Comparison of Clinical Estimation and Stereophotogrammic Instrumented Imaging of Burn Scar Height and Volume
Abstract
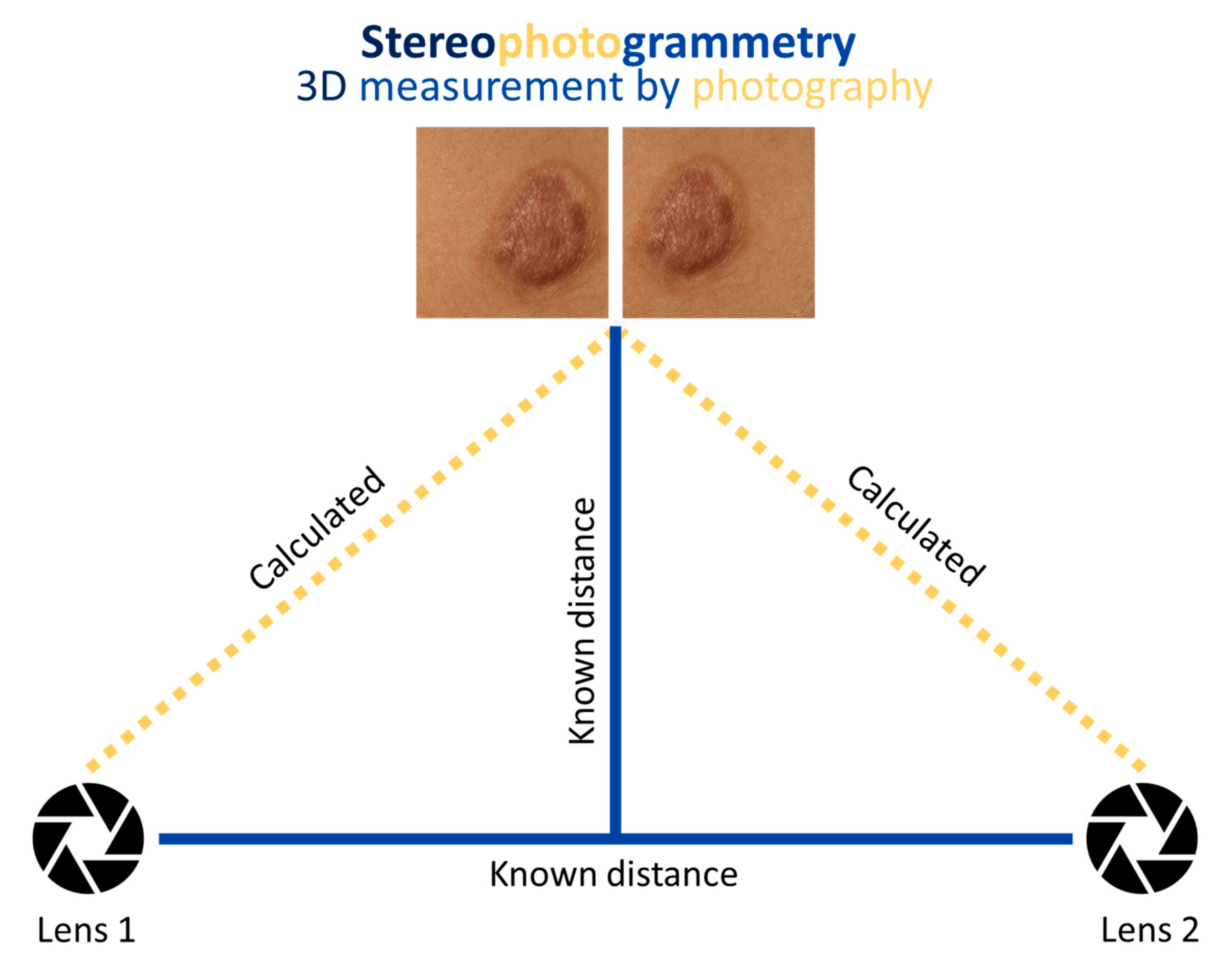
1. Introduction
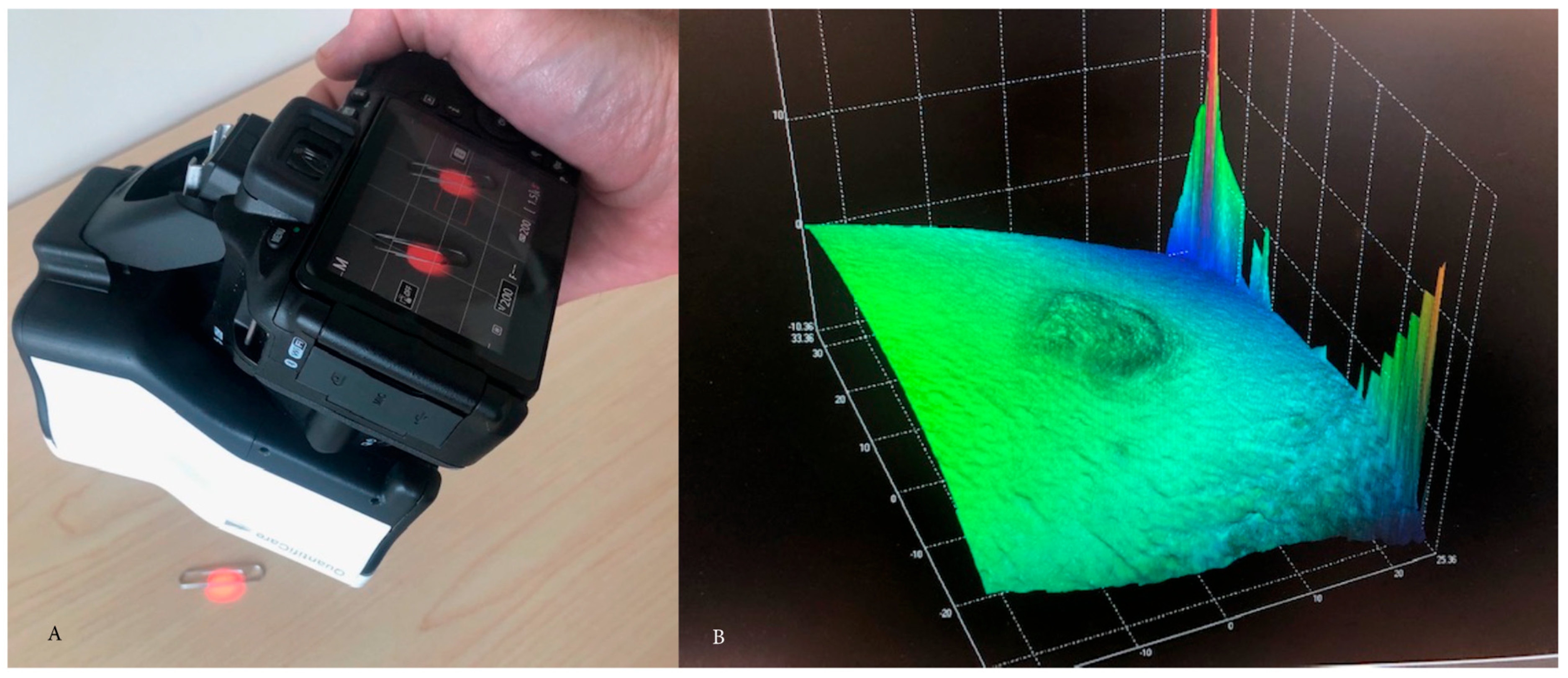
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bombaro, K.M.; Engrav, L.H.; Carrougher, G.J.; Wiechman, S.A.; Faucher, L.; Costa, B.A.; Heimbach, D.M.; Rivara, F.P.; Honari, S. What is the prevalence of hypertrophic scarring following burns? Burns 2003, 29, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, V. Hypertrophic scar. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.G.; Ghahary, A.; Tredget, E.E. Molecular and cellular aspects of fibrosis following thermal injury. Hand Clin. 2000, 16, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Tyack, Z.F.; Pegg, S.; Ziviani, J. Postburn dyspigmentation: Its assessment, management, and relationship to scarring—A review of the literature. J. Burn Care Rehabil. 1997, 18, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Boo, S. Quality of life and mediating role of patient scar assessment in burn patients. Burns 2017, 43, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Chung, H.S.; Kwon, B.; Kim, G. Association between depression, patient scar assessment and burn-specific health in hospitalized burn patients. Burns 2012, 38, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Tyack, Z.; Simons, M.; Spinks, A.; Wasiak, J. A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 2012, 38, 6–18. [Google Scholar] [CrossRef]
- Bae, S.H.; Bae, Y.C. Analysis of frequency of use of different scar assessment scales based on the scar condition and treatment method. Arch. Plast. Surg. 2014, 41, 111–115. [Google Scholar] [CrossRef]
- Lee, K.C.; Bamford, A.; Gardiner, F.; Agovino, A.; Ter Horst, B.; Bishop, J.; Sitch, A.; Grover, L.; Logan, A.; Moiemen, N.S. Investigating the intra- and inter-rater reliability of a panel of subjective and objective burn scar measurement tools. Burns 2019, 45, 1311–1324. [Google Scholar] [CrossRef]
- Nedelec, B.; Correa, J.A.; Rachelska, G.; Armour, A.; LaSalle, L. Quantitative measurement of hypertrophic scar: Interrater reliability and concurrent validity. J. Burn Care Res. 2008, 29, 501–511. [Google Scholar] [CrossRef]
- Nedelec, B.; Correa, J.A.; Rachelska, G.; Armour, A.; LaSalle, L. Quantitative measurement of hypertrophic scar: Intrarater reliability, sensitivity, and specificity. J. Burn Care Res. 2008, 29, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, M.B.; Vloemans, J.F.; Tuinebreijer, W.E.; van de Ven, P.; van Unen, E.; van Zuijlen, P.P.; Middelkoop, E. Outcome after burns: An observational study on burn scar maturation and predictors for severe scarring. Wound Repair Regen. 2012, 20, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Goei, H.; van der Vlies, C.H.; Tuinebreijer, W.E.; van Zuijlen, P.P.M.; Middelkoop, E.; van Baar, M.E. Predictive validity of short term scar quality on final burn scar outcome using the Patient and Observer Scar Assessment Scale in patients with minor to moderate burn severity. Burns 2017, 43, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Shankowsky, H.A.; Tredget, E.E. Rating the resolving hypertrophic scar: Comparison of the Vancouver Scar Scale and scar volume. J. Burn Care Rehabil. 2000, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Rennekampff, H.O.; Rabbels, J.; Reinhard, V.; Becker, S.T.; Schaller, H.E. Comparing the Vancouver Scar Scale with the cutometer in the assessment of donor site wounds treated with various dressings in a randomized trial. J. Burn Care Res. 2006, 27, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Draaijers, L.J.; Tempelman, F.R.; Botman, Y.A.; Kreis, R.W.; Middelkoop, E.; van Zuijlen, P.P. Colour evaluation in scars: Tristimulus colorimeter, narrow-band simple reflectance meter or subjective evaluation? Burns 2004, 30, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Agabalyan, N.A.; Su, S.; Sinha, S.; Gabriel, V. Comparison between high-frequency ultrasonography and histological assessment reveals weak correlation for measurements of scar tissue thickness. Burns 2017, 43, 531–538. [Google Scholar] [CrossRef]
- Du, Y.C.; Lin, C.M.; Chen, Y.F.; Chen, C.L.; Chen, T. Implementation of a burn scar assessment system by ultrasound techniques. In Proceedings of the International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2328–2331. [Google Scholar]
- Holavanahalli, R.K.; Helm, P.A.; Kowalske, K.J. Long-term outcomes in patients surviving large burns: The skin. J. Burn Care Res. 2010, 31, 631–639. [Google Scholar] [CrossRef]
- Ahuja, R.B.; Chatterjee, P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns 2014, 40, 583–588. [Google Scholar] [CrossRef]
- Peake, M.; Pan, K.; Rotatori, R.M.; Powell, H.; Fowler, L.; James, L.; Dale, E. Incorporation of 3D stereophotogrammetry as a reliable method for assessing scar volume in standard clinical practice. Burns 2019, 45, 1614–1620. [Google Scholar] [CrossRef]
- Su, S.; Sinha, S.; Gabriel, V. Evaluating accuracy and reliability of active stereophotogrammetry using MAVIS III Wound Camera for three-dimensional assessment of hypertrophic scars. Burns 2017, 43, 1263–1270. [Google Scholar] [CrossRef]
- Plassmann, P.; Jones, T.D. MAVIS: A non-invasive instrument to measure area and volume of wounds. Measurement of Area and Volume Instrument System. Med. Eng. Phys. 1998, 20, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, G.; Eklund, A.E.; Torlegård, K.; Dauphin, E. Evaluation of leg ulcer treatment with stereophotogrammetry. A pilot study. Br. J. Dermatol. 1979, 101, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tzou, C.H.; Artner, N.M.; Pona, I.; Hold, A.; Placheta, E.; Kropatsch, W.G.; Frey, M. Comparison of three-dimensional surface-imaging systems. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.J.; Nishimura, J.; Seton, J.; Goodman, B.L.; Ho, C.H.; Bogie, K.M. Repeatability and clinical utility in stereophotogrammetric measurements of wounds. J. Wound Care 2013, 22, 90–92, 94–97. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; Carter, R.R.; Bogie, K.M. Personalized prediction of chronic wound healing: An exponential mixed effects model using stereophotogrammetric measurement. J. Tissue Viability 2014, 23, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, B.; Nouraei, S.A.; Van Dam, H.; Dex, E.; Wood, S.; Nduka, C. Objective assessment of keloid scars with three-dimensional imaging: Quantifying response to intralesional steroid therapy. Plast. Reconstr. Surg. 2007, 119, 556–561. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Patient Summary |
|---|---|
| Patient age, n = 15 (years) | 20–68 (mean = 42.6, SD = 15.8) |
| Acute burn wound management, n = 26 (count) | |
| Non-operative treatment | 20 |
| Excision and grafting | 5 |
| Skin graft donor site | 1 |
| Image Number | Estimated Height (mm) | Estimated Volume (cm3) | Measured Height (cm) | Measured Volume (cm3) |
|---|---|---|---|---|
| 1 | 2 | 0.02 | 0.06 | 0.25 |
| 2 | 2 | 0.32 | 0.03 | 0.14 |
| 3 | 3 | 0.036 | 0.5 | 0.19 |
| 4 | 3 | 0.6 | 0.07 | 0.46 |
| 5 | 1 | 2 | 0.02 | 0.19 |
| 6 | 1 | 0.18 | 0.01 | 0.03 |
| 7 | 3 | 0.18 | 0.07 | 1.18 |
| 8 | 3 | 42 | 0.09 | 2.39 |
| 9 | 0 | 0 | 0.05 | 0.63 |
| 10 | 2 | 0.26 | 0.02 | 0.2 |
| 11 | 2 | 3.6 | 0.03 | 0.22 |
| 12 | 0.9 | 1.08 | 0.07 | 1.01 |
| 13 | 0.05 | 0.4 | 0.11 | 1.02 |
| 14 | 1 | 4.5 | 0.06 | 0.22 |
| 15 | 1 | 2.5 | 0.06 | 0.38 |
| 16 | 1 | 2.45 | 0.05 | 0.61 |
| 17 | 3 | 4.5 | 0.06 | 0.4 |
| 18 | 3 | 0.675 | 0.09 | 0.53 |
| 19 | 1 | 3.15 | 0.11 | 0.89 |
| 20 | 0.2 | 2 | 0.11 | 1.49 |
| 21 | 0.1 | 1.5 | 0.15 | 2.42 |
| 22 | 0.5 | 2.1 | 0.11 | 0.5 |
| 23 | 4 | 12.6 | 0.27 | 3.65 |
| 24 | 3 | 7.5 | 0.1 | 1.94 |
| 25 | 1 | 2.1 | 0.7 | 0.23 |
| 26 | 3 | 9.45 | 0.14 | 4.19 |
| Image Number | Estimated Height (mm) | Estimated Volume (cm3) | Measured Height (cm) | Measured Volume (cm3) |
|---|---|---|---|---|
| 1 | 3 | 0.234 | 0.06 | 0.14 |
| 2 | 2 | 0.4 | 0.03 | 0.05 |
| 3 | 2 | 0.48 | 0.05 | 0.18 |
| 4 | 3 | 0.504 | 0.06 | 0.13 |
| 5 | 2 | 0.4 | 0.03 | 0.14 |
| 6 | 2 | 0.09 | 0.02 | 0.01 |
| 7 | 2 | 2.1 | 0.07 | 0.88 |
| 8 | 3 | 2.02 | 0.1 | 2.08 |
| 9 | 0.5 | 4.625 | 0.05 | 0.86 |
| 10 | 0.2 | 0.48 | 0.03 | 0.52 |
| 11 | 2 | 13.12 | 0.04 | 0.83 |
| 12 | 0.5 | 1.8 | 0.11 | 1.02 |
| 13 | 2 | 2.1 | 0.11 | 1.01 |
| 14 | 2 | 0.304 | 0.06 | 0.18 |
| 15 | 1 | 3.08 | 0.06 | 0.4 |
| 16 | 3 | 0.576 | 0.02 | 0.13 |
| 17 | 3 | 0.72 | 0.07 | 0.5 |
| 18 | 2 | 0.14 | 0.05 | 0.12 |
| 19 | 3 | 0.6 | 0.13 | 1.02 |
| 20 | 0.5 | 4.75 | 0.12 | 0.02 |
| 21 | 1 | 2.5 | 0.13 | 1.78 |
| 22 | 3 | 0.66 | 0.08 | 0.19 |
| 23 | 4 | 7.56 | 0.23 | 2 |
| 24 | 3 | 5.25 | 0.09 | 1.57 |
| 25 | 2 | 0.084 | 0.02 | 0.08 |
| 26 | 2 | 1.79 | 0.1 | 2.69 |
| Image Number | Estimated Height (mm) | Estimated Volume (cm3) | Measured Height (cm) | Measured Volume (cm3) |
|---|---|---|---|---|
| 1 | 2.5 | 0.75 | 0.06 | 0.15 |
| 2 | 2 | 0.36 | 0.03 | 0.14 |
| 3 | 4 | 1.44 | 0.05 | 0.17 |
| 4 | 1.5 | 3.75 | 0.07 | 0.17 |
| 5 | 1 | 0.5 | 0.03 | 0.18 |
| 6 | 1 | 0.04 | 0.01 | 0.02 |
| 7 | 1 | 1.2 | 0.07 | 0.98 |
| 8 | 2 | 3.6 | 0.08 | 1.83 |
| 9 | 1 | 0.9 | 0.06 | 0.82 |
| 10 | 0.5 | 3 | 0.02 | 0.18 |
| 11 | 2 | 2.5 | 0.03 | 0.24 |
| 12 | 0.2 | 0.16 | 0.05 | 0.62 |
| 13 | 2 | 1.5 | 0.11 | 1.02 |
| 14 | 1.5 | 0.225 | 0.06 | 0.17 |
| 15 | 1 | 1.2 | 0.06 | 0.31 |
| 16 | 1 | 0.525 | 0.05 | 0.51 |
| 17 | 3 | 0.078 | 0.07 | 0.36 |
| 18 | 3 | 0.225 | 0.04 | 0.17 |
| 19 | 1 | 0.525 | 0.11 | 0.91 |
| 20 | 0.5 | 7.5 | 0.1 | 1.08 |
| 21 | 0.5 | 7.5 | 0.14 | 2.31 |
| 22 | 0.75 | 2.34 | 0.1 | 0.38 |
| 23 | 5 | 16.25 | 0.25 | 2.86 |
| 24 | 3 | 6.75 | 0.09 | 2.04 |
| 25 | 0.5 | 0.937 | 0.02 | 0.15 |
| 26 | 1.5 | 3.937 | 0.13 | 3.89 |
| Burn Scar Characteristic | ICC | Z-Score (p-Value) |
|---|---|---|
| Maximum height | −1.39 (0.161) | |
| Estimated | 0.595 | |
| Measured | 0.933 | |
| Positive volume | −2.87 (0.041) | |
| Estimated | 0.531 | |
| Measured | 0.890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharadia, S.K.; Gabriel, V. Comparison of Clinical Estimation and Stereophotogrammic Instrumented Imaging of Burn Scar Height and Volume. Eur. Burn J. 2024, 5, 38-48. https://doi.org/10.3390/ebj5010004
Bharadia SK, Gabriel V. Comparison of Clinical Estimation and Stereophotogrammic Instrumented Imaging of Burn Scar Height and Volume. European Burn Journal. 2024; 5(1):38-48. https://doi.org/10.3390/ebj5010004
Chicago/Turabian StyleBharadia, Shyla Kajal, and Vincent Gabriel. 2024. "Comparison of Clinical Estimation and Stereophotogrammic Instrumented Imaging of Burn Scar Height and Volume" European Burn Journal 5, no. 1: 38-48. https://doi.org/10.3390/ebj5010004
APA StyleBharadia, S. K., & Gabriel, V. (2024). Comparison of Clinical Estimation and Stereophotogrammic Instrumented Imaging of Burn Scar Height and Volume. European Burn Journal, 5(1), 38-48. https://doi.org/10.3390/ebj5010004






