Efficacy of Dialkylcarbamoylchloride (DACC)-Impregnated Dressings in Surgical Wound Management: A Review
Abstract
1. Introduction
2. Methodology
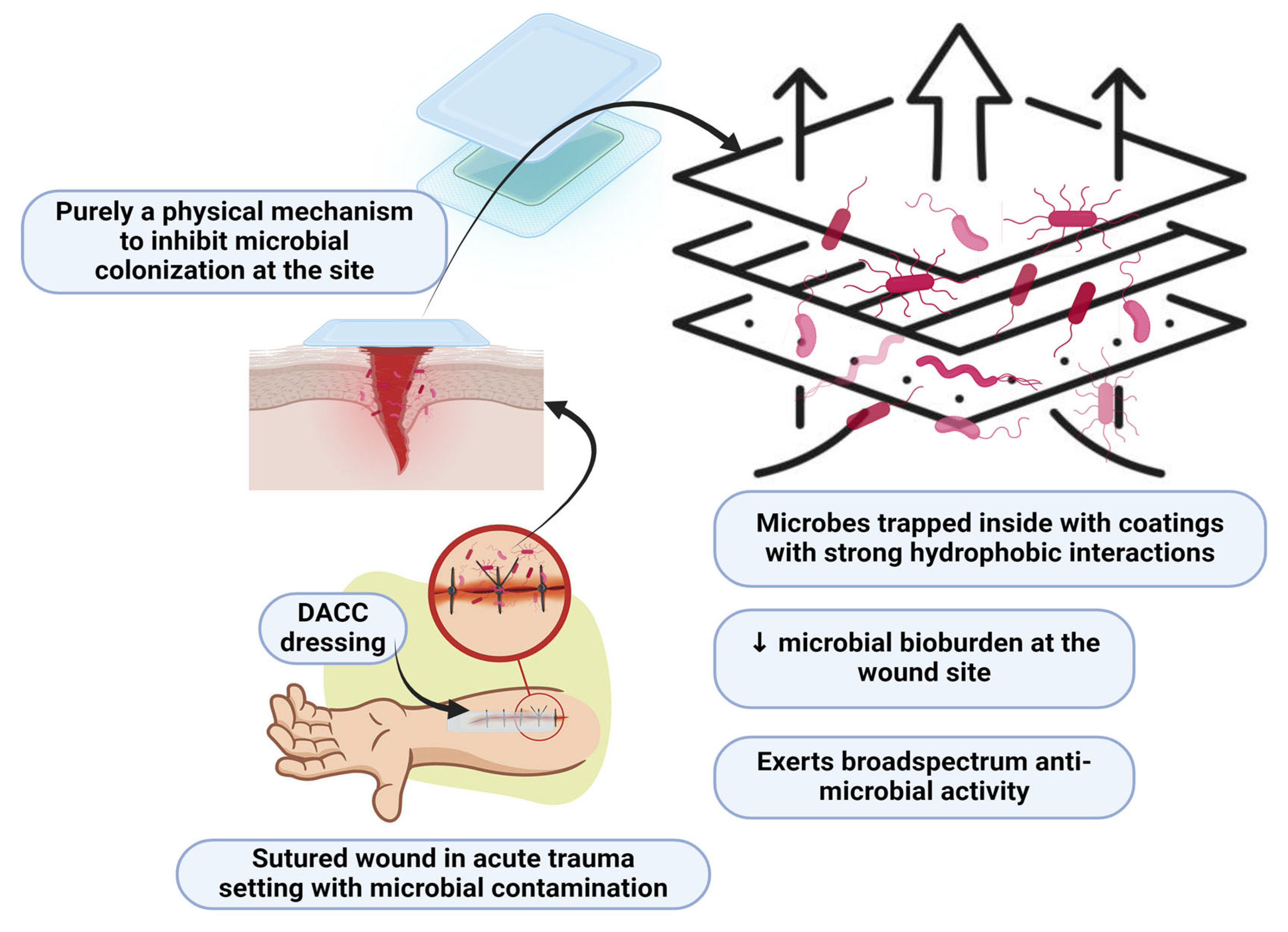
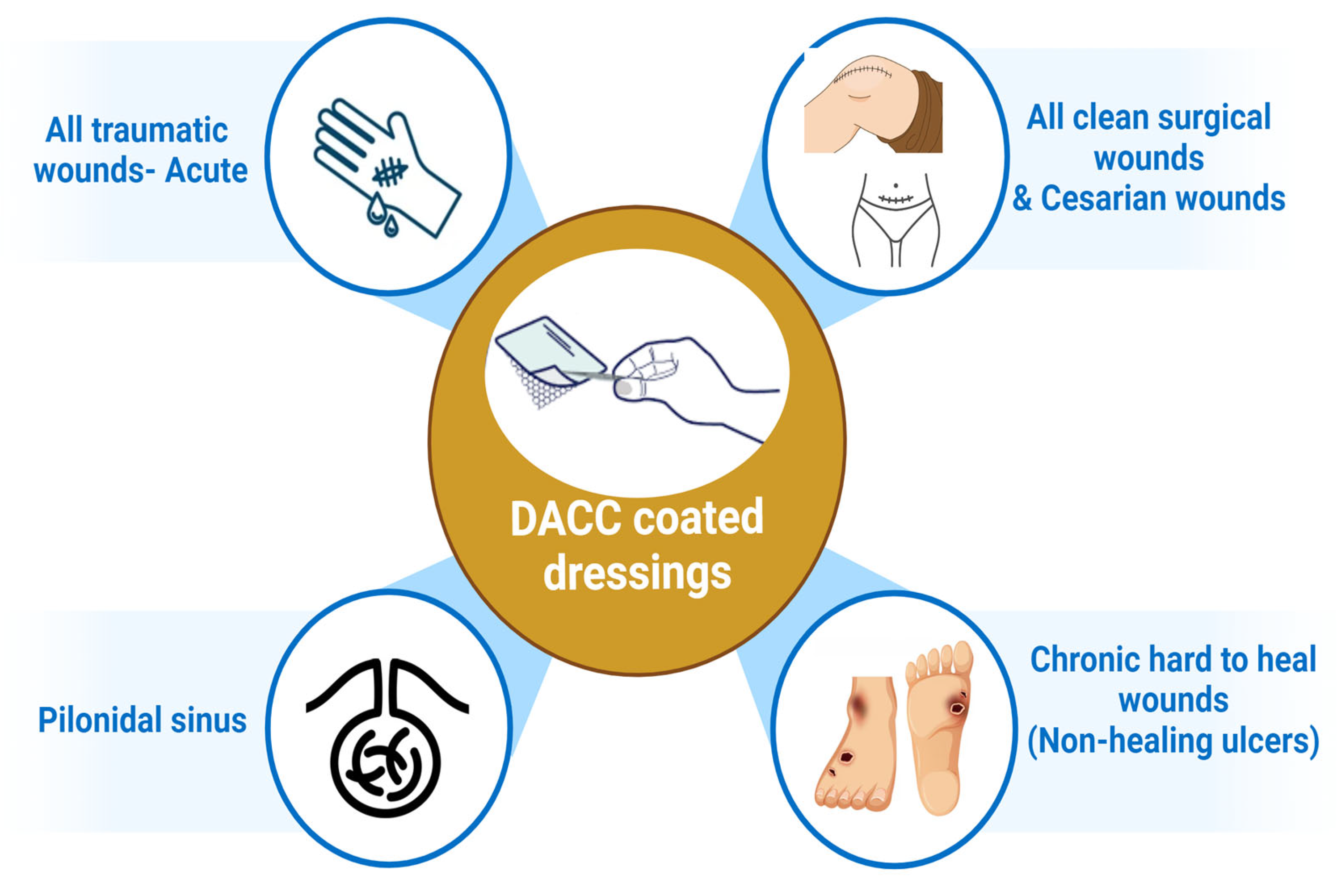
3. DACC-Coated Dressings
3.1. General Efficacy of DACC-Coated Dressings
3.2. Clinical Outcomes in Surgical Site Infections
3.3. Performance in Hard-to-Heal Wounds
3.4. Effectiveness in Pilonidal Sinus Disease
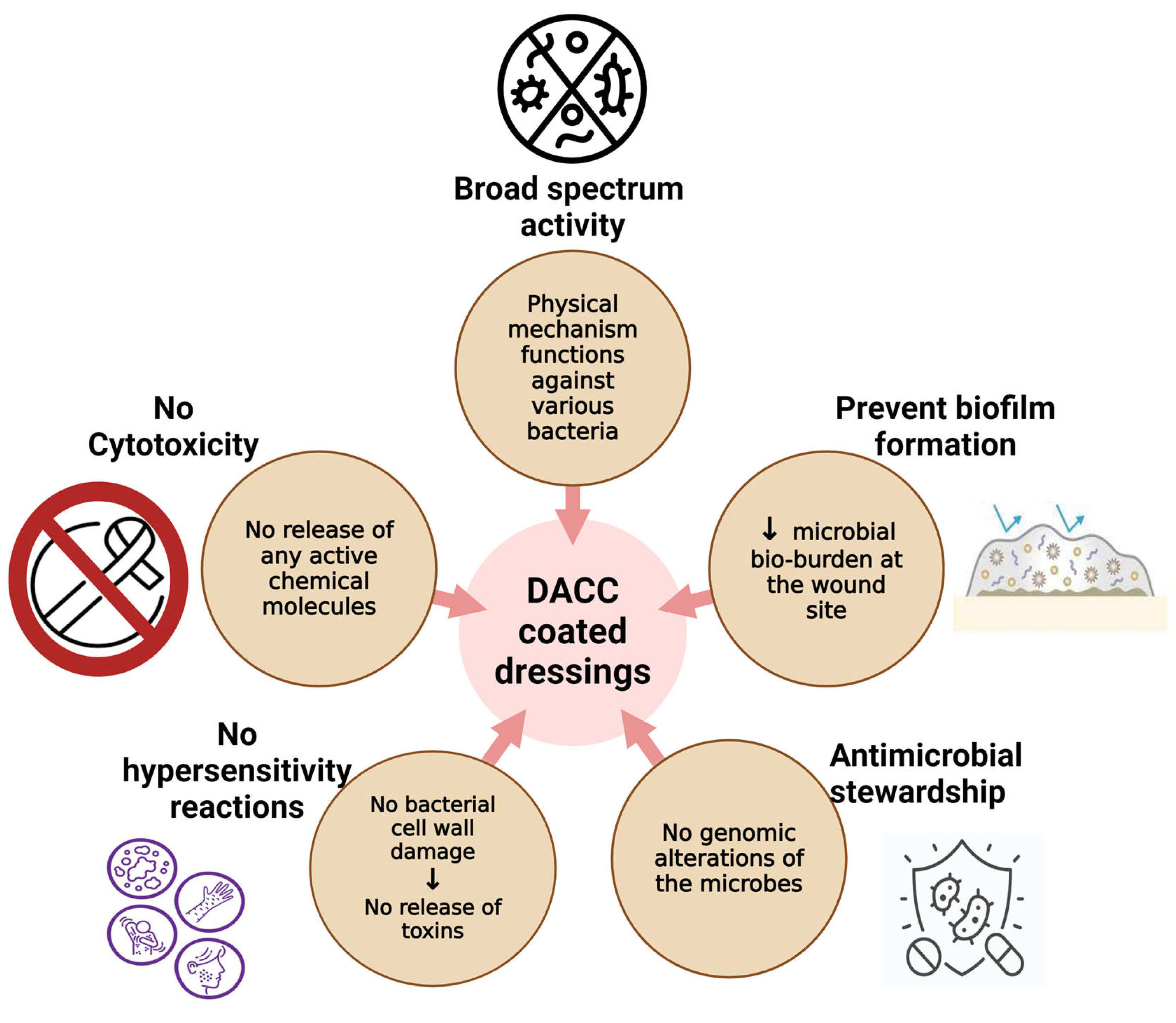
3.5. Antimicrobial Resistance and Stewardship
3.6. Early Post-Operative Infection Prevention
3.7. Cost-Effectiveness
4. Comparison with Antimicrobial Dressings
4.1. Silver-Coated Dressings
4.2. Iodine-Based Dressings
4.3. Honey-Based Dressings
5. Comparison with Non-Antimicrobial Dressings
5.1. Alginate Dressings
5.2. Standard Surgical Dressings
6. Gaps in Literature
6.1. Long-Term Follow-Up and Outcomes
6.2. Specific Surgical Populations
6.3. Cost-Effectiveness Data
6.4. Impact on Antimicrobial Resistance (AMR)
6.5. Limitations of the Evidence
6.5.1. Small Sample Sizes
6.5.2. Methodological Weaknesses
6.5.3. Heterogeneity in Study Designs and Outcomes
6.5.4. Potential Biases
6.6. Limitations of DACC Dressings
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costabella, F.; Patel, K.B.; Adepoju, A.V.; Singh, P.; Attia Hussein Mahmoud, H.; Zafar, A.; Patel, T.; Watekar, N.A.; Mallesh, N.; Fawad, M.; et al. Healthcare Cost and Outcomes Associated with Surgical Site Infection and Patient Outcomes in Low- and Middle-Income Countries. Cureus 2023, 15, e42493. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gálvez, J.; Martínez-Isasi, S.; Gómez-Salgado, J.; Rumbo-Prieto, J.M.; Sobrido-Prieto, M.; Sánchez-Hernández, M.; García-Martínez, M.; Fernández-García, D. Cytotoxicity and Concentration of Silver Ions Released from Dressings in the Treatment of Infected Wounds: A Systematic Review. Front. Public Health 2024, 12, 1331753. [Google Scholar] [CrossRef]
- Vermeulen, H.; Westerbos, S.J.; Ubbink, D.T. Benefit and Harm of Iodine in Wound Care: A Systematic Review. J. Hosp. Infect. 2010, 76, 191–199. [Google Scholar] [CrossRef]
- Pietsch, F.; O’Neill, A.J.; Ivask, A.; Jenssen, H.; Inkinen, J.; Kahru, A.; Ahonen, M.; Schreiber, F. Selection of Resistance by Antimicrobial Coatings in the Healthcare Setting. J. Hosp. Infect. 2020, 106, 115–125. [Google Scholar] [CrossRef]
- Totty, J.P.; Bua, N.; Smith, G.E.; Harwood, A.E.; Carradice, D.; Wallace, T.; Chetter, I.C. Dialkylcarbamoyl Chloride (DACC)-Coated Dressings in the Management and Prevention of Wound Infection: A Systematic Review. J. Wound Care 2017, 26, 107–114. [Google Scholar] [CrossRef]
- Ortega-Peña, S.; Chopin-Doroteo, M.; Tejeda-Fernández de Lara, A.; Giraldo-Gómez, D.M.; Salgado, R.M.; Krötzsch, E. Dialkyl Carbamoyl Chloride-Coated Dressing Prevents Macrophage and Fibroblast Stimulation via Control of Bacterial Growth: An In Vitro Assay. Microorganisms 2022, 10, 1825. [Google Scholar] [CrossRef]
- Malone, M.; Radzieta, M.; Schwarzer, S.; Walker, A.; Bradley, J.; Jensen, S.O. In Vivo Observations of Biofilm Adhering to a Dialkylcarbamoyl Chloride-Coated Mesh Dressing When Applied to Diabetes-Related Foot Ulcers: A Proof of Concept Study. Int. Wound J. 2023, 20, 1943–1953. [Google Scholar] [CrossRef]
- Totty, J.P.; Harwood, A.E.; Cai, P.L.; Hitchman, L.H.; Smith, G.E.; Chetter, I.C. Assessing the Effectiveness of Dialkylcarbamoylchloride (DACC)-Coated Post-Operative Dressings versus Standard Care in the Prevention of Surgical Site Infection in Clean or Clean-Contaminated, Vascular Surgery (the DRESSINg Trial): Study Protocol for a Pilot Feasibility Randomised Controlled Trial. Pilot Feasibility Stud. 2019, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Ljungh, A.; Yanagisawa, N.; Wadström, T. Using the Principle of Hydrophobic Interaction to Bind and Remove Wound Bacteria. J. Wound Care 2006, 15, 175–180. [Google Scholar] [CrossRef]
- Chadwick, P.; Ousey, K. Bacterial-Binding Dressings in the Management of Wound Healing and Infection Prevention: A Narrative Review. J. Wound Care 2019, 28, 370–382. [Google Scholar] [CrossRef]
- Stanirowski, P.J.; Bizoń, M.; Cendrowski, K.; Sawicki, W. Randomized Controlled Trial Evaluating Dialkylcarbamoyl Chloride Impregnated Dressings for the Prevention of Surgical Site Infections in Adult Women Undergoing Cesarean Section. Surg. Infect. 2016, 17, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bua, N.; Smith, G.E.; Totty, J.P.; Pan, D.; Wallace, T.; Carradice, D.; Chetter, I.C. Dialkylcarbamoyl Chloride Dressings in the Prevention of Surgical Site Infections after Nonimplant Vascular Surgery. Ann. Vasc. Surg. 2017, 44, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, S.; Martinez, J.L.; Killeen, A.; Alves, P.; Gledhill, A.; Nygren, E.; Lavery, L.A.; Malone, M. Does the Use of DACC-coated Dressings Improve Clinical Outcomes for Hard to Heal Wounds: A Systematic Review. Int. Wound J. 2024, 21, e70053. [Google Scholar] [CrossRef]
- Skinner, R.; Hampton, S. The Diabetic Foot: Managing Infection Using Cutimed Sorbact Dressings. Br. J. Nurs. Mark Allen Publ. 2010, 19, S30–S36. [Google Scholar]
- Haycocks, S.; Chadwick, P. Use of DACC Coated Dressings in Diabetic Foot Ulcers: A Case Series: Original Research. Wound Heal. South. Afr. 2013, 6, 37–40. [Google Scholar]
- Mosti, G.; Magliaro, A.; Mattaliano, V.; Picerni, P.; Angelotti, N. Comparative Study of Two Antimicrobial Dressings in Infected Leg Ulcers: A Pilot Study. J. Wound Care 2015, 24, 121–127. [Google Scholar] [CrossRef]
- Geroult, S.; Phillips, R.O.; Demangel, C. Adhesion of the Ulcerative Pathogen Mycobacterium Ulcerans to DACC-Coated Dressings. J. Wound Care 2014, 23, 417–424. [Google Scholar] [CrossRef]
- Meberg, A.; Schøyen, R. Hydrophobic Material in Routine Umbilical Cord Care and Prevention of Infections in Newborn Infants. Scand. J. Infect. Dis. 1990, 22, 729–733. [Google Scholar] [CrossRef]
- Hampton, S. An Evaluation of the Efficacy of Cutimed® Sorbact® in Different Types of Non-Healing Wounds. Wounds UK 2007, 3, 113–119. [Google Scholar]
- Kammerlander, G.; Locher, E.; Suess-Burghart, A.; Hallern, B.; Wipplinger, P. An Investigation of Cutimed® Sorbact® as an Antimicrobial Alternative in Wound Management. Wounds UK 2008, 4, 10–18. [Google Scholar]
- Pirie, G.; Duguid, K.; Timmons, J. Cutimed® Sorbact® Gel: A New Infection Management Dressing. Wounds UK 2009, 5, 74–78. [Google Scholar]
- Powell, G. Evaluating Cutimed Sorbact: Using a Case Study Approach. Br. J. Nurs. Mark Allen Publ. 2009, 18, S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, A. Innovative Solutions to Daily Challenges. Br. J. Community Nurs. 2010, 15 (Suppl. S38), S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Haycocks, S.; Chadwick, P.; Guttormsen, K. Use of a DACC-Coated Antimicrobial Dressing in People with Diabetes and a History of Foot Ulceration. Wounds UK 2011, 7, 108–114. [Google Scholar]
- Sibbald, G.; Woo, K.; Coutts, P. The Effectiveness of a New Antimicrobial Dressing with Microbinding Action for the Management of Chronic Wounds. Wound Care Can. 2012, 10, 20–22. [Google Scholar]
- Bruce, Z. Using Cutimed® Sorbact® Hydroactive on Chronic Infected Wounds. Wounds UK 2012, 8, 119–129. [Google Scholar]
- Bullough, L.; Little, G.; Hodson, J.; Morris, A. The Use of DACC-Coated Dressings for the Treatment of Infected, Complex Abdominal Wounds. Wounds UK 2012, 8, 102–109. [Google Scholar]
- Gentili, V.; Gianesini, S.; Balboni, P.G.; Menegatti, E.; Rotola, A.; Zuolo, M.; Caselli, E.; Zamboni, P.; Di Luca, D. Panbacterial Real-Time PCR to Evaluate Bacterial Burden in Chronic Wounds Treated with CutimedTM SorbactTM. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1523–1529. [Google Scholar] [CrossRef]
- Kleintjes, W.G.; Schoeman, D.; Collier, L. A Pilot Study of Cutimed® Sorbact® versus ACTICOAT versus Silverlon® for the Treatment of Burn Wounds in a South African Adult Burn Unit: General Review. Wound Health South. Afr. 2015, 8, 22–29. [Google Scholar]
- Choi, J.-S.; Lee, J.-H.; Kim, S.-M.; Kim, Y.-J.; Choi, J.-Y.; Jun, Y.-J. Hydrogel-Impregnated Dressings for Graft Fixation: A Case Series. J. Wound Care 2015, 24, 326–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanirowski, P.J.; Davies, H.; McMaster, J.; Mealing, S.; Sawicki, W.; Cendrowski, K.; Posnett, J. Cost-Effectiveness of a Bacterial-Binding Dressing to Prevent Surgical Site Infection Following Caesarean Section. J. Wound Care 2019, 28, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mulpur, P.; Jayakumar, T.; Sancheti, P.K.; Shankar, N.; Hippalgaonkar, K.; Reddy, A.V.G.; Mulpur, P.; Jayakumar, T.; Sancheti, P.K.; Shankar, N.; et al. Dialkyl Carbamoyl Chloride (DACC)-Impregnated Dressings for the Prevention of Surgical Site Infections: Experience from a Multi-Disciplinary Study in India. Cureus 2024, 16, e72654. [Google Scholar] [CrossRef]
- Romain, B.; Mielcarek, M.; Delhorme, J.B.; Meyer, N.; Brigand, C.; Rohr, S. SORKYSA group Dialkylcarbamoyl Chloride-Coated versus Alginate Dressings after Pilonidal Sinus Excision: A Randomized Clinical Trial (SORKYSA Study). BJS Open 2020, 4, 225–231. [Google Scholar] [CrossRef]
- Rippon, M.G.; Rogers, A.A.; Ousey, K. Antimicrobial Stewardship Strategies in Wound Care: Evidence to Support the Use of Dialkylcarbamoyl Chloride (DACC)- Coated Wound Dressings. J. Wound Care 2021, 30, 284–296. [Google Scholar] [CrossRef]
- Kostenko, V.; Lyczak, J.; Turner, K.; Martinuzzi, R.J. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. Antimicrob. Agents Chemother. 2010, 54, 5120–5131. [Google Scholar] [CrossRef]
- Yonathan, K.; Mann, R.; Mahbub, K.R.; Gunawan, C. The Impact of Silver Nanoparticles on Microbial Communities and Antibiotic Resistance Determinants in the Environment. Environ. Pollut. 2022, 293, 118506. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Mohamed, D.S.; Abd El-Baky, R.M.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial Activity of Silver-Treated Bacteria against Other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef] [PubMed]
- Hochvaldová, L.; Panáček, D.; Válková, L.; Večeřová, R.; Kolář, M.; Prucek, R.; Kvítek, L.; Panáček, A. E. coli and S. aureus Resist Silver Nanoparticles via an Identical Mechanism, but through Different Pathways. Commun. Biol. 2024, 7, 1552. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, G.; Monstrey, S.; Van Landuyt, K.; Hamdi, M.; Blondeel, P. The Role of Iodine in Antisepsis and Wound Management: A Reappraisal. Acta Chir. Belg. 2003, 103, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-R.; Jeong, J.; Brockow, K. Diagnosis and Prevention of Hypersensitivity Reactions to Iodinated Contrast Media. Allergy Asthma Immunol. Res. 2022, 14, 348–360. [Google Scholar] [CrossRef]
- Murthy, M.B.; Krishnamurthy, B. Severe Irritant Contact Dermatitis Induced by Povidone Iodine Solution. Indian J. Pharmacol. 2009, 41, 199–200. [Google Scholar] [CrossRef]
- Khan, M.A.O.; Ramadugu, R.; Suvvari, T.K.; Thomas, V. Irritant Contact Dermatitis Due to Povidone-Iodine Following a Surgical Intervention: An Unusual Case Report. SAGE Open Med. Case Rep. 2023, 11, 2050313X231185620. [Google Scholar] [CrossRef]
- Kaur, M.; Karadia, P.; Singh, S. Povidone-Iodine-Induced Disseminated Irritant Contact Dermatitis. BMJ Case Rep. 2022, 15, e251926. [Google Scholar] [CrossRef]
- Rao, S.; Bartkus, T.; Gandhi, K.; Gibson, A.; Ventolini, G. A Post-Operative Reaction to Povidone-iodine in a Postpartum Woman: A Case Report. Case Rep. Womens Health 2022, 34, e00394. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Atepileva, A.; Ogay, V.; Kudaibergen, G.; Kaukabaeva, G.; Nurkina, A.; Mukhambetova, A.; Balgazarov, S.; Batpen, A.; Saginova, D.; Ramazanov, Z.; et al. Exploring the Antibacterial and Regenerative Properties of a Two-Stage Alginate Wound Dressing in a Rat Model of Purulent Wounds. Biomedicines 2024, 12, 2122. [Google Scholar] [CrossRef]
- Rippon, M.; Rogers, A.A.; Ousey, K.; Chadwick, P. Experimental and Clinical Evidence for DACC-Coated Dressings: An Update. J. Wound Care 2023, 32, S13–S22. [Google Scholar] [CrossRef] [PubMed]
- Husmark, J.; Morgner, B.; Susilo, Y.B.; Wiegand, C. Antimicrobial Effects of Bacterial Binding to a Dialkylcarbamoyl Chloride-Coated Wound Dressing: An in Vitro Study. J. Wound Care 2022, 31, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Rao, F.; Xiao, J.; Yang, J.; Wang, W.; Li, Z.; Huang, R.; Liu, Z.; Guo, T. Evaluation of Different Surgical Dressings in Reducing Postoperative Surgical Site Infection of a Closed Wound: A Network Meta-Analysis. Int. J. Surg. 2020, 82, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic Wounds: Treatment Consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Cutting, K.; White, R.; Edmonds, M. The Safety and Efficacy of Dressings with Silver - Addressing Clinical Concerns. Int. Wound J. 2007, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sütterlin, S.; Tano, E.; Bergsten, A.; Tallberg, A.-B.; Melhus, A. Effects of Silver-Based Wound Dressings on the Bacterial Flora in Chronic Leg Ulcers and Its Susceptibility in Vitro to Silver. Acta Derm. Venereol. 2012, 92, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of Wound Dressings Containing Silver on Skin and Immune Cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef]
- Scanlon, E.; Karlsmark, T.; Leaper, D.J.; Carter, K.; Poulsen, P.B.; Hart-Hansen, K.; Hahn, T.W. Cost-Effective Faster Wound Healing with a Sustained Silver-Releasing Foam Dressing in Delayed Healing Leg Ulcers—A Health-Economic Analysis. Int. Wound J. 2005, 2, 150–160. [Google Scholar] [CrossRef]
- Morilla-Herrera, J.C.; Morales-Asencio, J.M.; Gómez-González, A.J.; Díez-De Los Ríos, A.; Lupiáñez-Pérez, I.; Acosta-Andrade, C.; Aranda-Gallardo, M.; Moya-Suárez, A.B.; Kaknani-Uttumchandani, S.; García-Mayor, S. Effectiveness of a Hydrophobic Dressing for Microorganisms’ Colonization of Vascular Ulcers: Protocol for a Randomized Controlled Trial (CUCO-UV Study). J. Adv. Nurs. 2020, 76, 2191–2197. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone Iodine in Wound Healing: A Review of Current Concepts and Practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Barrois, B.; Lambert, J.; Malhotra-Kumar, S.; Santos-Fernandes, V.; Monstrey, S. Addressing the Challenges in Antisepsis: Focus on Povidone Iodine. Int. J. Antimicrob. Agents 2020, 56, 106064. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Bhatia, M.; Gupta, P.; Omar, B.J. Emerging Biocide Resistance among Multidrug-Resistant Bacteria: Myth or Reality? A Pilot Study. J. Pharm. Bioallied Sci. 2018, 10, 96. [Google Scholar] [CrossRef]
- Kramer, S.A. Effect of Povidone-Iodine on Wound Healing: A Review. J. Vasc. Nurs. 1999, 17, 17–23. [Google Scholar] [CrossRef]
- Butcher, M. Introducing a New Paradigm for Bioburden Management. J. Wound Care 2011, 20, 4–19. [Google Scholar] [CrossRef]
- Cutting, K.; McGuire, J. Safe, Long-Term Management of Bioburden That Helps Promote Healing Evidence Review of DACC Technology. J. Wound Care 2015, 24, S3–S5. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds Compend. Clin. Res. Pract. 2015, 27, 141–151. [Google Scholar]
- Basualdo, C.; Sgroy, V.; Finola, M.S.; Marioli, J.M. Comparison of the Antibacterial Activity of Honey from Different Provenance against Bacteria Usually Isolated from Skin Wounds. Vet. Microbiol. 2007, 124, 375–381. [Google Scholar] [CrossRef]
- Cooper, R. Honey in Wound Care: Antibacterial Properties. GMS Krankenhaushygiene Interdiszip. 2007, 2, Doc51. [Google Scholar]
- Dwiyana, R.F.; Gondokaryono, S.P.; Rahardja, J.I.; Arline Diana, I.; Yogya, Y.; Gunawan, H. Clinical Efficacy of Dialkylcarbamoylchloride-Coated Cotton Acetate Dressing versus Combination of Normal Saline Dressing and 2% Mupirocin Ointment in Infected Wounds of Epidermolysis bullosa. Dermatol. Ther. 2019, 32, e13047. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Gray, T.A.; Walter, C.J.; Sharp, C.A.; Page, T.; Macefield, R.; Blencowe, N.; Milne, T.K.; Reeves, B.C.; Blazeby, J. Dressings for the Prevention of Surgical Site Infection. Cochrane Database Syst. Rev. 2016, 12, CD003091. [Google Scholar] [CrossRef] [PubMed]
- Tantillo, T.J.; Klein, B.; Wilson, M.; Grewal, K.S.; Bitterman, A.D.; Sgaglione, N.A. Orthopaedic Surgical Dressings. Orthoplastic Surg. 2021, 5, 9–17. [Google Scholar] [CrossRef]
- Totty, J.P.; Moss, J.W.E.; Barker, E.; Mealing, S.J.; Posnett, J.W.; Chetter, I.C.; Smith, G.E. The Impact of Surgical Site Infection on Hospitalisation, Treatment Costs, and Health-Related Quality of Life after Vascular Surgery. Int. Wound J. 2021, 18, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, I.; Khan, T.A.; Krukiewicz, K. Etiology, Pathology, and Host-Impaired Immunity in Medical Implant-Associated Infections. J. Infect. Public Health 2024, 17, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections-Can Immune Modulation Be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtlif, M.; Kathju, S.; Stoodley, P. Biofilms in Periprosthetic Orthopedic Infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef]
- de Freitas, P.S.S.; Rezende, L.D.A.; de Silva, K.E.J.; Fiorin, B.H.; dos Santos, R.A.; Ramalho, A.O. Utilização do Cloreto de Dialquil carbamoil na prevenção e tratamento de biofilme em feridas. Estima Braz. J. Enteros. Ther. 2021, 19. [Google Scholar] [CrossRef]
- Gueltzow, M.; Khalilpour, P.; Kolbe, K.; Zoellner, Y. Budget Impact of Antimicrobial Wound Dressings in the Treatment of Venous Leg Ulcers in the German Outpatient Care Sector: A Budget Impact Analysis. J. Mark. Access Health Policy 2018, 6, 1527654. [Google Scholar] [CrossRef]
- Amoako, Y.A.; Agbanyo, A.; Novignon, J.; Owusu, L.; Tuffour, J.; Asante-Poku, A.; Hailemichael, Y.; Mosweu, I.; Canter, R.; Opondo, C.; et al. Buruli-RifDACC: Evaluation of the Efficacy and Cost-Effectiveness of High-Dose versus Standard-Dose Rifampicin on Outcomes in Mycobacteriumulcerans Disease, a Protocol for a Randomised Controlled Trial in Ghana. NIHR Open Res. 2022, 2, 59. [Google Scholar] [CrossRef]
| Studies | Methods | Participants | Interventions | Outcomes | Primary Findings |
|---|---|---|---|---|---|
| Meberg et al. [19] (1990) | Randomized control trial | 2441 newborn infants | Alternately allocated to umbilical cord stump dressing with DACC-coated dressing or daily cleansing with chlorhexidine | Newborn infection (conjunctivitis, pyoderma, paronychia, omphalitis) | No significant difference in overall infection rates or omphalitis |
| Hampton et al. [20] (2007) | Case series | 21 patients with non-healing wounds over 3 months old | Treated with Cutimed® Sorbact® as part of the treatment plan | Inflammation, exudate, malodor, wound size, pain | 60% of wounds healed; 100% had reduced exudate levels; 58% had reduced odor |
| Kammerlander et al. [21] (2008) | Non-randomized multi-center evaluation | 116 patients (62 male) treated in four European hospitals | Patients treated with Cutimed® Sorbact® as part of their therapeutic regime | Assessment of inflammation reduction; infection control | 81% of wounds successfully treated for infection; 21% of wounds did not respond to treatment |
| Pirie et al. [22] (2009) | Case series | 3 patients with chronic non-healing wounds | DACC-coated dressing used as the primary wound contact layer along with other therapies | Wound healing, infection evidence, wound size, exudate levels | All patients showed clinical improvement (reduced wound size and slough) |
| Powell et al. [23] (2009) | Case series | 6 patients with various clinically infected or delayed-healing wounds | Cutimed® Sorbact® used as wound contact layer for 2–8 weeks | Inflammation, exudate, odor, wound healing | All wounds reduced in size, exudate, and odor; 80% completely healed |
| Skinner et al. [15] (2010) | Case series | 4 patients with diabetic foot ulcers | Treated with Cutimed® Sorbact® as part of their treatment plan | Bacterial colonization, infection, wound healing | One wound completely healed; 3 progressed towards healing |
| Derbyshire et al. [24] (2010) | Case series | 3 patients with chronic wounds over 4 years old | Treated with Cutimed® Sorbact® as part of their treatment plan | Wound size, healing, resource use, pain, exudate levels | Wounds became cleaner, dryer, and required fewer dressing changes |
| Haycocks et al. [25] (2011) | Case series | 19 patients with diabetic foot ulcers, up to age 80 | Treated with DACC-coated dressing as a wound contact layer for 4 weeks | Infection, healing, patient and clinician assessment | All wounds showed reduced infection signs; 69% reduced in size; 27.6% healed completely |
| Sibbald et al. [26] (2012) | Case series | 14 patients with lower limb ulcers (diabetic foot or venous leg ulcers) | Ulcers dressed 3 times a week for 4 weeks with DACC-coated dressing | Superficial infection, total ulcer surface area, pain | Total surface area reduced from 1.74 cm2 to 1.15 cm2 (p = 0.337); no significant difference in infection rates |
| Bruce et al. [27] (2012) | Multi-center evaluation | 13 patients with chronic infected wounds | Treated with DACC-coated dressings for 28 days or until infection signs resolved | Erythema, pain, heat, edema, odor, exudate | 86% infection reduction; 79% wounds reduced in size |
| Bullough et al. [28] (2012) | Case series | 4 patients with complex open abdominal wounds | DACC-coated dressings and swabs used as a wound contact layer throughout treatment | Wound infection recurrence; wound dimensions; wound healing; pain during dressing changes; exudate and odor | 3 out of 4 wounds healed, and signs of infection resolved by day 14 |
| Gentili et al. [29] (2012) | Non-comparative, double-blind, pilot study | 19 patients with chronic lower limb ulcers | Wounds treated with saline rinse, surgical debridement, and DACC dressing for 4 weeks | Wound condition, quality of life, bacterial load | 66% of wounds reduced in size; bacterial load decreased in all cases. |
| Kleintjes et al. [30] (2015) | Prospective pilot study | 13 patients over 16 with burn wounds | Burns dressed with DACC-coated dressings, Cuticcot®, and Silverlon® | Wound swab MC&S, visual inspection of wounds | DACC-coated areas appeared cleaner and had less bacterial growth |
| Choi et al. [31] (2015) | Case series | 7 patients (4 male) requiring skin grafts on clean surgical wounds | Skin grafts dressed with DACC-coated dressing and tie-over dressing for 5 days | Wounds checked for infection at 5, 14, and 30 days post-procedure | No infections were noted in the wounds |
| Mosti et al. [17] (2015) | Randomized, comparative, single-center study | 40 patients over 18 with infected vascular ulcers over 6 months old | Randomized to silver hydrofiber dressing or DACC-coated dressing | Ulcer bacterial load | 73.1% bacterial load reduction in DACC group vs. 41.6% in silver group (p < 0000.1) |
| Stanirowski et al. [12] (2016) | Single blinded, randomized control trial | 543 women over 18 undergoing planned or emergency C-section | Randomized to either DACC-coated post-op dressing or standard surgical dressing | Superficial or deep SSI within 14 days after C-section (as per CDC) | SSI rates were 1.8% in DACC group vs. 5.2% in control group (p = 0.04) |
| Stanirowski et al. [32] (2019) | Single blinded, randomized, controlled pilot study | 142 women over 18 years undergoing planned or emergency C-section | Randomized to either DACC-coated post-op dressing or standard surgical dressing | Superficial or deep SSI within 14 days after C-section (as per CDC) | SSI rates were 2.8% in DACC group vs. 9.8% in control group (p = 0.08) |
| Mulpur et al. [33] (2024) | Prospective, multicentric observational study | 106 patients (71 orthopaedic cases and 35 gastrointestinal casses) | DACC dressing applied immediately post-surgery and assessed over 30 days for the incidence of superficial or deep SSI | 1.9% cases of SSI were reported in orthopaedic patients | 73.5% patients reported an improved pain experiences during dressing changes compared to previous dressings. |
| Feature | Silver-Coated Dressings | Iodine Dressings | Honey-Based Dressings |
|---|---|---|---|
| Mechanism | Releases silver ions with antimicrobial properties | Releases iodine with antiseptic properties | Contains natural antibacterial properties |
| Antimicrobial action | Broad-spectrum antibacterial, anti-inflammatory, and anti-oxidative | Broad-spectrum antibacterial, wound debridement, and odour control | Broad-spectrum antibacterial, promotes healing, and reduces inflammation |
| Differences | Uses silver nanoparticles for enhanced efficacy | Uses cadexomer iodine for controlled release | Uses natural honey for its healing properties |
| Similarities | Both are used for chronic wound management and infection control | Both are used for chronic wound management and infection control | Both are used for chronic wound management and infection control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Nallakumarasamy, A.; Murugan, S.; Jayakumar, T.; Muthu, S. Efficacy of Dialkylcarbamoylchloride (DACC)-Impregnated Dressings in Surgical Wound Management: A Review. Eur. Burn J. 2025, 6, 1. https://doi.org/10.3390/ebj6010001
Jeyaraman M, Jeyaraman N, Ramasubramanian S, Nallakumarasamy A, Murugan S, Jayakumar T, Muthu S. Efficacy of Dialkylcarbamoylchloride (DACC)-Impregnated Dressings in Surgical Wound Management: A Review. European Burn Journal. 2025; 6(1):1. https://doi.org/10.3390/ebj6010001
Chicago/Turabian StyleJeyaraman, Madhan, Naveen Jeyaraman, Swaminathan Ramasubramanian, Arulkumar Nallakumarasamy, Shrideavi Murugan, Tarun Jayakumar, and Sathish Muthu. 2025. "Efficacy of Dialkylcarbamoylchloride (DACC)-Impregnated Dressings in Surgical Wound Management: A Review" European Burn Journal 6, no. 1: 1. https://doi.org/10.3390/ebj6010001
APA StyleJeyaraman, M., Jeyaraman, N., Ramasubramanian, S., Nallakumarasamy, A., Murugan, S., Jayakumar, T., & Muthu, S. (2025). Efficacy of Dialkylcarbamoylchloride (DACC)-Impregnated Dressings in Surgical Wound Management: A Review. European Burn Journal, 6(1), 1. https://doi.org/10.3390/ebj6010001








