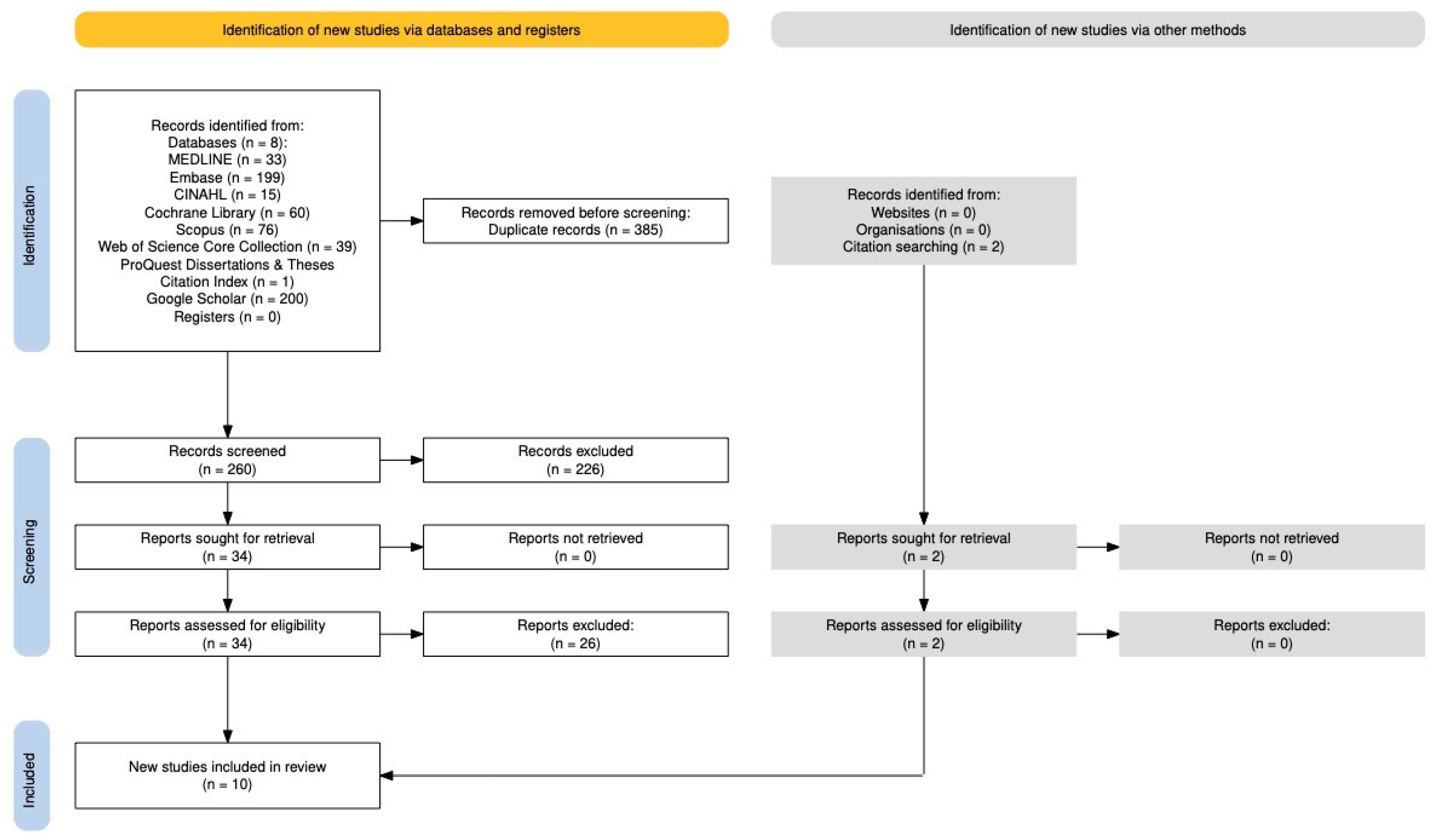
2.4. Study Selection and Data Collection
2.5. Risk of Bias Assessment
2.6. Data Synthesis
3. Results
3.1. Study Characteristics
3.2. Risk of Bias Across Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategies
| Database | Search Strategy |
|---|---|
| MEDLINE Ovid MEDLINE(R) ALL 25 June 1946 to 2025 |
|
| Embase Embase 25 June 1974 to 2025 |
|
| CINAHL | (tranexamic or tranexemic or TXA or cyklokapron or cyklo-F) AND (epinephrine or adrenalin*) AND (topical or soak* or gauze*) |
| Cochrane Library | (tranexamic or tranexemic or TXA or cyklokapron or cyklo-F) AND (epinephrine or adrenalin*) AND (topical or soak* or gauze*) |
| Scopus | (tranexamic or tranexemic or TXA or cyklokapron or cyklo-F) AND (epinephrine or adrenalin*) AND (topical or soak* or gauze*) |
| Web of Science Core Collection | (tranexamic or tranexemic or TXA or cyklokapron or cyklo-F) AND (epinephrine or adrenalin*) AND (topical or soak * or gauze*) |
| ProQuest Dissertations & Theses Citation Index | (tranexamic or tranexemic or TXA or cyklokapron or cyklo-F) AND (epinephrine or adrenalin*) AND (topical or soak* or gauze*) |
| Google Scholar | (tranexamic OR tranexemic OR TXA OR cyklokapron OR cyklo-F) AND (epinephrine OR adrenalin*) AND (topical OR soak* OR gauze*) |
References
- Koh, M.; Hunt, B.J. The management of perioperative bleeding. Blood Rev. 2003, 17, 179–185. [Google Scholar] [CrossRef]
- Allotey, J.K.; King, A.H.; Kumins, N.H.; Wong, V.L.; Harth, K.C.; Cho, J.S.; Kashyap, V.S. Systematic review of hemostatic agents used in vascular surgery. J. Vasc. Surg. 2021, 73, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- De Sutter, P.; Van Lersberghe, C.; Van Tussenbroek, F.; Makar, A. Prevention and management of severe intra-operative and post-operative bleeding in gynaecologic surgery. Eur. Clin. Obstet. Gynaecol. 2006, 2, 102–110. [Google Scholar] [CrossRef]
- Lewis, K.M.; Li, Q.; Jones, D.S.; Corrales, J.D.; Du, H.; Spiess, P.E.; Menzo, E.L.; DeAnda, A. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery 2017, 161, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, I.; Chae, C.; Okocha, O.; Sweitzer, B. Risk assessment and risk stratification for perioperative complications and mitigation: Where should the focus be? How are we doing? Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 517–529. [Google Scholar] [CrossRef]
- Oprea, A.D.; Noto, C.J.; Halaszynski, T.M. Risk stratification, perioperative and periprocedural management of the patient receiving anticoagulant therapy. J. Clin. Anesth. 2016, 34, 586–599. [Google Scholar] [CrossRef]
- Shah, A.; Palmer, A.J.R.; Klein, A.A. Strategies to minimize intraoperative blood loss during major surgery. Br. J. Surg. 2020, 107, e26–e38. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology: First update 2016. Eur. J. Anaesthesiol.|EJA 2017, 34, 332–395. [Google Scholar] [CrossRef]
- Malik, A.; Rehman, F.U.; Shah, K.U.; Naz, S.S.; Qaisar, S. Hemostatic strategies for uncontrolled bleeding: A comprehensive update. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1465–1477. [Google Scholar] [CrossRef]
- Jamali, B.; Nouri, S.; Amidi, S. Local and Systemic Hemostatic Agents: A Comprehensive Review. Cureus 2024, 16, e72312. [Google Scholar] [CrossRef]
- Sundaram, C.P.; Keenan, A.C. Evolution of hemostatic agents in surgical practice. Indian J. Urol. 2010, 26, 374–378. [Google Scholar] [CrossRef]
- Hunt, B.J. The current place of tranexamic acid in the management of bleeding. Anaesthesia 2015, 70 (Suppl. 1), 50–53.e18. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Medcalf, R.L.; Cloud, G.C.; Myles, P.S.; Keragala, C.B. Tranexamic acid for haemostasis and beyond: Does dose matter? Thromb. J. 2023, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ribkoff, J.; Olson, S.; Raghunathan, V.; Al-Samkari, H.; DeLoughery, T.G.; Shatzel, J.J. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur. J. Haematol. 2020, 104, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Meaidi, A.; Mørch, L.; Torp-Pedersen, C.; Lidegaard, O. Oral tranexamic acid and thrombosis risk in women. EClinicalMedicine 2021, 35, 100882. [Google Scholar] [CrossRef]
- Relke, N.; Chornenki, N.L.J.; Sholzberg, M. Tranexamic acid evidence and controversies: An illustrated review. Res. Pract. Thromb. Haemost. 2021, 5, e12546. [Google Scholar] [CrossRef]
- Zehtabchi, S. In nonsurgical settings, tranexamic acid reduces mortality and does not increase thrombotic events. Ann. Intern. Med. 2019, 171, JC40. [Google Scholar] [CrossRef]
- Jans, Ø.; Grevstad, U.; Mandøe, H.; Kehlet, H.; Johansson, P.I. A randomized trial of the effect of low dose epinephrine infusion in addition to tranexamic acid on blood loss during total hip arthroplasty. BJA Br. J. Anaesth. 2016, 116, 357–362. [Google Scholar] [CrossRef]
- Teng, Y.; Ma, J.; Ma, X.; Wang, Y.; Lu, B.; Guo, C. The efficacy and safety of epinephrine for postoperative bleeding in total joint arthroplasty: A PRISMA-compliant meta-analysis. Medicine 2017, 96, e6763. [Google Scholar] [CrossRef]
- Zeng, W.-N.; Liu, J.-L.; Wang, F.-Y.; Chen, C.; Zhou, Q.; Yang, L. Low-dose epinephrine plus tranexamic acid reduces early postoperative blood loss and inflammatory response: A randomized controlled trial. J. Bone Jt. Surg. 2018, 100, 295–304. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 72. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The Role of Google Scholar in Evidence Reviews and Its Applicability to Grey Literature Searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis Campbell Systematic Reviews. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Mohan, A.; Vishwanath, G.; Rajendran, N. A study to evaluate reduction in blood loss by topical application of tranexamic acid in burn wound surgery. J. Mar. Med. Soc. 2021, 23, 129–134. [Google Scholar] [CrossRef]
- Schroeder, R.J.; Langsdon, P.R. Effect of local tranexamic acid on hemostasis in rhytidectomy. Facial Plast. Surg. Aesthetic Med. 2020, 22, 195–199. [Google Scholar] [CrossRef]
- Couto, R.A.; Charafeddine, A.; Sinclair, N.R.; Nayak, L.M.; Zins, J.E. Local infiltration of tranexamic acid with local anesthetic reduces intraoperative facelift bleeding: A preliminary report. Aesthetic Surg. J. 2020, 40, 587–593. [Google Scholar] [CrossRef]
- Fayman, M.; Beeton, A.; Potgieter, E.; Ndou, R.; Mazengenya, P. Efficacy of topical tranexamic acid (Cyclokapron) in “wet” field infiltration with dilute local anaesthetic solutions in plastic surgery. Aesthetic Plast. Surg. 2021, 45, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Abboud, N.M.; Kapila, A.K.; Abboud, S.; Yaacoub, E.; Abboud, M.H. The Combined Effect of Intravenous and Topical Tranexamic Acid in Liposuction: A Randomized Double-Blinded Controlled Trial; Oxford University Press US: New York, NY, USA, 2021. [Google Scholar]
- Hazrati, E.; Masnour-Ghanaei, A.; Soleimanlo, A.; Rafiei, M. Evaluation of local tranexamic acid on septoplastic surgery quality. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Zhaohui, L.; Wanshou, G.; Qidong, Z.; Guangduo, Z. Topical hemostatic procedures control blood loss in bilateral cemented single-stage total knee arthroplasty. J. Orthop. Sci. 2014, 19, 948–953. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chang, Y.; Chen, D.W.; Ueng, S.W.N.; Lee, M.S. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.a.M.; Al-Talibi, I.; Darak, S.M.; Mohammed, A.A.; Alnori, H. Tranexamic acid versus adrenaline-soaked pledgets for the reduction of intraoperative bleeding in functional endoscopic sinus surgery. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2024, 52, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Salamah, M.A.; Al Bialy, H.A.; Khairy, M.A.; Ali, A.G. Topical combined tranexamic acid and epinephrine versus topical epinephrine in control of intraoperative bleeding of external dacryocystorhinostomy. Int. Ophthalmol. 2023, 43, 3785–3791. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Marcucci, M.; Painter, T.W.; Conen, D.; Lomivorotov, V.; Sessler, D.I.; Chan, M.T.V.; Borges, F.K.; Martínez-Zapata, M.J.; Wang, C.Y. Tranexamic acid in patients undergoing noncardiac surgery. N. Engl. J. Med. 2022, 386, 1986–1997. [Google Scholar] [CrossRef]
- Murao, S.; Nakata, H.; Roberts, I.; Yamakawa, K. Effect of tranexamic acid on thrombotic events and seizures in bleeding patients: A systematic review and meta-analysis. Crit. Care 2021, 25, 380. [Google Scholar] [CrossRef] [PubMed]
- Park, L.J.; Marcucci, M.; Ofori, S.N.; Borges, F.K.; Nenshi, R.; Kanstrup, C.T.B.; Rosen, M.; Landoni, G.; Lomivorotov, V.; Painter, T.W. Safety and efficacy of tranexamic acid in general surgery. JAMA Surg. 2025, 160, 267–274. [Google Scholar] [CrossRef]
- Tsan, S.E.H.; Viknaswaran, N.L.; Cheong, C.C.; Cheah, S.; Ng, K.T.; Mong, S.X.Y.; Wang, C.Y. Prophylactic intravenous tranexamic acid and thromboembolism in non-cardiac surgery: A systematic review, meta-analysis and trial sequential analysis. Anaesthesia 2023, 78, 1153–1161. [Google Scholar] [CrossRef]
- Battistini, A.; Gottlieb, L.J.; Vrouwe, S.Q. Topical hemostatic agents in burn surgery: A systematic review. J. Burn Care Res. 2023, 44, 262–273. [Google Scholar] [CrossRef]
- Fijany, A.J.; Givechian, K.B.; Zago, I.; Olsson, S.E.; Boctor, M.J.; Gandhi, R.R.; Pekarev, M. Tranexamic acid in burn surgery: A systematic review and meta-analysis. Burns 2023, 49, 1249–1259. [Google Scholar] [CrossRef]
- Ker, K.; Beecher, D.; Roberts, I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst. Rev. 2013, 2013, CD010562. [Google Scholar] [PubMed]
- Tapking, C.; Hundeshagen, G.; Kirchner, M.; Fischer, S.; Kneser, U.; Bliesener, B. Tranexamic acid reduced blood transfusions in acute burn surgery: A retrospective case-controlled trial. Burns 2022, 48, 522–528. [Google Scholar] [CrossRef] [PubMed]
| Study | Procedure Type | Study Design | Sample Size | TXA Dose and Route | Comparator | Outcomes Reported | Key Findings | Complications |
|---|---|---|---|---|---|---|---|---|
| Mohan et al., 2021 [26] | Burn wound excision | Prospective observational with within-patient control | 38 | 1000 mg TXA in 200 mL NS (0.5%) with 1:200,000 epinephrine; applied topically via soaked sponge after excision | Epinephrine alone (1:200,000) | Blood loss per unit area, total blood loss, graft take, complications | TXA + epinephrine reduced blood loss by 36% compared to epinephrine alone; no impact on graft take | None reported |
| Schroeder et al., 2020 [27] | Rhytidectomy | Retrospective cohort | 76 (44 TXA, 32 control) | TXA 9.1 mg/mL added to local anesthetic and tumescent; injected subcutaneously and into the sub-SMAS plane | Same anesthetic solutions with epinephrine, without TXA | POD1 drain output, days to drain removal, intraoperative EBL, hematoma, thromboembolic events | TXA reduced POD1 drain output (14.8 vs. 50.4 cc), earlier drain removal, and lower EBL; no difference in hematoma rate | 1 PE in TXA group, no significant difference overall |
| Couto et al., 2020 [28] | Facelift (extended SMAS and SMAS plication) | Retrospective case series | 27 | 1.5 mL TXA (100 mg/mL) in 150 mL lidocaine + epinephrine; subcutaneous injection | None | Time to hemostasis, estimated surgical time saved, subjective field dryness | TXA reduced time to hemostasis; improved field dryness; estimated 25–60 min surgical time saved | Minor skin healing delay (7.4%), 1 temporary neuropraxia (3.7%) |
| Fayman et al., 2021 [29] | Liposuction | Blinded prospective randomized case–control (within-patient) | 33 | 500 mL tumescent solution with 0.1% TXA + epinephrine; injected into one flank | TXA-free tumescent solution (same patient) | Bruise area (day 1 and day 7) | TXA significantly reduced bruise area on days 1 and 7 | None reported |
| Abboud et al., 2021 [30] | Liposuction | Randomized double-blind within-patient controlled trial | 36 | 5 mL IV TXA at induction + 5 mL TXA in 1L NS with epinephrine (370 mL avg infiltrated per breast) | Epinephrine alone (same-patient control) | Decantation ratio, dermal bleeding, postoperative ecchymosis | TXA reduced decanted blood volume by 38% and intraoperative bleeding, but associated with increased ecchymosis | None reported |
| Hazrati et al., 2021 [31] | Septoplasty | Randomized double-blind controlled trial | 60 (30 per group) | 100 mg TXA in lidocaine + epinephrine; locally injected at surgical site | Lidocaine + epinephrine only | Blood loss, Boezaart score, satisfaction, operative time, hemodynamics | TXA group had significantly less blood loss, better scores, higher satisfaction, shorter OR time | None reported |
| Zhaohui et al., 2014 [32] | Bilateral total knee arthroplasty | Randomized controlled trial | 90 (43 TXA, 47 control) | Multi-step: TXA + epinephrine soft tissue infiltration, intra-articular injection, bone sealing, drain clamping | Epinephrine-only infiltration; no TXA, no bone sealing; drain clamping | Blood loss, drainage, Hb/Hct drop, transfusions, swelling, function, adverse events | TXA group had 27% less blood loss, fewer transfusions, improved Hb/Hct, no significant diff in complications | TXA: 2 DVTs, 2 blisters, 3 bruises; Control: 3 DVTs, 3 hematomas, 2 blisters, 4 bruises |
| Chang et al., 2014 [33] | Total hip arthroplasty | Retrospective cohort | 388 hips (154 TXA, 234 control) | 10 mL of 5% TXA in local cocktail with epinephrine, anesthetics, antibiotics; injected intraarticularly | Cocktail without TXA | Hb drop, blood loss, transfusion rate, transfusion volume, hospital stay | TXA group had reduced Hb drop, EBL (695 vs. 819 mL), and transfusion rate (17% vs. 35%) | 1 PE in control group |
| Aziz et al., 2024 [34] | Functional Endoscopic Sinus Surgery (FESS) | Randomized within-subject split-body trial | 40 | 10 mg/mL TXA + 1:10,000 epinephrine; pledgets applied intranasally for 10 min | Epinephrine-only pledgets and TXA-only pledgets (same patient, opposite side) | Intraoperative blood loss, Boezaart score, surgery duration | TXA improved surgical field score, reduced blood loss, with shorter surgery duration | None reported |
| Salamah et al., 2023 [35] | External DCR | Double-blind randomized controlled trial | 30 | 100 mg/mL TXA + epinephrine; gauze soaked and applied topically for 2 min | Epinephrine only | Intraoperative blood loss, surgery duration, gauze use, satisfaction | TXA significantly reduced blood loss, gauze use, surgical time | None reported |
| Risk of Bias Domain | Description | Mohan et al., 2021 [26] | Schroeder et al., 2020 [27] | Couto et al., 2020 [28] | Fayman et al., 2021 [29] | Abboud et al., 2021 [30] | Hazrati et al., 2021 [31] | Zhaohui et al., 2014 [32] | Chang et al., 2014 [33] | Aziz et al., 2024 [34] | Salamah et al., 2023 [35] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Confounding/Randomization | Were confounders adjusted or were groups randomized and balanced at baseline? | Moderate | Serious | Critical | Serious | Low | Low | Moderate | Low to Moderate | Low | Low |
| Participant Selection/Allocation Concealment | Were participants selected appropriately and/or was allocation concealed? | Moderate | Moderate to Serious | Moderate to Critical | Critical | Low | Moderate | Moderate | Moderate | Low | Low |
| Intervention Classification/Blinding | Was intervention clearly defined and were participants and researchers blinded? | Low to Moderate | Low to Moderate | Low | Moderate | Low | Low | Moderate | Low | Moderate | Low |
| Deviations from Intended Interventions | Were interventions implemented as planned (including intention-to-treat)? | Moderate | Moderate | Critical | Serious | Low | Low | Moderate | Low | Low | Low |
| Missing Data | Was follow-up complete or were missing data appropriately handled? | Low | Low | Serious | Critical | Low | Low | Low | Low | Low | Low |
| Measurement of Outcomes | Were outcomes measured objectively and were assessors blinded? | Moderate to Serious | Serious | Critical | Low to Moderate | Moderate | Low | Low | Low to Moderate | Moderate | Moderate |
| Selective Reporting | Were all pre-specified outcomes reported? | Moderate | Low to Moderate | Low | Moderate | Moderate | Low | Low | Low to Moderate | Low | Low |
| Overall Risk of Bias | Final judgment based on the above domains | Moderate | Serious | Critical | Serious | Low | Low | Low to Moderate | Moderate | Moderate | Low |
| Notes | Justifications or key limitations | Within-patient design minimize confounding. Definitions of outcomes were vague, and measurement methods lacked full objectivity. | Historical control design. All female participants. Most outcomes measured objectively but thromboembolic surveillance lacked detail. | No control group or objective measurements of outcomes. Bleeding was assessed subjectively by unblinded staff. | No information on initial sample size or loss to follow-up; systemic TXA absorption may have blurred control vs. experimental effects. | Strong RCT design with within-patient controls. | Well-executed RCT. Minor concerns remain regarding allocation concealment and unreported confounders. | Randomization method and allocation concealment were not described, raising risk of selection bias. concerns persist due to lack of blinding and unspecified exclusions. | Historical control design introduces time-based confounding risk. No blinding was mentioned, and VTE was not systematically screened. | Split-body randomized design minimized confounding, and interventions were clearly defined and applied consistently. However, lack of assessor blinding for subjective outcomes introduced moderate detection bias. | Well-conducted double-blind RCT with clearly defined interventions and complete follow-up. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavarz, H.; Lin, W.W.C.; Dodd, S.; Kung, J.Y.; Wong, J.N. Effectiveness and Safety of Topically Applied Tranexamic Acid with Epinephrine in Surgical Procedures: A Systematic Review. Eur. Burn J. 2025, 6, 52. https://doi.org/10.3390/ebj6030052
Keshavarz H, Lin WWC, Dodd S, Kung JY, Wong JN. Effectiveness and Safety of Topically Applied Tranexamic Acid with Epinephrine in Surgical Procedures: A Systematic Review. European Burn Journal. 2025; 6(3):52. https://doi.org/10.3390/ebj6030052
Chicago/Turabian StyleKeshavarz, Hedieh, Weber Wei Chiang Lin, Shawn Dodd, Janice Y. Kung, and Joshua N. Wong. 2025. "Effectiveness and Safety of Topically Applied Tranexamic Acid with Epinephrine in Surgical Procedures: A Systematic Review" European Burn Journal 6, no. 3: 52. https://doi.org/10.3390/ebj6030052
APA StyleKeshavarz, H., Lin, W. W. C., Dodd, S., Kung, J. Y., & Wong, J. N. (2025). Effectiveness and Safety of Topically Applied Tranexamic Acid with Epinephrine in Surgical Procedures: A Systematic Review. European Burn Journal, 6(3), 52. https://doi.org/10.3390/ebj6030052






