Use of Integra® Dermal Regeneration Template Bilayer in Burn Reconstruction: Narrative Review, Expert Opinion, Tips and Tricks
Abstract
1. Introduction
2. Indications
3. IDRT for Different Burn Areas and Locations
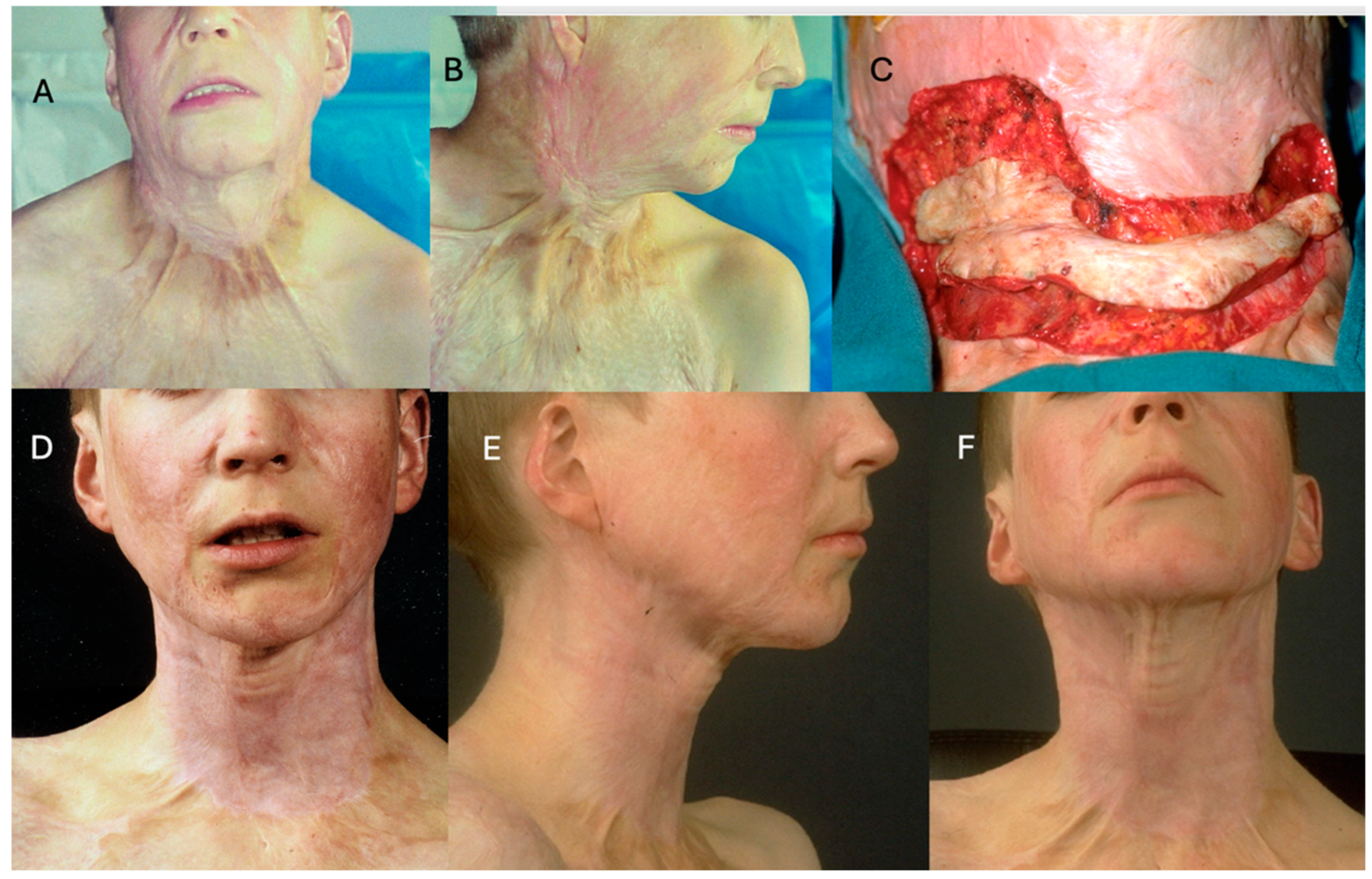
3.1. Face and Neck
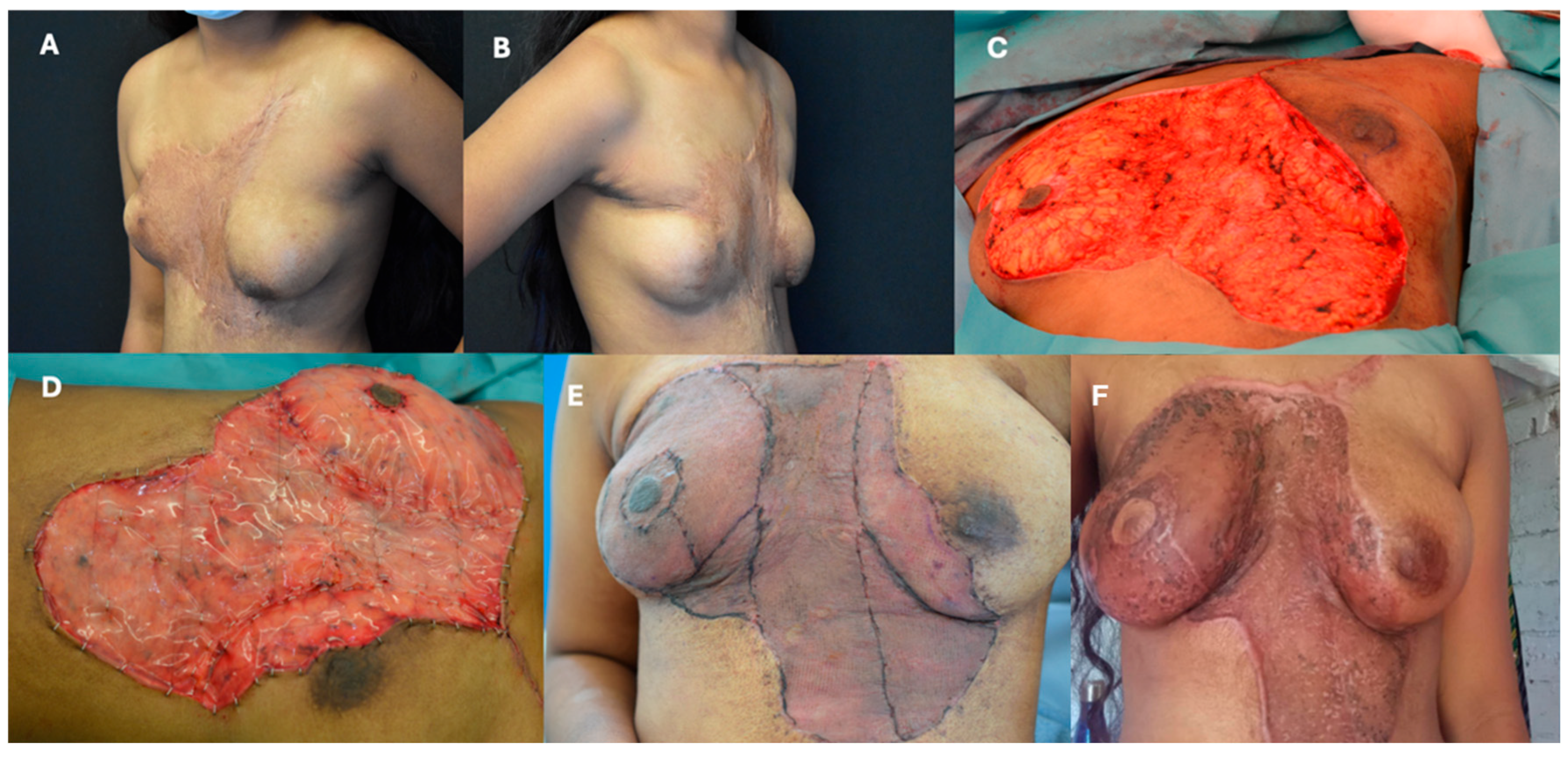
3.2. Torso
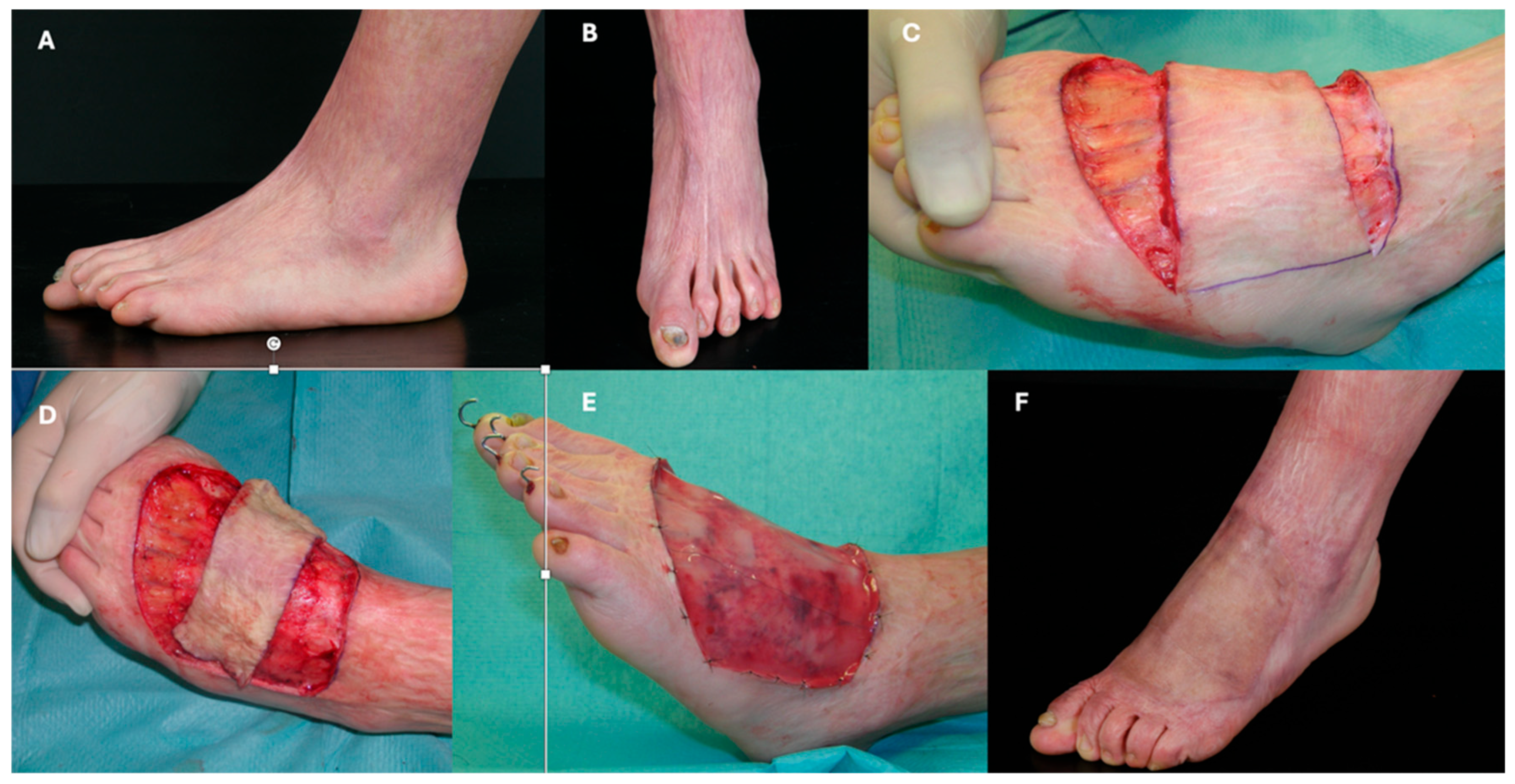
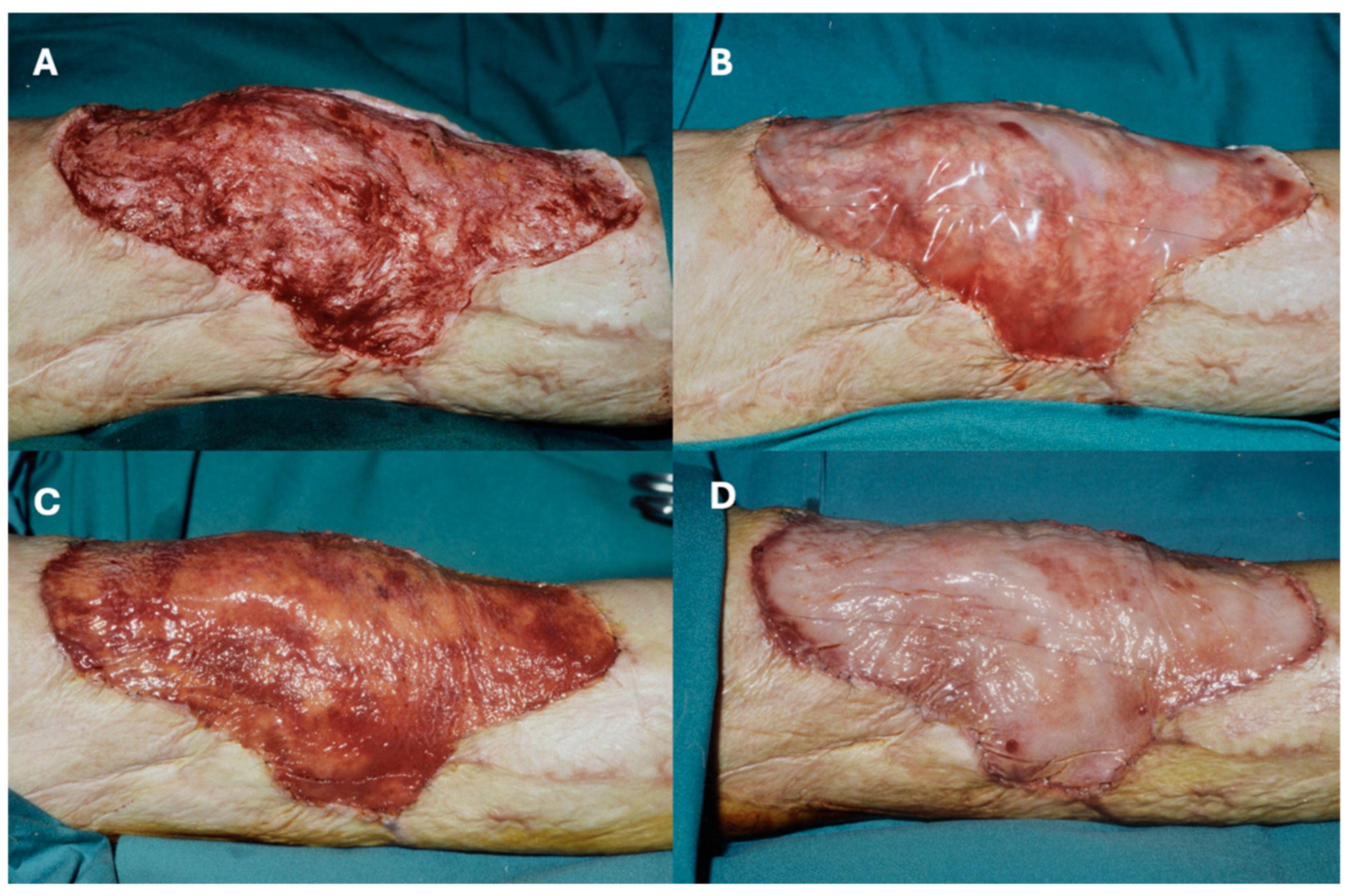
3.3. Limbs
4. Protocols
4.1. Protocols and Their Changes over the Last 25 Years
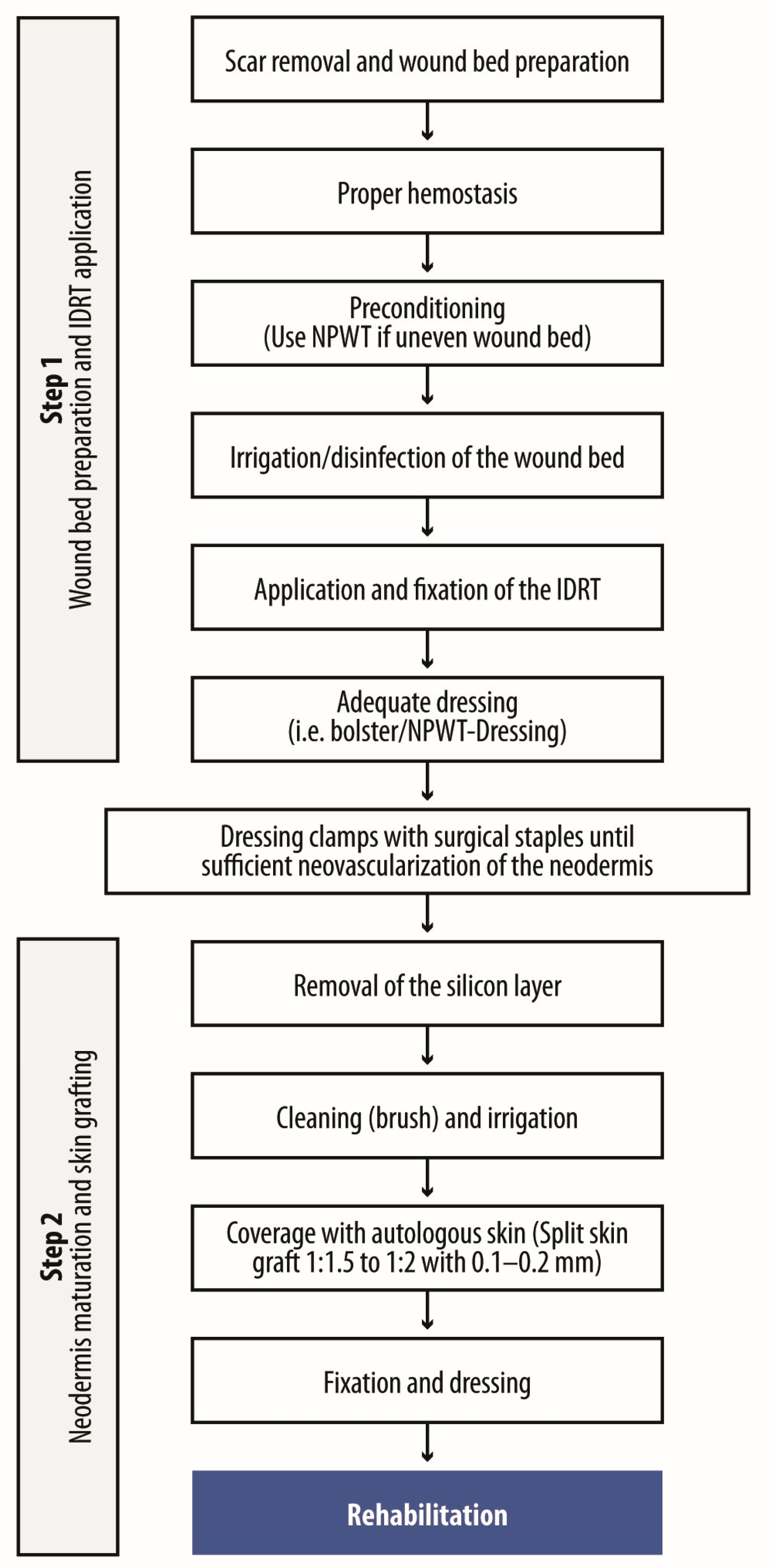
4.2. Wound Preparation and IDRT Application
4.3. Neodermis Maturation and Grafting
4.4. Postoperative Care
4.5. Special Considerations and Challenging Anatomical Areas
5. Dressings
5.1. Types of Dressings and Their Roles
5.2. Dressing Changes and Monitoring
5.3. Post-Grafting Dressing Protocol
6. Complication Management
6.1. Infections
6.2. Hematomas
6.3. Lack of Adherence
6.4. Poor STSG Take Rate
6.5. Shrinkage
7. Tips and Tricks
7.1. Treatment Planning and Coordination
7.2. Learning Curve
7.3. Tailored Approach
7.4. Wound Bed Preparation and Management
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Burns. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/burns#:~:text=The%20problem,and%20South%2DEast%20Asia%20Regions (accessed on 6 August 2025).
- Spronk, I.; Legemate, I.; Oen, N.; van Loey, S.; Polinder, S.; van Baar, M. Health related quality of life in adults after burn injuries: A systematic review. PLoS ONE 2018, 13, e0197507. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.E.; Huynh, M.N.; Jeschke, M.; Malic, C. Dermal regenerative matrix use in burn patients: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.C.; Di Gennaro, J.L.; von Saint André-von Arnim, A.; Stewart, B.T. Stewart. Global trends in pediatric burn injuries and care capacity from the world health organization global burn registry. Front. Pediatr. 2022, 10, 954995. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Mason, S.A.; Nathens, A.B.; Byrne, J.P.; Diong, C.; Fowler, R.A.; Karanicolas, P.J.; Moineddin, R.; Jeschke, M.G. Increased rate of long-term mortality among burn survivo;rs: A population-based matched cohort study. Ann. Surg. 2019, 269, 1192–1199. [Google Scholar] [CrossRef]
- Stone, J.; Gawaziuk, J.P.; Khan, S.; Chateau, D.; Bolton, J.M.; Sareen, J.; Enns, J.; Doupe, M.; Brownell, M.; Logsetty, S. Logsetty. Outcomes in adult survivors of childhood burn injuries as compared with matched controls. J. Burn. Care Res. 2016, 37, e166–e173. [Google Scholar] [CrossRef]
- Wainwright, D.J. Burn reconstruction: The problems, the techniques, and the applications. Clin. Plast. Surg. 2009, 36, 687–700. [Google Scholar] [CrossRef]
- Gupta, S.N.; Moiemen, J.P.; Fischer, C.; Attinger, M.G.; Jeschke, P. Taupin and D. P. Orgill. Dermal regeneration template in the management and reconstruction of burn injuries and complex wounds: A review. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5674. [Google Scholar] [CrossRef]
- Giudice, G.; Ranno, R.; Lombardo, G.; Di Lonardo, A.; Perniciaro, G.; Posadinu, M.A.; Melandri, D.; D’ALessio, R.; Preis, F.B.; Zamparelli, M.; et al. Use of integra dermal regeneration template in burn patients: An italian expert consensus delphi study. Burns 2024, 50, 107236. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C., Jr.; Bondoc, C.C.; Jung, W.K. Jung. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981, 194, 413. [Google Scholar] [CrossRef]
- Lohana, P.; Hassan, S.; Watson, S.B. Watson. Integra™ in burns reconstruction: Our experience and report of an unusual immunological reaction. Ann. Burn. Fire Disasters 2014, 27, 17–21. [Google Scholar]
- Heimbach, D.; Luterman, A.; Burke, J.; Cram, A.; Herndon, D.; Hunt, J.; Jordan, M.; McMANUS, W.; Solem, L.; Warden, G.; et al. Artificial dermis for major burns. A multicenter randomized clinical trial. Ann. Surg. 1988, 208, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra artificial skin for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, E.; Queruel, P.; Salinier, L.; Palmier, B.; Quinot, J. Dermal regeneration template for deep hand burns: Clinical utility for both early grafting and reconstructive surgery. Br. J. Plast. Surg. 2003, 56, 764–774. [Google Scholar] [CrossRef]
- Jeng, J.C.; Fidler, P.E.; Sokolich, J.C.; Jaskille, A.D.; Khan, S.; White, P.M.; Street, J.H.; Light, T.D.; Jordan, M.H. Seven years’ experience with integra as a reconstructive tool. J. Burn. Care Res. 2007, 28, 120–126. [Google Scholar] [CrossRef]
- Kowal-Vern, A.; Criswell, B.K. Burn scar neoplasms: A literature review and statistical analysis. Burns 2005, 31, 403–413. [Google Scholar] [CrossRef]
- Figus, A.; Leon-Villapalos, J.; Philp, B.; Dziewulski, P. Severe multiple extensive postburn contractures: A simultaneous approach with total scar tissue excision and resurfacing with dermal regeneration template. J. Burn. Care Res. 2007, 28, 913–917. [Google Scholar] [CrossRef]
- Moiemen, N.S.; Staiano, J.J.; Ojeh, N.O.; Thway, Y.; Frame, J.D. Reconstructive surgery with a dermal regeneration template: Clinical and histologic study. Plast. Reconstr. Surg. 2001, 108, 93–103. [Google Scholar] [CrossRef]
- Klein, M.B.; Engrav, L.H.; Holmes, J.H.; Friedrich, J.B.; Costa, B.A.; Honari, S.; Gibran, N.S. Management of facial burns with a collagen/glycosaminoglycan skin substitute-prospective experience with 12 consecutive patients with large, deep facial burns. Burns 2005, 31, 257–261. [Google Scholar] [CrossRef]
- Suarez-Cañon, N.; Pérez-Camelo, J.S.; Barrios, V.; Gómez-Ortega, V. Monolayer acellular dermal matrix for reconstruction of face burn: A case report. JPRAS Open 2024, 39, 307–312. [Google Scholar] [CrossRef]
- Palao, R.; Gómez, P.; Huguet, P. Burned breast reconstructive surgery with integra dermal regeneration template. Br. J. Plast. Surg. 2003, 56, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Moiemen, N.S.; Vlachou, E.; Staiano, J.J.; Thawy, Y.; Frame, J.D. Reconstructive surgery with Integra dermal regeneration template: Histologic study, clinical evaluation, and current practice. Plast. Reconstr. Surg. 2006, 117, 160S–174S. [Google Scholar] [CrossRef]
- Karki, D.; Mehta, N.; Narayan, R.P. Post-burn axillary contracture: A therapeutic challenge! Indian. J. Plast. Surg. 2014, 47, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Roa, G.; Heras, F.; Las, R.; Pineros, B.; Jose, L.; Correa, S.; Norambuena, B.; Marre, N. Axillary burn contracture treated with integra®. Rev. Chil. Cir. 2011, 63, 276–279. [Google Scholar] [CrossRef]
- Young, R.; Burd, A. Paediatric upper limb contracture release following burn injury. Burns 2004, 30, 723–728. [Google Scholar] [CrossRef]
- Chou, T.-D.; Chen, S.-L.; Lee, T.-W.; Chen, S.-G.; Cheng, T.-Y.; Lee, C.-H.; Chen, T.-M.; Wang, H.-J. Reconstruction of burn scar of the upper extremities with artificial skin. Plast. Reconstr. Surg. 2001, 108, 378–384; Discussion 85. [Google Scholar] [CrossRef]
- Kreymerman, P.A.; Andres, L.A.; Lucas, H.D.; Silverman, A.L.; Smith, A.A. Reconstruction of the burned hand. Plast. Reconstr. Surg. 2011, 127, 752–759. [Google Scholar] [CrossRef]
- Dantzer, E.; Braye, F.M. Reconstructive surgery using an artificial dermis (integra): Results with 39 grafts. Br. J. Plast. Surg. 2001, 54, 659–664. [Google Scholar] [CrossRef]
- Danin, A.; Georgesco, G.; Le Touze, A.; Penaud, A.; Quignon, R.; Zakine, G. Assessment of burned hands reconstructed with integra (r) by ultrasonography and elastometry. Burns 2012, 38, 998–1004. [Google Scholar] [CrossRef]
- Cuadra, A.; Correa, G.; Roa, R.; Piñeros, J.L.; Norambuena, H.; Searle, S.; Heras, R.L.; Calderón, W. Functional results of burned hands treated with integra®. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 228–234. [Google Scholar] [CrossRef]
- Weigert, R.; Choughri, H.; Casoli, V. Management of severe hand wounds with integra® dermal regeneration template. J. Hand Surg. Eur. Vol. 2011, 36, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P.; Gandhi, A.; Saini, S. Integra® dermal regeneration template: From design to clinical use. Cureus 2023, 15, e38608. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Wormald, J.C.R.; Wade, R.G.; Collins, D.P. Systematic review of fibrin glue in burn wound reconstruction. Br. J. Surg. 2019, 106, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Leavitt, L.R. Bayer and D. P. Orgill. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef]
- Schiestl, C.; Stiefel, D.; Meuli, M. Giant naevus, giant excision, eleg(i)ant closure? Reconstructive surgery with integra artificial skin to treat giant congenital melanocytic naevi in children. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 610–615. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.M.; Meuli, M. The positive effect of negative pressure: Vacuum-assisted fixation of integra artificial skin for reconstructive surgery. J. Pediatr. Surg. 2009, 44, 575–580. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist wound healing with commonly available dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Gefen, A.; Alves, P.; Beeckman, D.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Swanson, T.; Woo, K. Mechanical and contact characteristics of foam materials within wound dressings: Theoretical and practical considerations in treatment. Int. Wound J. 2023, 20, 1960–1978. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial wound dressings: A concise review for clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef]
- Eskes, A.M.; Schreuder, A.M.; Vermeulen, H.; van Dijkum, E.J.M.N.; Chaboyer, W. Developing an evidence-based and theory informed intervention to involve families in patients care after surgery: A quality improvement project. Int. J. Nurs. Sci. 2019, 6, 352–361. [Google Scholar] [CrossRef]
- Davis, C.R.; Trevatt, A.E.; McGoldrick, R.B.; Parrott, F.E.; Mohanna, P.-N. How to train plastic surgeons of the future. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1134–1140. [Google Scholar] [CrossRef]
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The basics of integra dermal regeneration template and its expanding clinical applications. Semin. Plast. Surg. 2019, 33, 185–189. [Google Scholar] [CrossRef]
- Bishop, S.; Walker, M.; Rogers, A.; Chen, W. Importance of moisture balance at the wound-dressing interface. J. Wound Care 2003, 12, 125–128. [Google Scholar] [CrossRef]
| Protocol Stage | Key Steps and Considerations | Notes |
|---|---|---|
| Preoperative preparation |
| Infection control essential |
| ||
| ||
| ||
| Wound Bed preparation |
| Electrocautery or scalpel-based |
| Fibrin glue recommended | |
| ||
| IDRT application and fixation |
| Immediate application post-excision |
| ||
| Improves adherence, reduces movement and the likelihood of seroma due to reduced movement or sliding | |
| Neodermis maturation |
| Typically 4–6 weeks for maturation |
| Longer than acute burn protocols | |
| Silicone layer removal and grafting |
| Sheet or mesh graft 0.1–0.2 mm thick |
| Mesh expansion 1:1.5 or 1:2 | |
| ||
| Postoperative care |
| Reduces infection risk |
| Hydration and nutrition critical | |
| Begin after 2 weeks | |
| ||
| Special considerations |
| |
| ||
Challenging Areas: | ||
| ||
| ||
| ||
|
| Aspect | Key Points & Considerations |
|---|---|
| Goals of dressing management | Support dermal regeneration; prevent contamination and infection; protect wound from trauma; ensure comfort, mobility, and moisture balance. |
| Silicone-based dressings | Non-adherent, minimize pain/trauma during changes; require frequent monitoring in high-mobility areas. |
| Foam dressings | Absorb exudate, maintain moist environment; can become bulky, may require frequent changes. |
| Hydrocolloid dressings | Absorptive, reduce dressing frequency; may adhere too strongly, risking disruption during removal. |
| Antimicrobial dressings | Reduce infection risk (high-contamination sites); prolonged use may delay healing (cytotoxicity). |
| Non-adherent dressings | Protect wound bed, minimize trauma; may not absorb enough for highly exudative wounds. |
| Negative pressure wound therapy | Adjunct to improve adherence and fluid control; duration and intensity must be carefully calibrated. |
| Dressing change frequency | Every 3–7 days, adjusted for exudate or infection. |
| Initial post-STSG change | At 5–7 days post-application; avoid trauma to graft during early changes. |
| Infection monitoring & management | Inspect regularly; use antimicrobial dressings or topical antibiotics as needed. |
| Moisture balance | Essential for healing; foam or hydrocolloid dressings recommended. |
| Patient comfort | Ensure proper fit and compliance. |
| Complication | Prevention | Management |
|---|---|---|
| Infection |
|
|
| Hematoma |
|
|
| Lack of adherence |
|
|
| Poor STSG take rate |
|
|
| Shrinkage (tissue contraction) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiestl, C.M.; Moiemen, N.; Duhamel, P.; Jones, I.; Zamparelli, M.; López-Gutiérrez, J.C.; Kuepper, S. Use of Integra® Dermal Regeneration Template Bilayer in Burn Reconstruction: Narrative Review, Expert Opinion, Tips and Tricks. Eur. Burn J. 2025, 6, 45. https://doi.org/10.3390/ebj6030045
Schiestl CM, Moiemen N, Duhamel P, Jones I, Zamparelli M, López-Gutiérrez JC, Kuepper S. Use of Integra® Dermal Regeneration Template Bilayer in Burn Reconstruction: Narrative Review, Expert Opinion, Tips and Tricks. European Burn Journal. 2025; 6(3):45. https://doi.org/10.3390/ebj6030045
Chicago/Turabian StyleSchiestl, Clemens Maria, Naiem Moiemen, Patrick Duhamel, Isabel Jones, Marcello Zamparelli, Juan Carlos López-Gutiérrez, and Simon Kuepper. 2025. "Use of Integra® Dermal Regeneration Template Bilayer in Burn Reconstruction: Narrative Review, Expert Opinion, Tips and Tricks" European Burn Journal 6, no. 3: 45. https://doi.org/10.3390/ebj6030045
APA StyleSchiestl, C. M., Moiemen, N., Duhamel, P., Jones, I., Zamparelli, M., López-Gutiérrez, J. C., & Kuepper, S. (2025). Use of Integra® Dermal Regeneration Template Bilayer in Burn Reconstruction: Narrative Review, Expert Opinion, Tips and Tricks. European Burn Journal, 6(3), 45. https://doi.org/10.3390/ebj6030045








