The Impact of Face and Neck Burns on Respiratory Complications and Mortality
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Burns: Key Facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 4 March 2025).
- Sheridan, R.L. Burns. Crit. Care Med. 2002, 30 (Suppl. 11), S500–S514. [Google Scholar] [CrossRef] [PubMed]
- Herdy Guerra Avila, J.E.; Aniceto Santana, L.; Rabelo Suzuki, D.; Maldaner da Silva, V.Z.; Duarte, M.L.; Mizusaki Imoto, A.; Ferreira Amorim, F. Frequency, complications, and mortality of inhalation injury in burn patients: A systematic review and meta-analysis protocol. PLoS ONE 2024, 19, e0295318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenhalgh, D.G.; Saffle, J.R.; Holmes, J.H., 4th; Gamelli, R.L.; Palmieri, T.L.; Horton, J.W.; Tompkins, R.G.; Traber, D.L.; Mozingo, D.W.; Deitch, E.A.; et al. American Burn Association consensus conference to define sepsis and infection in burns. J. Burn. Care Res. 2007, 28, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Zatriqi, V.; Arifi, H.; Zatriqi, S.; Duci, S.; Rrecaj, S.; Martinaj, M. Facial burns—Our experience. Mater. Sociomed. 2013, 25, 26–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horch, R.E.; Jeschke, M.G.; Spilker, G.; Herndon, D.N.; Kopp, J. Treatment of second degree facial burns with allografts--preliminary results. Burns 2005, 31, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Management of facial burns. Burn. Trauma. 2020, 8, tkaa023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoogewerf, C.J.; Hop, M.J.; Nieuwenhuis, M.K.; Oen, I.M.; Middelkoop, E.; Van Baar, M.E. Topical treatment for facial burns. Cochrane Database Syst. Rev. 2020, 7, CD008058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herndon, D.N. Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 550–570+580–600. [Google Scholar]
- Coston, T.D.; Gaskins, D.; Bailey, A.; Minus, E.; Arbabi, S.; West, T.E.; Stewart, B.T. Severity of Inhalation Injury and Risk of Nosocomial Pneumonia: A Retrospective Cohort Study. Chest 2024, 166, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa Santos, D.; Barros, F.; Gomes, N.; Guedes, T.; Maia, M. Face and/or neck burns: A risk factor for respiratory infection? Ann. Burn. Fire Disasters 2016, 29, 97–102. [Google Scholar] [PubMed] [PubMed Central]
- Lange, M.; Hamahata, A.; Traber, D.L.; Esechie, A.; Jonkam, C.; Bansal, K.; Nakano, Y.; Traber, L.D.; Enkhbaatar, P. A murine model of sepsis following smoke inhalation injury. Biochem. Biophys. Res. Commun. 2010, 391, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Mehrotra, M.; Kumar, P.; Gogia, A.R.; Prasad, A.; Fisher, J.A. Smoke Inhalation Injury: Etiopathogenesis, Diagnosis, and Management. Indian J. Crit. Care Med. 2018, 22, 180–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lam, N.N.; Hung, T.D. ARDS among cutaneous burn patients combined with inhalation injury: Early onset and bad outcome. Ann. Burn. Fire Disasters 2019, 32, 37–42. [Google Scholar] [PubMed] [PubMed Central]
- Eastman, A.L.; Arnoldo, B.A.; Hunt, J.L.; Purdue, G.F. Pre-burn center management of the burned airway: Do we know enough? J. Burn. Care Res. 2010, 31, 701–705. [Google Scholar] [CrossRef] [PubMed]
- McLure, M.; Macneil, F.; Wood, F.M.; Cuttle, L.; Eastwood, K.; Bray, J.; Tracy, L.M. A Rapid Review of Burns First Aid Guidelines: Is There Consistency Across International Guidelines? Cureus 2021, 13, e15779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Avignon, L.C.; Hogan, B.K.; Murray, C.K.; Loo, F.L.; Hospenthal, D.R.; Cancio, L.C.; Kim, S.H.; Renz, E.M.; Barillo, D.; Holcomb, J.B.; et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: An autopsy series. Burns 2010, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Hoogewerf, C.J.; van Baar, M.E.; Hop, M.J.; Bloemen, M.C.; Middelkoop, E.; Nieuwenhuis, M.K. Burns to the head and neck: Epidemiology and predictors of surgery. Burns 2013, 39, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Esnault, P.; Prunet, B.; Cotte, J.; Marsaa, H.; Prat, N.; Lacroix, G.; Goutorbe, P.; Montcriol, A.; Dantzer, E.; Meaudre, E. Tracheal intubation difficulties in the setting of face and neck burns: Myth or reality? Am. J. Emerg. Med. 2014, 32, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Jennes, S.; Hanchart, B.; Keersebilck, E.; Rose, T.; Soete, O.; François, P.M.; Engel, H.; Van Trimpont, F.; Davin, C.; Trippaerts, M.; et al. Management of burn wounds of the head and neck region. B-ENT 2016, 12 (Suppl. 26), 107–126. [Google Scholar] [PubMed]
- Coban, Y.K. Infection control in severely burned patients. World J. Crit. Care Med. 2012, 1, 94–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fatusi, O.A.; Fatusi, A.O.; Olabanji, J.K.; Alatise, O.I. Management outcome and associated factors in burn injuries with and without facial involvement in a Nigerian population. J. Burn. Care Res. 2006, 27, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Fraulin, F.O.G.; Illmayer, S.J.; Tredget, E.E. Assessment of cosmetic and functional results of conservative versus surgical management of facial burns. J. Burn. Care Rehabil. 1996, 17, 19–29. [Google Scholar] [CrossRef]
- Chien, W.C.; Pai, L.; Lin, C.C.; Chen, H.C. Epidemiology of hospitalized burns patients in Taiwan. Burns 2003, 29, 582–588. [Google Scholar] [CrossRef]
- Tian, H.; Wang, L.; Xie, W.; Shen, C.; Guo, G.; Liu, J.; Han, C.; Ren, L.; Liang, Y.; Liu, J.; et al. Epidemiology and outcome analysis of facial burns: A retrospective multicentre study 2011-2015. Burns 2020, 46, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Ledrick, D.; Moore, A. Facial Burns. [Updated 3 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559290/# (accessed on 10 March 2025).
- Shams Ortiz, A.; Chan, R.K.; Dion, G.R. Skin burns of the head and neck. Oper. Tech. Otolaryngol. Head. Neck Surg. 2020, 31, 283–288. [Google Scholar] [CrossRef]
- Gigengack, R.K.; Cleffken, B.I.; Loer, S.A. Advances in airway management and mechanical ventilation in inhalation injury. Curr. Opin. Anaesthesiol. 2020, 33, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chen, M.H.; Wen, B.S.; Lee, M.H.; Ma, H. The impact of inhalation injury in patients with small and moderate burns. Burns 2014, 40, 1481–1486. [Google Scholar] [CrossRef]
- Jones, S.W.; Williams, F.N.; Cairns, B.A.; Cartotto, R. Inhalation Injury: Pathophysiology, Diagnosis, and Treatment. Clin. Plast. Surg. 2017, 44, 505–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dries, D.J.; Endorf, F.W. Inhalation injury: Epidemiology, pathology, treatment strategies. Scand. J. Trauma. Resusc. Emerg. Med. 2013, 21, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foncerrada, G.; Culnan, D.M.; Capek, K.D.; González-Trejo, S.; Cambiaso-Daniel, J.; Woodson, L.C.; Herndon, D.N.; Finnerty, C.C.; Lee, J.O. Inhalation injury in the burned patient. Ann. Plast. Surg. 2018, 80 (Suppl. 2), S98–S105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shubert, J.; Sharma, S. Inhalation Injury. [Updated 12 June 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513261/ (accessed on 12 March 2025).
- El-Helbawy, R.H.; Ghareeb, F.M. Inhalation injury as a prognostic factor for mortality in burn patients. Ann. Burn. Fire Disasters 2011, 24, 82–88. [Google Scholar] [PubMed] [PubMed Central]
- Puyana, S.; Ruiz, S.; Amador, F.; Mckenney, M.; Young, E.; Lim, R.; Mir, H. The Outcomes of Inhalation Injuries in Lesser Burns: Still a Deadly Injury. Eplasty 2021, 21, e7. [Google Scholar] [CrossRef]
- Ching, J.A.; Shah, J.L.; Doran, C.J.; Chen, H.; Payne, W.G.; Smith, D.J., Jr. The evaluation of physical exam findings in patients assessed for suspected burn inhalation injury. J. Burn. Care Res. 2015, 36, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi, S.; Sheckter, C.C.; Shepard, K.; Pereira, C.; Davis, D.J.; Karanas, Y.; Rochlin, D.H. Preventing Unnecessary Intubations: A 5-Year Regional Burn Center Experience Using Flexible Fiberoptic Laryngoscopy for Airway Evaluation in Patients With Suspected Inhalation or Airway Injury. J. Burn. Care Res. 2019, 40, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Badulak, J.H.; Schurr, M.; Sauaia, A.; Ivashchenko, A.; Peltz, E. Defining the criteria for intubation of the patient with thermal burns. Burns 2018, 44, 531–538. [Google Scholar] [CrossRef]
- Cotte, J.; Prunet, B.; Esnault, P.; Lacroix, G.; D’aranda, E.; Cungi, P.J.; Goutorbe, P.; Dantzer, E.; Meaudre, E. Early onset pneumonia in patients with severely burned face and neck: A 5-year retrospective study. Burns 2013, 39, 892–896. [Google Scholar] [CrossRef]
- Huzar, T.F.; Cross, J.M. Ventilator-associated pneumonia in burn patients: A cause or consequence of critical illness? Expert. Rev. Respir. Med. 2011, 5, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.D.; Argent, A.C.; Rode, H. Review article: Ventilator-associated pneumonia in major burns. Ann. Burn. Fire Disasters 2012, 25, 135–139. [Google Scholar] [PubMed] [PubMed Central]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 2009, 13, R183. [Google Scholar] [CrossRef]
- Liodaki, E.; Kalousis, K.; Mauss, K.L.; Kisch, T.; Mailaender, P.; Stang, F. Epidemiology of pneumonia in a burn care unit: The influence of inhalation trauma on pneumonia and of pneumonia on burn mortality. Ann. Burn. Fire Disasters 2015, 28, 128–133. [Google Scholar] [PubMed] [PubMed Central]
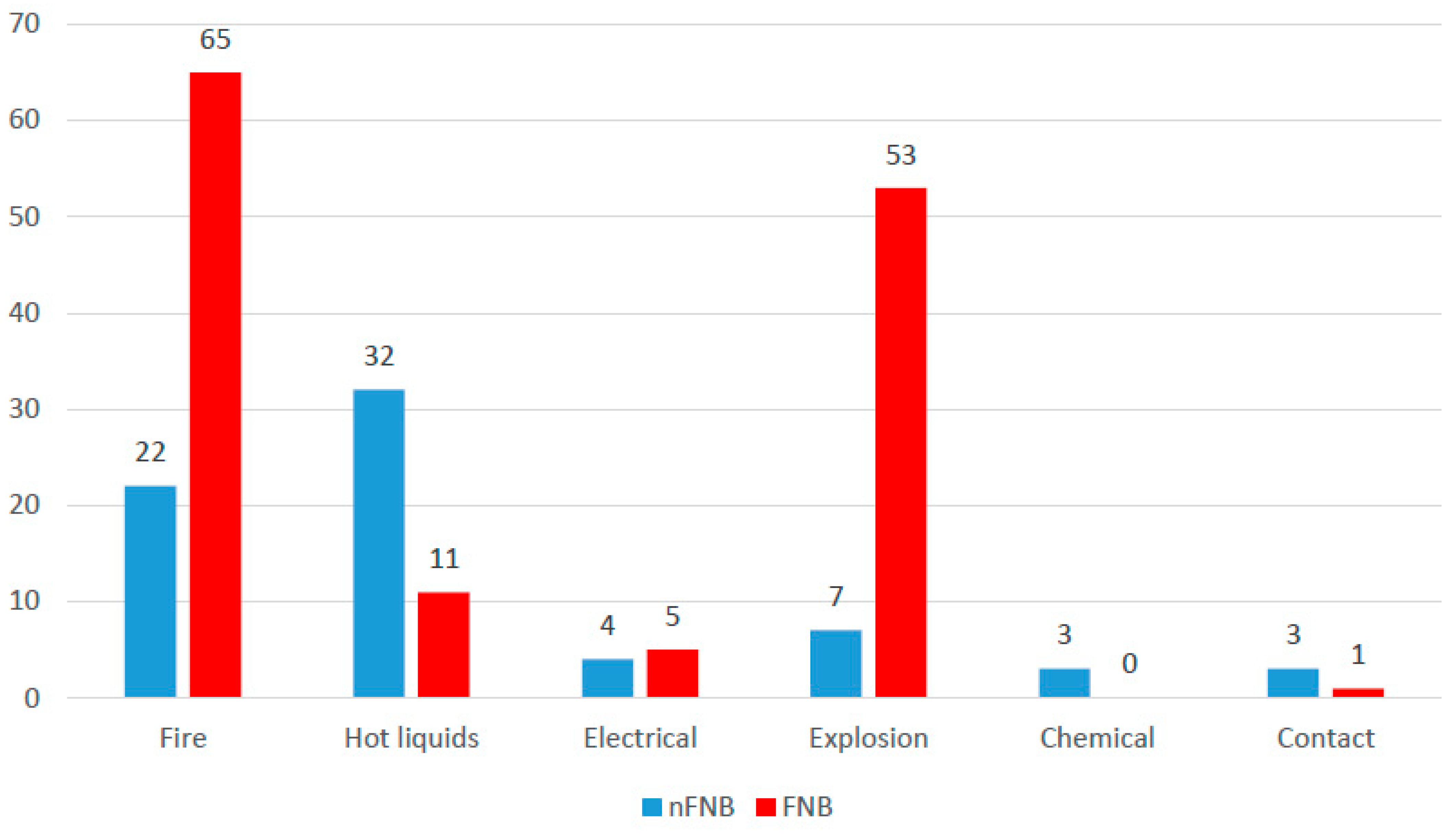
| Parameter | nFNB (n = 71) | FNB (n = 135) | p |
|---|---|---|---|
| Gender (male) (Nr., %) | 43 (60.6%) | 97 (71.9%) | 0.117 |
| Age (median (IQR)) | 57 (45–71) | 49 (37–64) | 0.013 |
| Age category (Nr., %) | |||
| 18–39 years | 13 (18.3%) | 42 (31.1%) | 0.060 |
| 40–64 years | 32 (45.1%) | 61 (45.2%) | |
| ≥65 years | 26 (36.6%) | 32 (23.7%) |
| Medical Condition (Nr., %) | nFNB (n = 71) | FNB (n = 135) |
|---|---|---|
| Hypertension | 32 (45.1%) | 30 (22.2%) |
| Other cardiovascular diseases | 18 (25.4%) | 22 (16.3%) |
| Diabetes mellitus | 16 (22.5%) | 11 (8.1%) |
| Neurologic disease | 8 (11.3%) | 15 (11.1%) |
| Asthma | 2 (2.8%) | 0 (0%) |
| COPD | 2 (2.8%) | 3 (2.2%) |
| Drug abuse | 1 (1.4%) | 1 (0.7%) |
| Psychiatric illness | 4 (5.6%) | 16 (11.8%) |
| Neurologic disease | 8 (11.3%) | 15 (11.1%) |
| Asthma | 2 (2.8%) | 0 (0%) |
| COPD | 2 (2.8%) | 3 (2.2%) |
| Burn Degree (Nr., %) | nFNB (n = 71) | FNB (n = 135) | p |
|---|---|---|---|
| 1st + 2nd | 2 (2.8%) | 11 (8.1%) | 0.239 |
| 2nd | 34 (47.9%) | 51 (37.8%) | |
| 2nd + 3rd | 33 (46.5%) | 71 (52.6%) | |
| 3rd | 2 (2.8%) | 2 (1.5%) |
| Parameter (Nr., %) | Without Infection | With Infection | p |
|---|---|---|---|
| Gender | |||
| - Female | 54 (32.7%) | 12 (29.3%) | 0.713 |
| - Male | 111 (67.3%) | 29 (70.7%) | |
| Age (median (IQR)) | 52 (37–64.5) | 59 (48–74) | 0.010 |
| Age | |||
| 18–39 years | 50 (30.3%) | 5 (12.2%) | 0.022 |
| 40–64 years | 74 (44.8%) | 19 (46.3)% | |
| ≥65 years | 41 (24.8%) | 17 (41.5%) | |
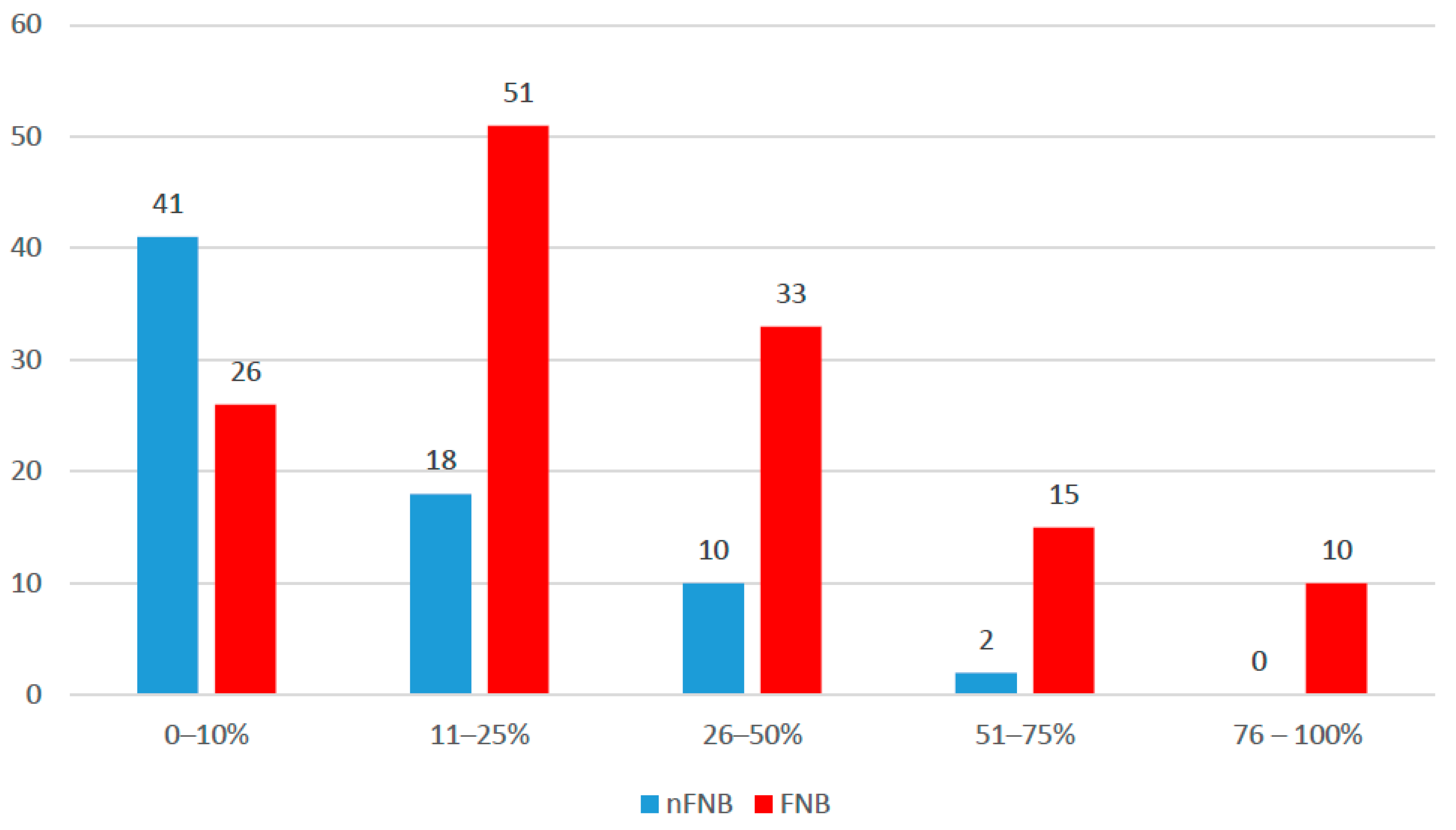
| TBSA (median (IQR)) | 15 (8–25) | 45 (25–59) | <0.001 |
| TBSA | |||
| 0–10% | 65 (39.4%) | 2 (4.9%) | <0.001 |
| 11–25% | 60 (36.4%) | 9 (22%) | |
| 26–50% | 21 (12.7%) | 14 (34.1%) | |
| 51–75% | 13 (7.9%) | 12 (29.3%) | |
| 76–100% | 6 (3.6%) | 4 (9.8%) |
| Dependent Variable = Respiratory Infections | ||||
|---|---|---|---|---|
| Parameter | Univariable | Multivariable | ||
| Log10 (TBSA) | 25.105 (7.574–83.215) | <0.001 | 19.769 (5.729–68.218) | <0.001 |
| FNB | 4.800 (1.791–12.865) | 0.002 | 2.172 (0.751–6.281) | 0.152 |
| Parameter (Nr., %) | nFNB (n = 71) | FNB (n = 135) | p |
|---|---|---|---|
| Inhalation injury | 20 (12.1%) | 29 (70.7%) | <0.001 |
| Endotracheal intubation (ETI) | 10 (14.1%) | 70 (51.9%) | <0.001 |
| ETI scene | |||
| - Burn injury scene | 1 (10%) | 56 (80%) | |
| - Emergency room | 8 (80%) | 14 (20%) | <0.001 |
| - Burn unit | 1 (10%) | 0 (0%) |
| Species (Nr., %) | nFNB Respiratory | FNB Respiratory | nFNB Blood | FNB Blood |
|---|---|---|---|---|
| Acinetobacter baumannii | 1 (16.7%) | 9 (18.75%) | - | 4 (19.05%) |
| Aspergillus spp. | - | 1 (2.08%) | - | - |
| Burkholderia cepacia | 1 (2.08%) | - | - | |
| Candida albicans | - | - | - | 1 (4.76%) |
| Candida parapsilosis | - | - | - | 2 (9.52%) |
| Citrobacter spp. | 1 (2.08%) | - | - | |
| Corynebacterium striatum | 1 (16.7%) | 2 (4.17%) | - | - |
| Escherichia coli | - | - | 1 (16.7%) | 1 (4.76%) |
| Haemophylus influenzae | - | 1 (2.08%) | - | - |
| Klebsiella aerogenes | - | 1 (2.08%) | - | - |
| Klebsiella oxytoca | - | - | 1 (16.7%) | - |
| Klebsiella pneumoniae | 1 (16.7%) | 3 (6.25%) | 1 (16.7%) | 3 (14.29%) |
| Proteus vulgaris | - | 2 (4.17%) | - | |
| Providencia stuartii | - | 1 (2.08%) | 1 (16.7%) | - |
| Serratia marcescens | - | - | - | 1 (4.76%) |
| Pseudomonas aeruginosa | 3 (50%) | 19 (39.58%) | 2 (33.3%) | 5 (23.81%) |
| Pseudomonas putida | - | 2 (4.17%) | - | - |
| Serratia marcescens | - | 2 (4.17%) | - | - |
| Staphylococcus aureus | - | - | - | 1 (4.76%) |
| Staphylococcus haemolyticus | - | - | - | 2 (9.52%) |
| Staphylococcus hominis | - | - | - | 1 (4.76%) |
| Stenotrophomonas maltophilia | - | 3 (6.25%) | - | - |
| Total | 6 | 48 | 6 | 21 |
| Mortality (Nr., %) | nFNB (n = 71) | FNB (n = 135) | p |
|---|---|---|---|
| Survivors | 62 (87.3%) | 93 (68.9%) | 0.004 * |
| Deceased | 9 (12.7%) | 42 (31.1%) | |
| Overall survival (days) | 84.361(68.404–100.317) | 66.781 (52.420–81.142) | 0.003 ** |
| Mean (95% C.I.) | |||
| Dependent Variable = Respiratory Infections | ||||
|---|---|---|---|---|
| Parameter | Univariable | Multivariable | ||
| TBSA | 1.050 (1.037–1.062) | <0.001 | 1.052 (1.039–1.066) | <0.001 |
| FNB | 2.812 (1.366–5.789) | 0.005 | 1.842 (0.848–4.002) | 0.123 |
| Age ≥ 65 years | 2.294 (1.312–4.009) | 0.004 | 4.000 (2.218–7.213) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giurgiu, R.-A.; Bordeanu-Diaconescu, E.-M.; Grosu-Bularda, A.; Frunza, A.; Grama, S.; Costache, R.-A.; Cristescu, C.-I.; Neagu, T.-P.; Lascar, I.; Hariga, C.-S. The Impact of Face and Neck Burns on Respiratory Complications and Mortality. Eur. Burn J. 2025, 6, 27. https://doi.org/10.3390/ebj6020027
Giurgiu R-A, Bordeanu-Diaconescu E-M, Grosu-Bularda A, Frunza A, Grama S, Costache R-A, Cristescu C-I, Neagu T-P, Lascar I, Hariga C-S. The Impact of Face and Neck Burns on Respiratory Complications and Mortality. European Burn Journal. 2025; 6(2):27. https://doi.org/10.3390/ebj6020027
Chicago/Turabian StyleGiurgiu, Rares-Adrian, Eliza-Maria Bordeanu-Diaconescu, Andreea Grosu-Bularda, Adrian Frunza, Sabina Grama, Raducu-Andrei Costache, Carina-Ioana Cristescu, Tiberiu-Paul Neagu, Ioan Lascar, and Cristian-Sorin Hariga. 2025. "The Impact of Face and Neck Burns on Respiratory Complications and Mortality" European Burn Journal 6, no. 2: 27. https://doi.org/10.3390/ebj6020027
APA StyleGiurgiu, R.-A., Bordeanu-Diaconescu, E.-M., Grosu-Bularda, A., Frunza, A., Grama, S., Costache, R.-A., Cristescu, C.-I., Neagu, T.-P., Lascar, I., & Hariga, C.-S. (2025). The Impact of Face and Neck Burns on Respiratory Complications and Mortality. European Burn Journal, 6(2), 27. https://doi.org/10.3390/ebj6020027






