The Successful Treatment of Multi-Resistant Colonized Burns with Large-Area Atmospheric Cold Plasma Therapy and Dermis Substitute Matrix—A Case Report
Abstract
1. Introduction
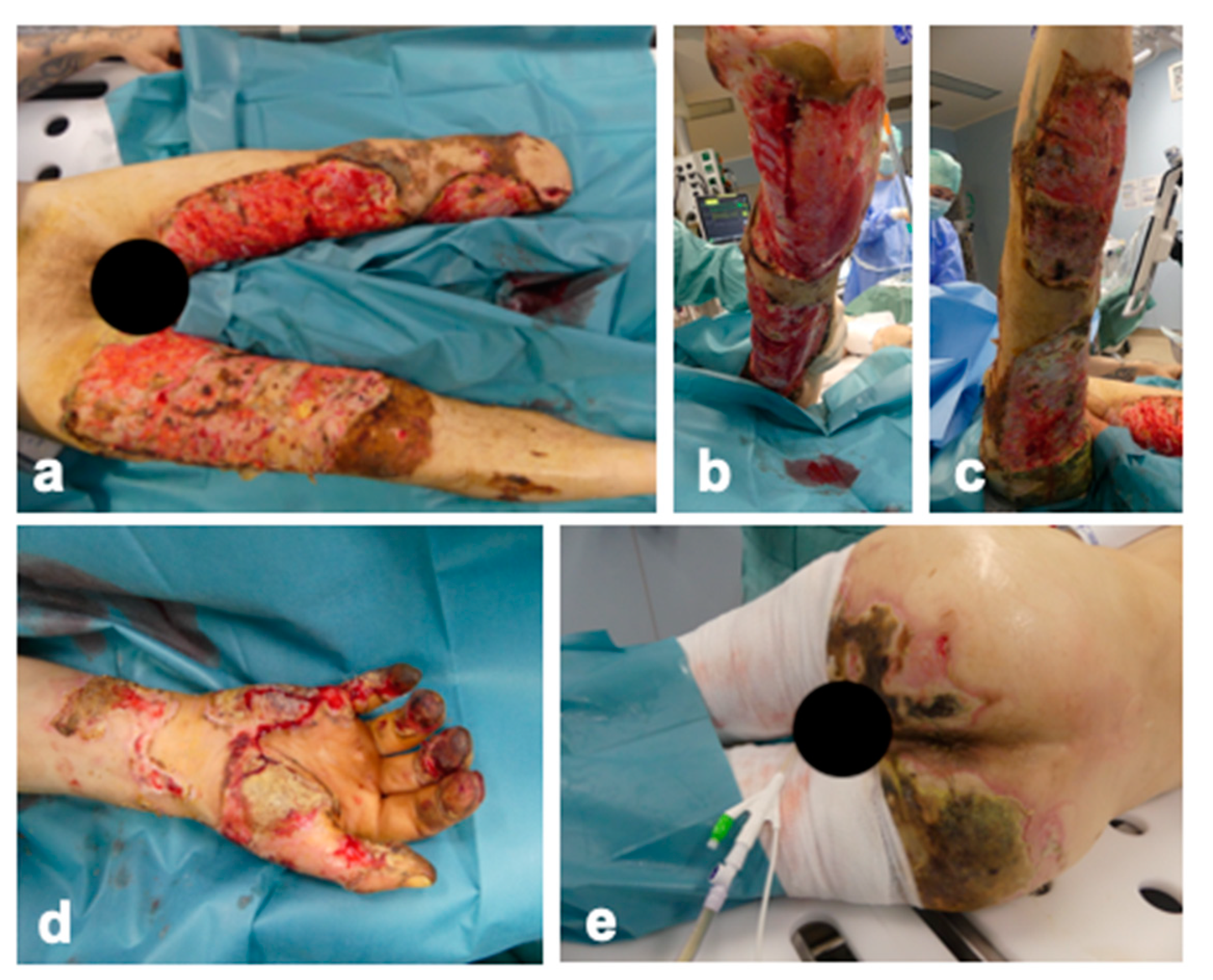
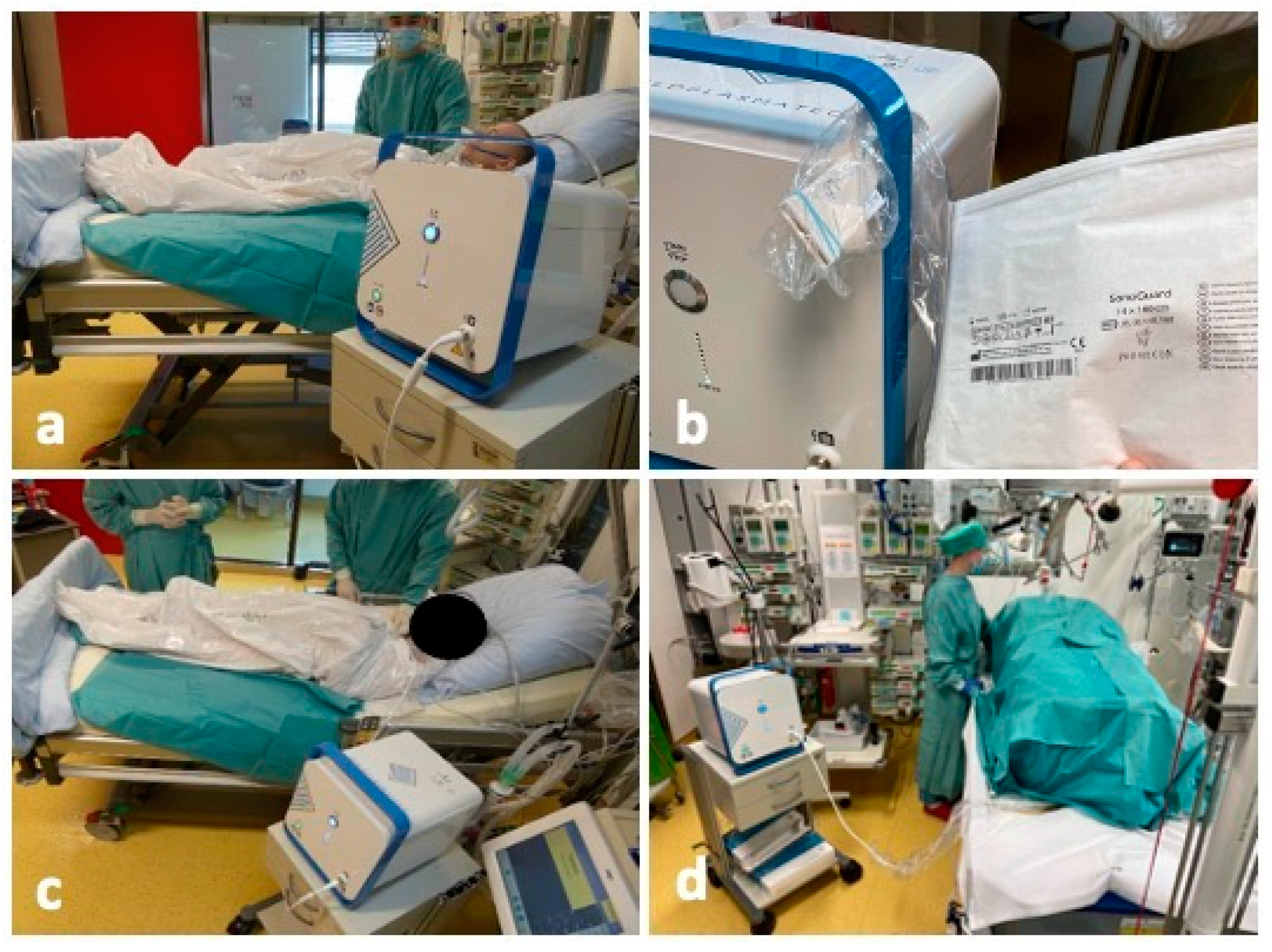
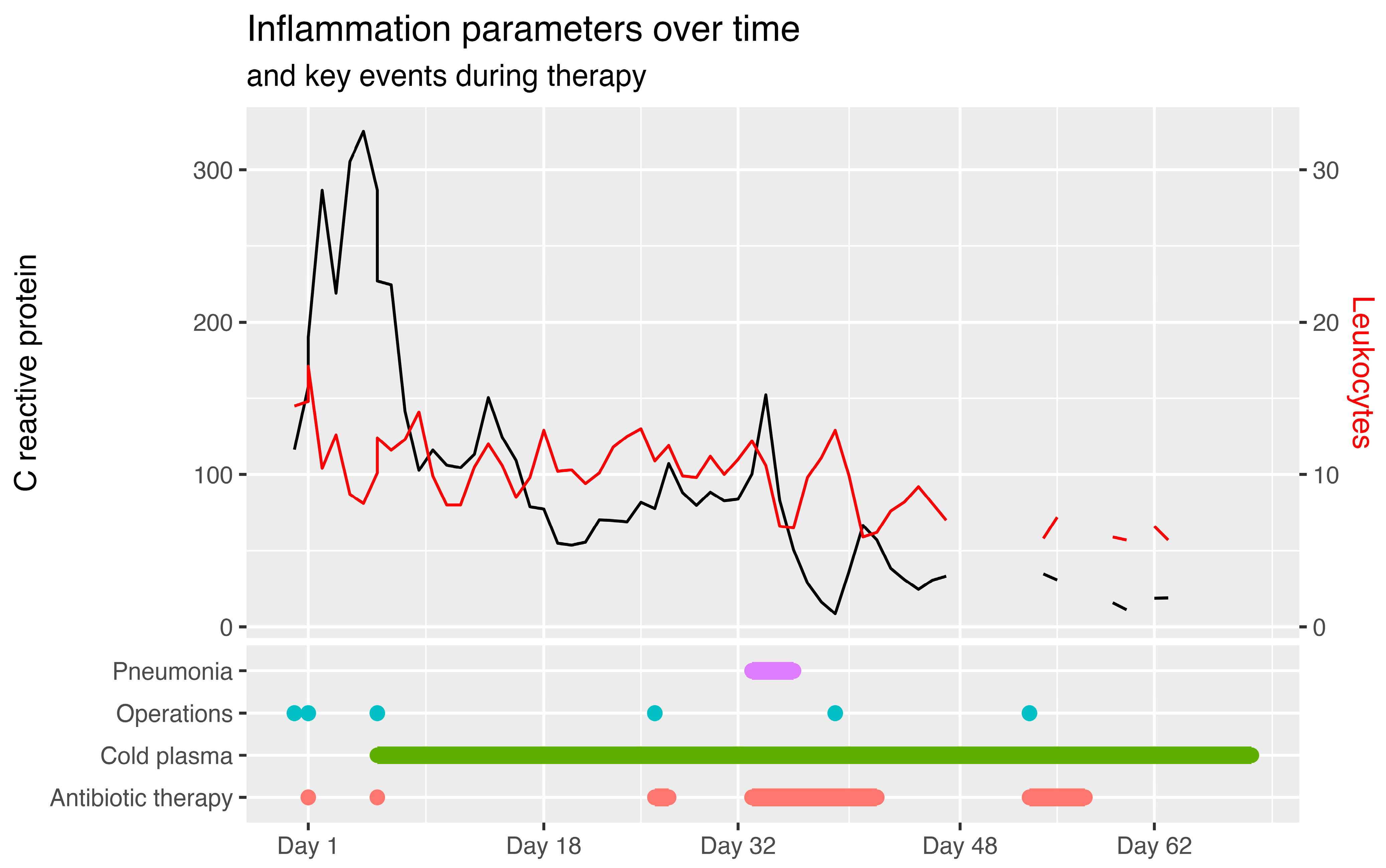
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Langmuir, I. Oscillations in Ionized Gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Imam, A.M.; Rizvi, S.Z.H.; Ali, J. Plasma Kinetic Theory. In Kinetic Theory; InTech: London, UK, 2018. [Google Scholar]
- Garner, A.L.; Loveless, A.M.; Dahal, J.N.; Venkattraman, A. A Tutorial on Theoretical and Computational Techniques for Gas Breakdown in Microscale Gaps. IEEE Trans. Plasma Sci. 2020, 48, 808–824. [Google Scholar] [CrossRef]
- Isbary, G.; Shimizu, T.; Li, Y.-F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold Atmospheric Plasma Devices for Medical Issues. Expert. Rev. Med. Devices 2013, 10, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of Production and Application in Dentistry and Oncology. Med. Gas. Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.L.; Mehlhorn, T.A. A Review of Cold Atmospheric Pressure Plasmas for Trauma and Acute Care. Front. Phys. 2021, 9, 786381. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and Safety of Atmospheric Low-temperature Plasma on Bacterial Reduction in Chronic Wounds and Wound Size Reduction: A Systematic Review and Meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Pai, K.; Timmons, C.; Roehm, K.D.; Ngo, A.; Narayanan, S.S.; Ramachandran, A.; Jacob, J.D.; Ma, L.M.; Madihally, S.V. Investigation of the Roles of Plasma Species Generated by Surface Dielectric Barrier Discharge. Sci. Rep. 2018, 8, 16674. [Google Scholar] [CrossRef]
- Dabek, R.J.; Decik, M.; Driscoll, D.N.; Fuzaylov, G. Global Burn Prevention: Ukraine. J. Burn. Care Res. 2023, 44, 1323–1326. [Google Scholar] [CrossRef]
- Friemert, B.; Pennig, D. Organisatorische Weiterverteilung Ukrainischer Kriegsverletzter Zur Versorgung in Deutschland. Die Unfallchirurgie 2023, 126, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.-T.; Hannappel, L.; Friemert, B.; Lorenz, D.; Brenner, S.; Gottschalk, A. Kriegen Gegen Die Ukraine: Nutzung Des Kleeblattmechanismus Für Verlegungen Aus Der Ukraine. Dtsch. Ärztebl. Int. 2022, 119, 1122–1126. [Google Scholar]
- Vogt, P.M.; Mailänder, P.; Jostkleigrewe, F.; Reichert, B.; Adams, H.A. Centers for Severely Burned Patients in Germany--Management Structure and Needs. Chirurg 2007, Suppl, 411–413. [Google Scholar] [PubMed]
- Stein, C.; Zechel, M.; Spott, R.; Pletz, M.W.; Kipp, F. Multidrug-Resistant Isolates from Ukrainian Patients in a German Health Facility: A Genomic Surveillance Study Focusing on Antimicrobial Resistance and Bacterial Relatedness. Infection 2023, 51, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A Short History of Skin Grafting in Burns: From the Gold Standard of Autologous Skin Grafting to the Possibilities of Allogeneic Skin Grafting with Immunomodulatory Approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef]
- Schlottmann, F.; Obed, D.; Bingöl, A.S.; März, V.; Vogt, P.M.; Krezdorn, N. Treatment of Complex Wounds with NovoSorb® Biodegradable Temporising Matrix (BTM)—A Retrospective Analysis of Clinical Outcomes. J. Pers. Med. 2022, 12, 2002. [Google Scholar] [CrossRef]
- Gładysz, M.; März, V.; Ruemke, S.; Rubalskii, E.; Vogt, P.M.; Krezdorn, N. Limb Salvage through Intermediary Wound Coverage with Acellular Dermal Matrix Template after Persistent Pseudomonas Aeruginosa Infection in a Burn Patient. Eur. Burn. J. 2022, 3, 27–33. [Google Scholar] [CrossRef]
- Tapking, C.; Thomas, B.F.; Hundeshagen, G.; Haug, V.F.M.; Gazyakan, E.; Bliesener, B.; Bigdeli, A.K.; Kneser, U.; Vollbach, F.H. NovoSorb® Biodegradable Temporising Matrix (BTM): What We Learned from the First 300 Consecutive Cases. J. Plast. Reconstr. Aesthetic Surg. 2024, 92, 190–197. [Google Scholar] [CrossRef]
- Greenwood, J.E.; Dearman, B.L. Split Skin Graft Application Over an Integrating, Biodegradable Temporizing Polymer Matrix. J. Burn. Care Res. 2012, 33, 7–19. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage Therapy: From Biological Mechanisms to Future Directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.; Stratmann, B.; Timm, J.; Costea, T.; Tschoepe, D. Enhanced Growth Factor Expression in Chronic Diabetic Wounds Treated by Cold Atmospheric Plasma. Diabet. Med. 2022, 39, e14787. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Hussein, L.K.; Molina, E.A.; Keyloun, J.W.; McKnight, S.M.; Jimenez, L.M.; Moffatt, L.T.; Shupp, J.W.; Carney, B.C. Cold Atmospheric Plasma Is Bactericidal to Wound-Relevant Pathogens and Is Compatible with Burn Wound Healing. Burns 2024, 50, 1192–1212. [Google Scholar] [CrossRef]
- Badr, G.; El-Hossary, F.M.; Lasheen, F.E.M.; Negm, N.Z.; Khalaf, M.; Salah, M.; Sayed, L.H.; Abdel-Maksoud, M.A.; Elminshawy, A. Cold Atmospheric Plasma Induces the Curing Mechanism of Diabetic Wounds by Regulating the Oxidative Stress Mediators INOS and NO, the Pyroptotic Mediators NLRP-3, Caspase-1 and IL-1β and the Angiogenesis Mediators VEGF and Ang-1. Biomed. Pharmacother. 2023, 169, 115934. [Google Scholar] [CrossRef] [PubMed]
- Abu Rached, N.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12, 5121. [Google Scholar] [CrossRef] [PubMed]
- Cartotto, R.; Johnson, L.; Rood, J.M.; Lorello, D.; Matherly, A.; Parry, I.; Romanowski, K.; Wiechman, S.; Bettencourt, A.; Carson, J.S.; et al. Clinical Practice Guideline: Early Mobilization and Rehabilitation of Critically Ill Burn Patients. J. Burn. Care Res. 2023, 44, 1–15. [Google Scholar] [CrossRef]
- Tillmann, J.; Weckbecker, K.; Wiesheu, P.; Bleckwenn, M.; Deutsch, T.; Münster, E. Hausärztliche Versorgung Ukrainischer Geflüchteter. Z. Allg. 2023, 99, 28–33. [Google Scholar] [CrossRef]
- Schultze, T.; Hogardt, M.; Velázquez, E.S.; Hack, D.; Besier, S.; Wichelhaus, T.A.; Rochwalsky, U.; Kempf, V.A.; Reinheimer, C. Molecular Surveillance of Multidrug-Resistant Gram-Negative Bacteria in Ukrainian Patients, Germany, March to June 2022. Eurosurveillance 2023, 28, 2200850. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to Manage Pseudomonas Aeruginosa Infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold Plasma Inactivation of Bacterial Biofilms and Reduction of Quorum Sensing Regulated Virulence Factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological Interactions with Cold Plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Vlig, M.; Sobota, A.; Middelkoop, E.; Boekema, B.K.H.L. Safety and Bactericidal Efficacy of Cold Atmospheric Plasma Generated by a Flexible Surface Dielectric Barrier Discharge Device against Pseudomonas Aeruginosa in Vitro and in Vivo. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Lunder, M.; Dahle, S.; Fink, R. Cold Atmospheric Plasma for Surface Disinfection: A Promising Weapon against Deleterious Meticillin-Resistant Staphylococcus Aureus Biofilms. J. Hosp. Infect. 2024, 143, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Shimizu, T.; Li, Y.-F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S. Aureus and E. Coli by Cold-Atmospheric Plasma Using a Porcine Skin Model In Vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef]
- Bayliss, D.L.; Shama, G.; Kong, M.G. Restoration of Antibiotic Sensitivity in Meticillin-Resistant Staphylococcus Aureus Following Treatment with a Non-Thermal Atmospheric Gas Plasma. Int. J. Antimicrob. Agents 2013, 41, 398–399. [Google Scholar] [CrossRef]
- de Souza, L.B.; de Souza Silva, J.I.; Bagne, L.; Pereira, A.T.; de Oliveira, M.A.; Lopes, B.B.; do Amaral, M.E.C.; de Aro, A.A.; Esquisatto, M.A.M.; dos Santos, G.M.T.; et al. Argon Atmospheric Plasma Treatment Promotes Burn Healing by Stimulating Inflammation and Controlling the Redox State. Inflammation 2020, 43, 2357–2371. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.; Rousseau, A.; Frescaline, N. Cold Atmospheric Plasma Modulates Endothelial Nitric Oxide Synthase Signalling and Enhances Burn Wound Neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef]
- Kaushik, N.; Mitra, S.; Baek, E.J.; Nguyen, L.N.; Bhartiya, P.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. The Inactivation and Destruction of Viruses by Reactive Oxygen Species Generated through Physical and Cold Atmospheric Plasma Techniques: Current Status and Perspectives. J. Adv. Res. 2023, 43, 59–71. [Google Scholar] [CrossRef]
- Frescaline, N.; Duchesne, C.; Favier, M.; Onifarasoaniaina, R.; Guilbert, T.; Uzan, G.; Banzet, S.; Rousseau, A.; Lataillade, J. Physical Plasma Therapy Accelerates Wound Re-epithelialisation and Enhances Extracellular Matrix Formation in Cutaneous Skin Grafts. J. Pathol. 2020, 252, 451–464. [Google Scholar] [CrossRef]
- Flynn, P.B.; Graham, W.G.; Gilmore, B.F. Acinetobacter Baumannii Biofilm Biomass Mediates Tolerance to Cold Plasma. Lett. Appl. Microbiol. 2019, 68, 344–349. [Google Scholar] [CrossRef]
- Khabipov, A.; Schreiber, A.; Kersting, S.; Hummel, R.; Höhn, J.; Partecke, L.-I.; Bekeschus, S.; Glitsch, A.; Keßler, W. Cold Atmospheric Plasma Is a Promising Alternative Treatment Option in Case of Split Skin Graft Failure. Case Rep. Surg. 2024, 2024, 1013445. [Google Scholar] [CrossRef]
- Vasile Nastuta, A.; Pohoata, V.; Topala, I. Atmospheric Pressure Plasma Jet—Living Tissue Interface: Electrical, Optical, and Spectral Characterization. J. Appl. Phys. 2013, 113, 183302. [Google Scholar] [CrossRef]
- Fiebrandt, M.; Lackmann, J.; Stapelmann, K. From Patent to Product? 50 Years of Low-pressure Plasma Sterilization. Plasma Process. Polym. 2018, 15, 1800139. [Google Scholar] [CrossRef]
- Dai, X.; Wu, J.; Lu, L.; Chen, Y. Current Status and Future Trends of Cold Atmospheric Plasma as an Oncotherapy. Biomol. Ther. 2023, 31, 496–514. [Google Scholar] [CrossRef] [PubMed]

| Location | Day | Acinetobacter baumannii 4-MRGN | Escherichia coli | Enterobacter cloacae 3-MRGN | Vancomycin Resistant Enterococcus Faecium | Pseudomonas aeruginosa 4-MRGN | Corynebacterium striatum | Staphylococcus Coagulase Negative |
|---|---|---|---|---|---|---|---|---|
| Buttocks | 0 | +++ | + | |||||
| Buttocks | 11 | ++ | ++ | ++ | ||||
| Buttocks | 67 | ++ | ++ | |||||
| Left leg | 0 | ++ | ||||||
| Left leg | 0 | +++ | ||||||
| Left leg | 1 | +++ | positive | |||||
| Left leg | 4 | +++ | ++ | |||||
| Left leg | 11 | ++ | ++ | |||||
| Left leg | 67 | + | ||||||
| Rectum | 0 | +++ | positive | positive | ||||
| Rectum | 4 | positive | ||||||
| Rectum | 11 | positive | positive | positive | positive | |||
| Right arm | 4 | ++ | ||||||
| Right arm | 11 | + | + | |||||
| Right arm | 67 | ++ | ||||||
| Right leg | 0 | ++ | ||||||
| Right leg | 1 | +++ | ||||||
| Right leg | 4 | +++ | ++ | |||||
| Right leg | 11 | ++ | + | ++ | ||||
| Right leg | 67 | + | + | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewski, M.R.; Schlottmann, F.; März, V.; Dieck, T.; Vogt, P.M. The Successful Treatment of Multi-Resistant Colonized Burns with Large-Area Atmospheric Cold Plasma Therapy and Dermis Substitute Matrix—A Case Report. Eur. Burn J. 2024, 5, 271-282. https://doi.org/10.3390/ebj5030025
Milewski MR, Schlottmann F, März V, Dieck T, Vogt PM. The Successful Treatment of Multi-Resistant Colonized Burns with Large-Area Atmospheric Cold Plasma Therapy and Dermis Substitute Matrix—A Case Report. European Burn Journal. 2024; 5(3):271-282. https://doi.org/10.3390/ebj5030025
Chicago/Turabian StyleMilewski, Moritz R., Frederik Schlottmann, Vincent März, Thorben Dieck, and Peter M. Vogt. 2024. "The Successful Treatment of Multi-Resistant Colonized Burns with Large-Area Atmospheric Cold Plasma Therapy and Dermis Substitute Matrix—A Case Report" European Burn Journal 5, no. 3: 271-282. https://doi.org/10.3390/ebj5030025
APA StyleMilewski, M. R., Schlottmann, F., März, V., Dieck, T., & Vogt, P. M. (2024). The Successful Treatment of Multi-Resistant Colonized Burns with Large-Area Atmospheric Cold Plasma Therapy and Dermis Substitute Matrix—A Case Report. European Burn Journal, 5(3), 271-282. https://doi.org/10.3390/ebj5030025







