Abstract
Burns and scarring are considered some of the greatest problems in public health because of their frequent occurrence. Today, photo-electric technology shows promising results in the treatment of burn scars. Over the years, more clinical trials and more technologies for scarring have emerged. The aim of this study was to determine better timing and methods of photo-electric therapy for burn scars. This study was registered in PROSPERO (CRD42023397244), following the PRISMA statement, and was carried out in concordance with the PRISMA checklist. In October 2022, we searched PubMed.gov, Embase, and the Cochrane library (1980–present) for published studies related to the photo-electric treatment of burn scars. Two review authors independently selected the studies, extracted the data, assessed the risk of bias among the studies included, and carried out NIH assessments to assess the certainty of the evidence. A third review author arbitrated any disagreements. Our research included 39 studies. We found evidence suggesting that photo-electric therapy between six months and one year offers significantly better outcomes than treatment of scarring after one year. The evidence also suggests the use of IPL for the treatment of early burn scarring. However, it is important to emphasize that the scientific evidence remains insufficient. We need more clinical trials of higher quality and with less heterogeneity to confirm our results.
1. Introduction
Burn scars are considered one of the greatest problems in public health [1,2]. Hypertrophic scarring occurs in 30 to 90 percent of patients following burns [3,4,5]. Most burn patients have to suffer physical pain and pruritus in the first stage. As long-term effects, dysfunction and aesthetic deformations in some severe cases also negatively impact patients’ self-confidence, making them feel inferior. Today, first-line therapy for scarring includes surgery, pression therapy, silicone sheets and gel formulations, intralesional pharmacologic treatments, and many others [6,7,8]. However, the recurrence rate of scars after surgery is high at up to 45–100% [9,10,11]. Intralesional pharmacologic treatments are also commonly used, including triamcinolone acetonide (TAC) and fluorouracil (FU) [12,13]. Overall, burn scar characteristics can certainly be improved [14,15,16,17] but also cause many side-effects. For example, TAC can cause atrophy, hypopigmentation, hypertension, hirsutism, and even Cushing’s syndrome [18], and FU may also have myelosuppression activities, causing leukopenia, infection, anemia, and other side effects. Additionally, some treatments cannot obtain satisfactory results. Other methods, including radiotherapy, cryotherapy, and massage therapy, are not commonly used clinically for a variety of reasons [9,19,20]. Meanwhile, photo-electric technology, which shows promising results in the treatment of burn scars [21], produces photophysical (such as thermal, mechanical, and electromagnetic) and photobiological effects (such as photochemistry and photobiological regulation) by skin exposure.
Today, the increasing interest in photo-electric therapy is making a great difference in the treatment of scarring. Photo-electric therapy in scarring includes pulsed dye lasers (PDLs), neodymium-doped yttrium aluminum garnet (Nd: YAG) lasers, low-level lasers (LLLT), intense pulsed light (IPL), ablative fractional carbon dioxide lasers (CO2AFL), and radiofrequency (RF). On Pubmed.gov, over two thousand results since 1967 can be found for the photo-electric treatment of scars. However, nearly 1800 of these results were published after the year 2000, and over a thousand were published in the last decade. Thus, in recent years, more clinical trials have been conducted and more technologies for scarring have emerged. These technologies include narrow-spectrum intense pulsed light (DPL) and Q-switched frequency-doubled Nd: YAG lasers. Due to the abovementioned factors, photo-electric treatment has become an efficient modality of therapy for burn scars with few side effects. Some systematic reviews have noted that CO2AFL is a safe, cost-effective, and efficacious procedure for burn scars [22] that offers objective improvements specifically for chronic burn scars [23]. However, systematic reviews of other treatments are still scarce. We located a systematic review about the effectiveness of laser therapy for hypertrophic burn scars; this review noted that the evidence is not adequate to reach a conclusion [24]. Additionally, we found a systematic review and meta-analysis about surgical scars that showed that laser therapy may be a useful modality to minimize surgical scars when applied earlier on [25]. However, this study only used four articles to perform the meta-analysis, so more research should be carried out to support this result. We also found a systematic review of early laser intervention in scarring [26]. The results were uncertain as to whether early laser treatment can reduce scar formation, and more high-quality research is needed for a definitive conclusion. There were also some reviews on this topic [27,28]. Globally, there are still many deficits in photo-electric therapy, and no detailed protocol is available. We still have no agreed-upon methods or parameters for the treatment of burn scars, which may cause many side-effects. This study focuses on photo-electric therapy, which can be used in the first period of scarring to prevent progression in a worse direction. We also explore when and how to use these treatments to achieve the most effective outcomes.
2. Materials and Methods
2.1. Search Strategy
Firstly, this study was registered in PROSPERO (CRD42023397244), following the PRISMA statement, and was carried out in concordance with the PRISMA checklist, which is included in the Supplementary Materials (File S1).
We employed the following search strategy to identify the clinical evidence reported in the biomedical literature: In October 2022, we searched PubMed.gov, Embase, and the Cochrane library (1980–October 2022) for published case reports, clinical studies, clinical trials, controlled clinical trials, and randomized controlled trials related to the photo-electric treatment of burns. We included no restriction for language. The mesh terms we utilized were ‘burn’ AND (‘laser’ OR ‘light’ OR ‘radiofrequency’) AND ‘therapy*’ AND ‘cicatrix’. The details of our search strategy are provided in Table 1.

Table 1.
Search details on PubMed.gov.
2.2. Selection Inclusion
To be included in the analysis, an original article had to meet the following inclusion criteria: (1) subject: patients who had clinically obvious scars, with more than 50% of the sample featuring scarring due to burns; (2) intervention: treatment of scars needed to involve photo-electric therapy; (3) outcome: Vancouver Scar Scale (VSS) score, Patient and Observer Scar Assessment Scale (POSAS), Visual Analogue Score (VAS), and scar thickness (mm) measured with ultrasonography; (4) control: pretreatment in individuals or other treatments or an untreated area control; (5) study design: randomized controlled trial (RCT), non-randomized control trial, pre–post study of the same person, cohort study, case–control study, and/or comparative study; (6) a mention of scar duration.
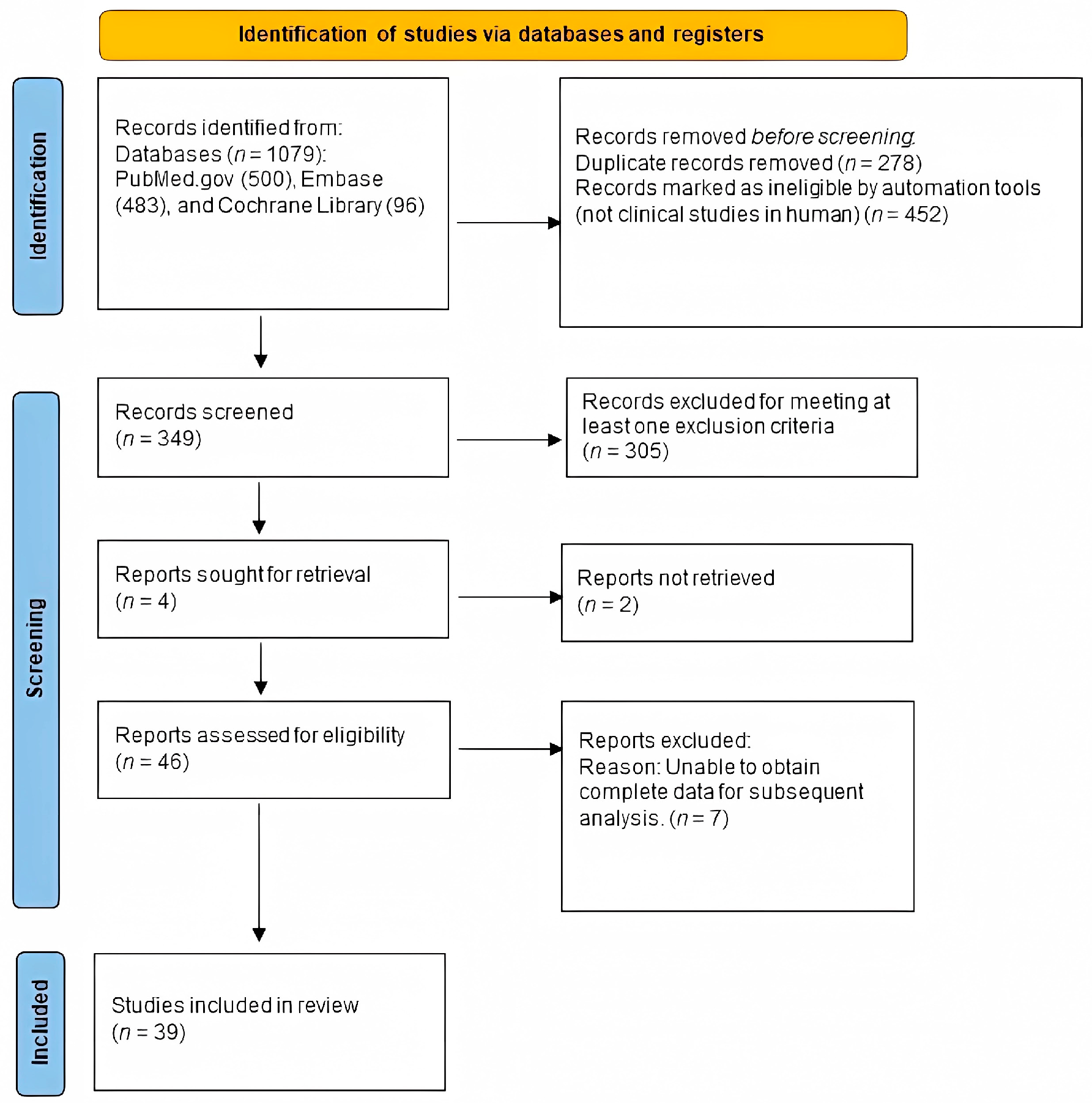
The exclusion criteria were as follows: (1) subject: more than 50% of the sample due to etiologies other than burns; (2) intervention: treatment of scars did not involve photo-electric therapy; (3) outcomes: measurement methods did not include the Vancouver Scar Scale (VSS), the Patient and Observer Scar Assessment Scale (POSAS), the Visual Analogue Score (VAS), or scar thickness (mm) measured with ultrasonography; (4) control: no control; (5) study design: case report or case series; (6) no mention of scar duration (Figure 1).
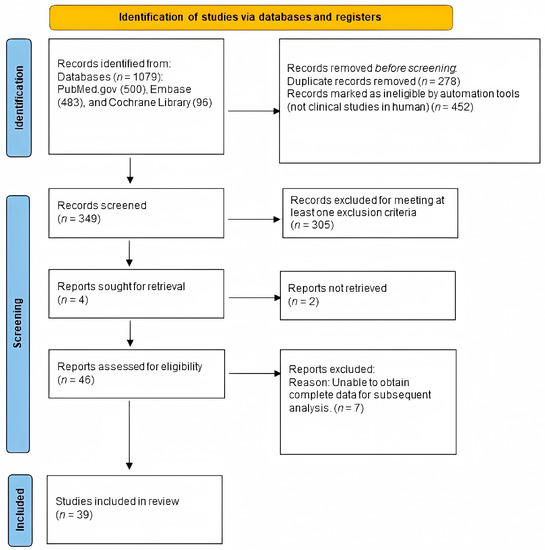
Figure 1.
Eligibility of studies for inclusion in the meta-analysis.
2.3. Data Extraction
Two independent investigators browsed all included studies and recorded the features and outcomes of the trials using a data extraction form. The following variables were summarized in a standard Excel file: first author’s name, year of publication, study design, control, duration of follow-up, sample size, country, patients’ baseline characteristics, the type of treatment, the parameters used, the requirements, whether or not any other scar treatments were used concurrently, and the main outcomes (VSS, total POSAS, POSAS-patient, POSAS-observer, VAS, and thickness). If the study used multiple evaluation data, then our selection order was as follows: (1) total POSAS, (2) POSAS-observer, (3) POSAS-patient, (4) VSS, and (5) VAS and thickness. These instruments are the most widely used and objective assessment criteria for burn scars. We also contacted the corresponding authors for more detailed information when the necessary data were not presented in the original study. Discrepancies between investigators were resolved by discussion and consensus.
2.4. Methodological Quality Assessment of Included Studies
The quality assessment for all studies was performed using the study quality assessment of the National Heart, Lung, and Blood Institute (NIH) [29]. This assessment has different scales for each type of study, with ratings of good, fair, and poor.
2.5. Statistical Analysis
Consensus in China indicates that the length of immature scarring varies greatly between individuals and is dependent on a number of factors. Most scars reach maturity in 6–12 months, but the average immature period for hyperplastic scars can be 22–46 months [30]. As a result, to determine the best time to start treatment and the best method for scar treatment within one year, we analyzed relevant data by dividing the samples into the following groups: scarring for less than six months, scarring for six months to one year, and scarring for longer than one year. We used Review Manager 5.4 to calculate the std. mean difference (SMD) or weighted mean difference (WMD) with a 95% confidence interval (95% CI) for continuous outcomes. As substantial heterogeneity was identified, we used only the random-effects model. A p-value less than 0.05 was judged to be statistically significant.
3. Results
3.1. Identification of Eligible Studies
A flowchart of the literature search process is presented in Figure 1. Our search yielded 349 unique articles. Of these, 46 articles met our inclusion criteria (Figure 1), and 39 articles were available. We found that 12 were cohort studies [31,32,33,34,35,36,37,38,39,40,41,42], 3 were case–control studies [43,44,45], 5 were RCTs [46,47,48,49,50], 4 were non-randomized controlled trials [51,52,53,54], and 15 were in-patient controlled studies [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Table 2 summarizes the characteristics of the 39 studies. These studies were published between 2004 and 2020. The population involved mainly burn scars. The studies were mainly of a good or fair level when assessed by the NIH, suggesting that these studies were of moderate or high quality. Ultimately, we included 22 studies with a total of 916 patients who suffered scarring for over one year, 10 studies with 355 patients who suffered scarring for over 6 months but less than 1 year, and 13 studies with 1101 patients who had scars for up to 6 months.

Table 2.
Baseline characteristics of patients in the trials included in the meta-analysis.
3.2. Time of Intervention
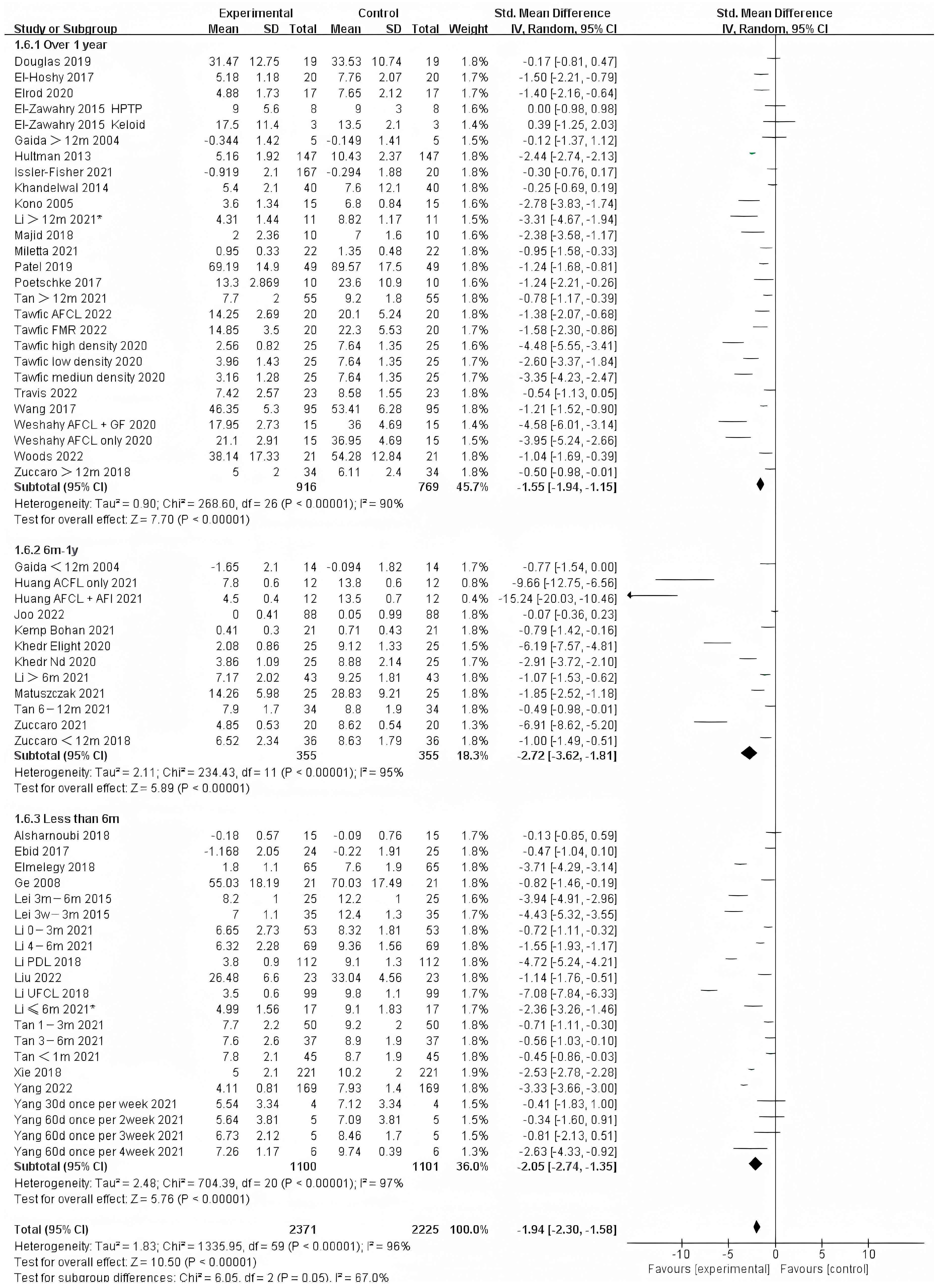
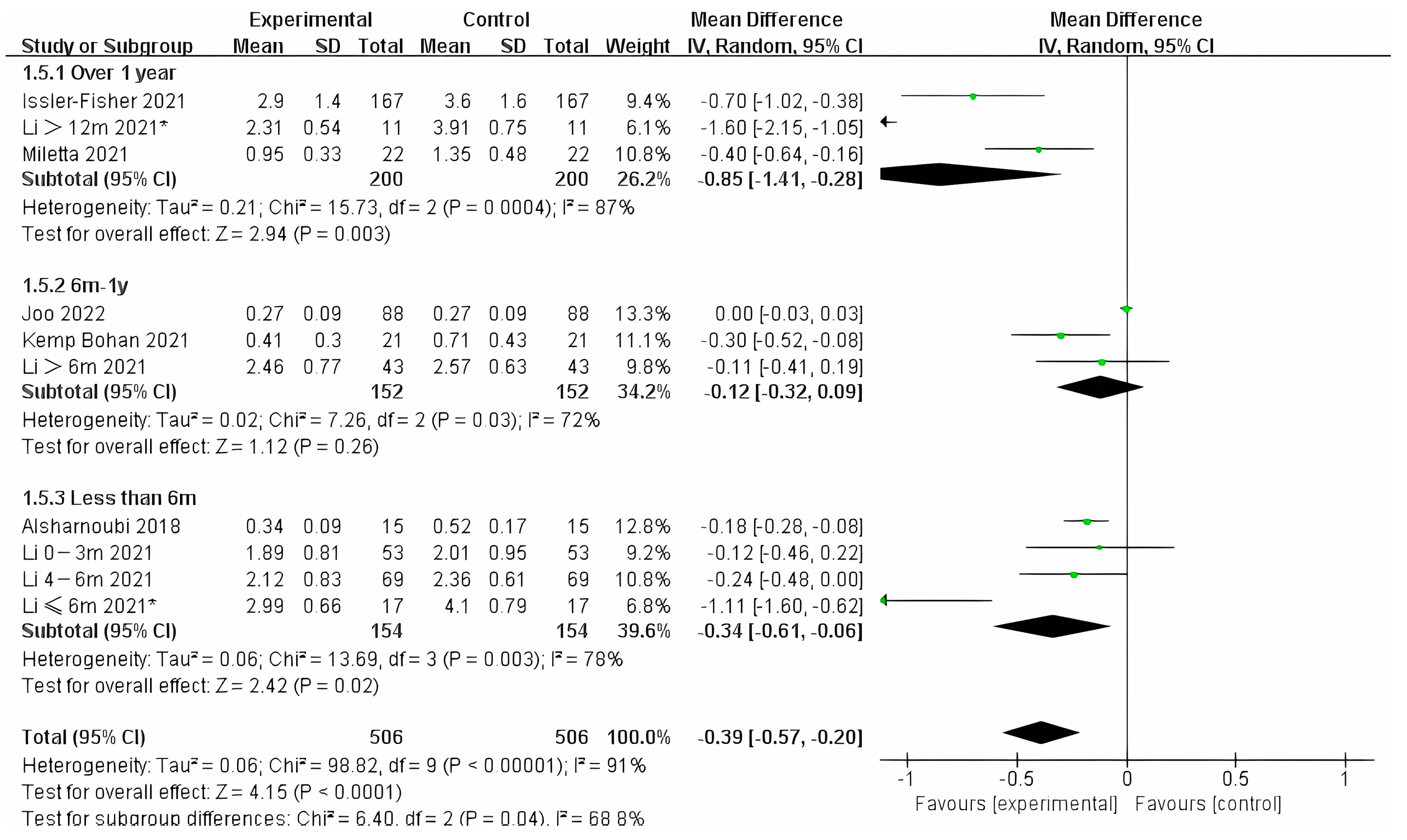
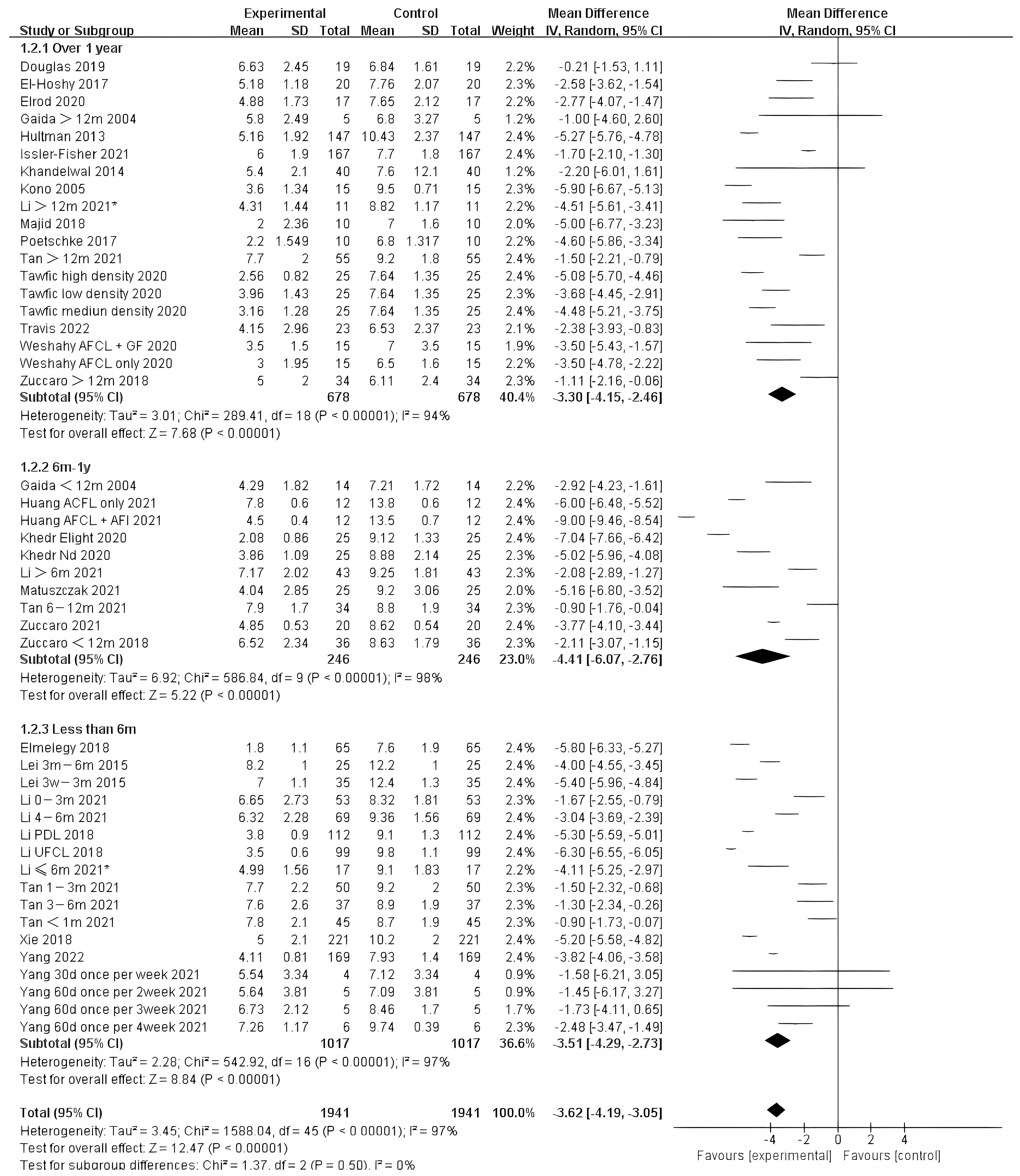
Photo-electric therapy offered significant improvement for each period of burn scarring (Figure 2) (Chi² = 6.05, df = 2 (p = 0.05), I² = 67.0%). Furthermore, for the group with scarring for over one year and the group with scarring between six months and one year, there was a significant difference in improvement (Chi² = 5.43, df = 1 (p = 0.02), I² = 81.6%) (Figure S1). However, there was no significant difference between the group with less than 6 months of scarring compared to the other two groups. For deeper insight, we also analyzed the improvement using only thickness and VSS. Interestingly, we found that in terms of thickness, the photo-electric therapy presented a significant difference in improvement of the scar over one year (Figure 3) (Chi² = 6.40, df = 2 (p = 0.04), I² = 68.8%). However, in VSS, although there was no significant difference between the two groups, scarring less than one year presented a higher effect size than scarring over one year (Figure 4) (Chi² = 1.37, df = 2 (p = 0.50), I² = 0%).
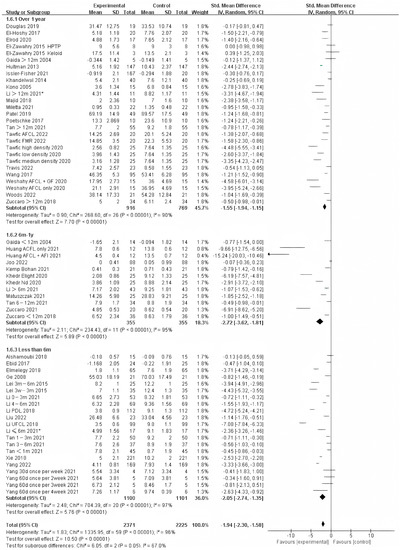
Figure 2.
Forest plot showing the effects of photo-electric therapy for burn scars with different scar durations according to VSS, POSAS, thickness, and VAS [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Each trial is represented by a green point, and the size of the point is proportional to the information in that trial. The ends of the horizontal bars denote 95% confidence intervals (Cis). Black diamonds indicate the overall results of all trials. * In order to distinguish between the two Li’s articles published in 2021, we have marked this one with an asterisk [43].
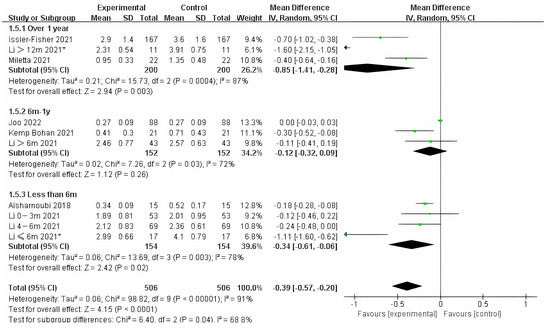
Figure 3.
Forest plot showing the effects of photo-electric therapy on the thickness of burn scars with different scar durations [35,43,45,48,53,58,63]. Each trial is represented by a green point, and the size of the point is proportional to the information in that trial. The ends of the horizontal bars denote 95% confidence intervals (Cis). Black diamonds give the overall results of all trials. * In order to distinguish between the two Li’s articles published in 2021, we have marked this one with an asterisk [43].
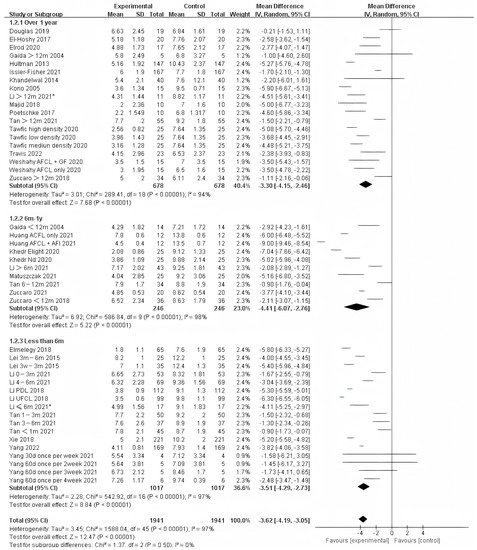
Figure 4.
Forest plot showing the effects of photo-electric therapy using VSS for burn scars with different scar durations [31,33,34,35,36,38,39,41,42,43,45,46,50,52,54,55,56,57,59,60,61,62,64,65,67,68,69]. Each trial is represented by a green point, and the size of the point is proportional to the information in that trial. The ends of the horizontal bars denote 95% confidence intervals (Cis). Black diamonds indicate the overall results of all trials. * In order to distinguish between the two Li’s articles published in 2021, we have marked this one with an asterisk [43].
3.3. Method for Burn Scarring
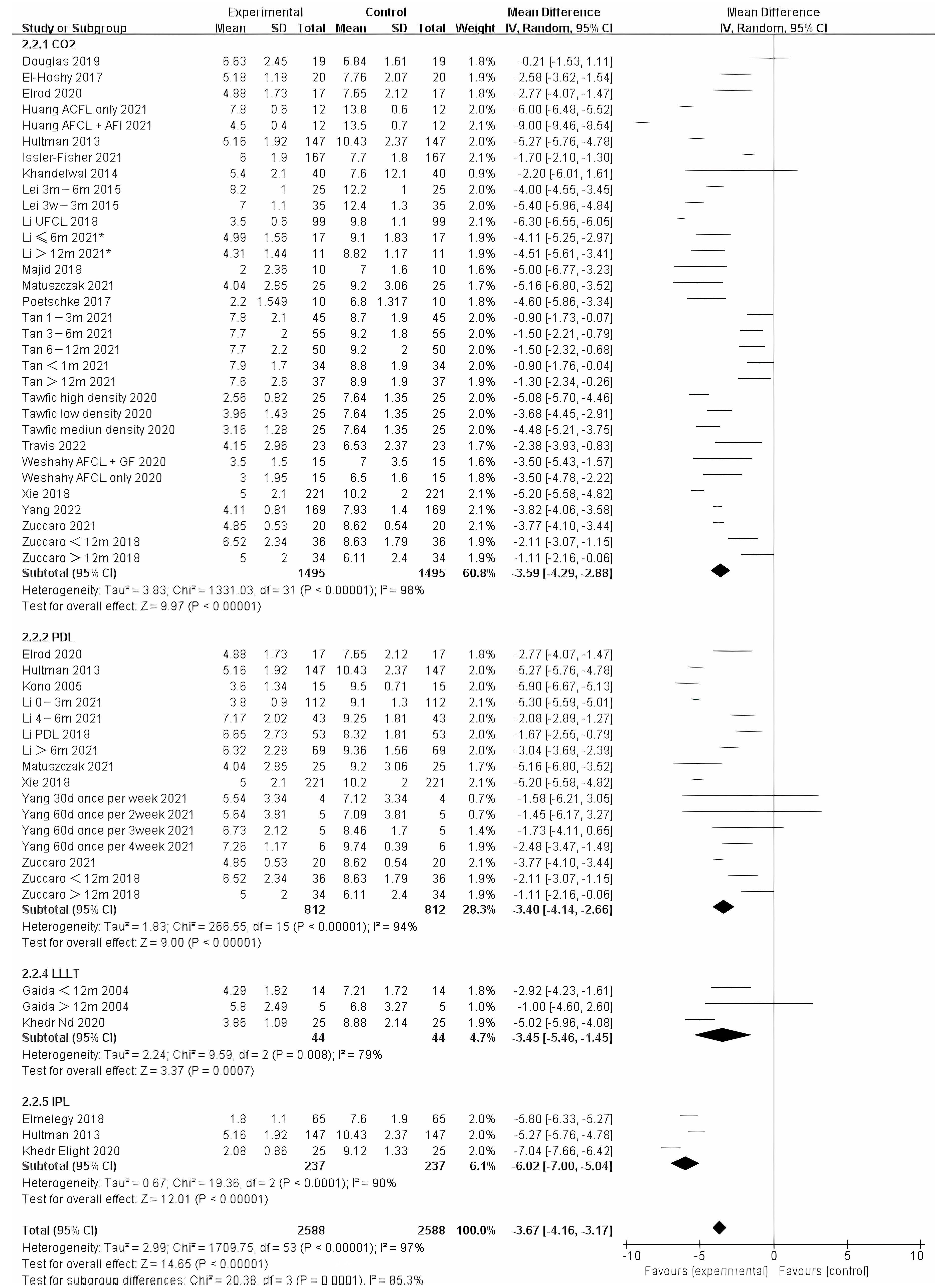
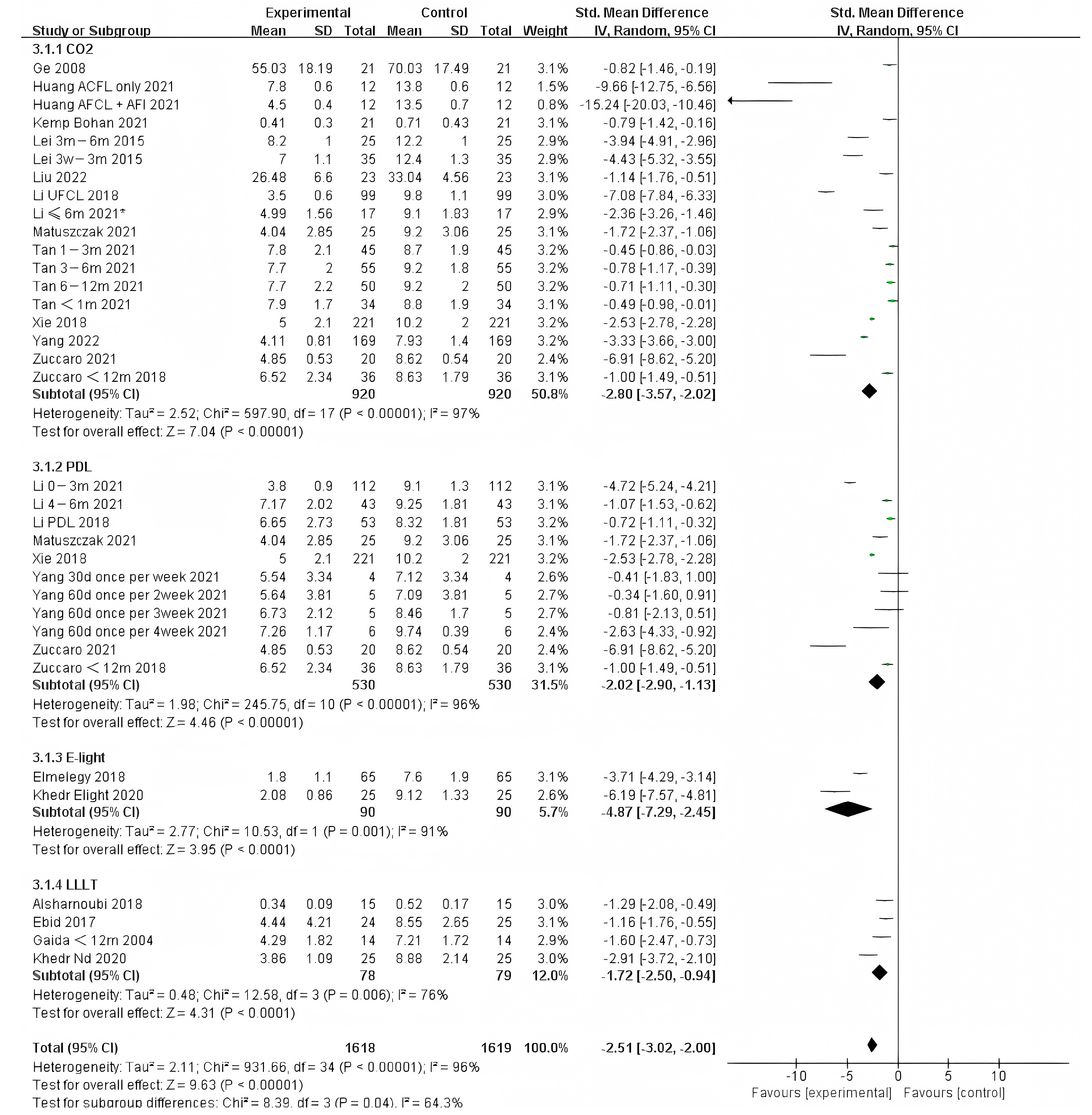
We also analyzed the effects of different photo-electric therapies for all periods of burn scars. The results showed significant differences between the methods (Figure 5) (Chi² = 20.38, df = 3 (p = 0.0001), I² = 85.3%). Overall, therapies that included IPL were found to work best.
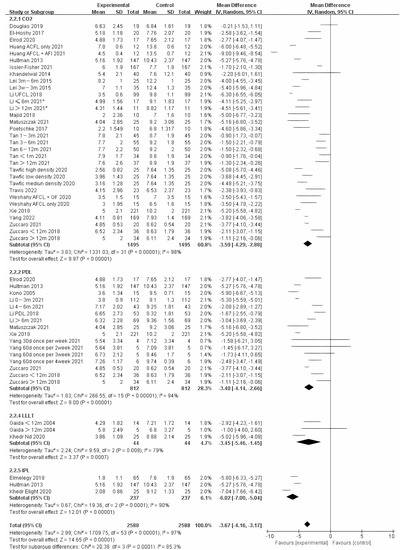
Figure 5.
Forest plot showing the effects of different photo-electric therapies for burn scars, using VSS [31,33,34,35,36,38,39,41,42,43,45,46,50,52,54,55,56,57,59,60,61,62,64,65,67,68,69]. Each trial is represented by a green point, and the size of the point is proportional to the information in that trial. The ends of the horizontal bars denote 95% confidence intervals (Cis). Black diamonds give the overall results of all trials. * In order to distinguish between the two Li’s articles published in 2021, we have marked this one with an asterisk [43].
For scarring less than one year, E-light (combined radiofrequency and IPL) offered significantly different improvement compared to other therapies. However, IPL was represented in only two studies of the 90 samples. Therefore, more studies on E-light should be performed (Figure 6). Although there was no significant difference between other therapies, it appears that CO2 treatment was more effective than PDL.
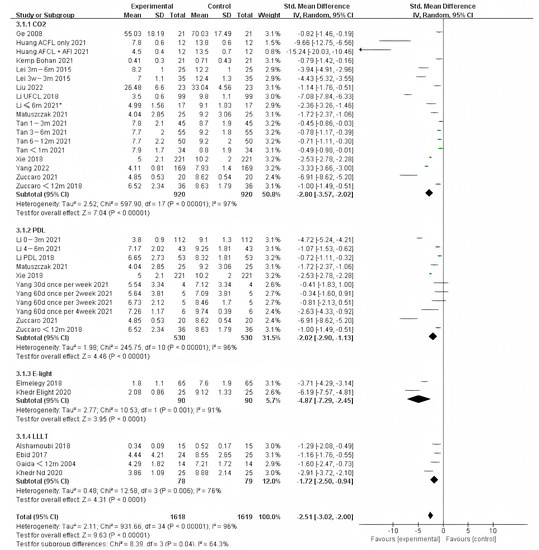
Figure 6.
Forest plot showing the effects of different photo-electric therapies for burn scars within 1 year. Each trial is represented by a green point, and the size of the point is proportional to the information in that trial. The ends of the horizontal bars denote 95% confidence intervals (CIs). Black diamonds give the overall results of all trials. * In order to distinguish between the two Li’s articles published in 2021, we have marked this one with an asterisk [43].
3.4. Publication Bias
Our assessment showed no evidence of significant publication bias based on formal statistical tests (Egger’s test, p = 0.056 > 0.05).
4. Discussion
In this study, we found that treatments for scarring over six months and one year have significant improvement differences in general presentation compared to other periods of scarring. The formation of scarring can be divided into three stages: inflammation, proliferation, and remodeling. During the first few days after an injury, corresponding to the inflammation stage, a variety of chemokines and vessel active mediators are produced at the site of the injury [70]. Then, in the proliferation stage, vessel active mediators, such as vascular endothelial growth factor (VEGF), from the previous stage induce microvascular scar tissue, which leads to scar proliferation [71,72,73]. The degree of microvascular density and scar hyperplasia are positively correlated [74]. Therefore, theoretically, intervention in this period can reduce angiogenesis, which can help relieve pruritus, contracture scars, and prevent scar growth and dysfunction. Histological analysis showed that the density of blood vessels in scar tissue increases significantly starting at one month after wound healing [74]. Then, hypertrophic scars generally develop in 2~6 months [27]. However, the results vary greatly between individuals due to different factors. Notably, burn scarring, the time of healing, and remodeling can be prolonged [30], so the best time for intervention in burn scarring is within one year, as shown by our results; interventions may also need to be personalized. Poetschke, J et al. [28] published a similar review on the treatment of immature scarring and concluded that a treatment algorithm should be formulated according to each patient’s needs. In conflict with our results, Brewin, M. P et al. [27] proposed that the treatment of PDL should begin before six months, when the scarring remains immature. This difference depends on the definition of immature scarring. As we mentioned, the duration of immature scarring varies greatly between individuals, making it difficult to clearly determine the ideal intervention time. Thus, the best way to deliver treatment is to follow-up with the patient as early as possible and avoid starting treatment too late. Treatment within one year is a good choice based on our results. Lastly, in the remodeling phase, the scar no longer presents redness, and for a hypertrophic or keloid scar, the scar may continue to thicken. This agrees with our outcome that in terms of thickness, photo-electric therapy corresponds to significant differences in improvement of the scar over one year.
In our study, treatment with the addition of IPL offered better improvements than other devices, especially for burn scars treated within one year. IPL therapy is non-invasive, non-surgical, and preliminarily filtered, forming an intense light with a wavelength of 400 to 1200 nm. IPL is not a laser but has similar characteristics to a laser [72]. Through the function of selective photothermolysis, light energy is absorbed by chromophore oxyhemoglobin, which is abundant in the blood vessels, causing photocoagulation of the vascular endothelium. This chromophore can also be absorbed by melanin in the epidermis. Thus, after the application of IPL, melanosomes in the epidermal melasma quickly move to the surface of the skin, undergo desquamation, and take the form of tiny crusts. Li. N et al. [75] used IPL to treat 35 Chinese patients who had a history of skin burns within the past year. The results showed that IPL is effective and safe in Chinese patients with postburn hyperpigmentation and telangiectasia. Meanwhile, as the maximum absorption by collagen occurs in the visible and near-infrared spectra [76], the light can also be absorbed by collagen. It was further confirmed that, with IPL, the activity of fibroblasts is increased, causing upregulation of type-I and type-III collagens at the mRNA and protein levels and rearranging elastin fibers both in vitro and in vivo [77,78]. However, we did not find a convincing systematic review that evaluated the effectiveness of IPL. In the systematic review of Vrijman, C et al. [76], the authors did not find any evidence for the efficacy of IPL therapy, as no study met the inclusion criteria. Zuccaro, Jv [24] found only one study about IPL, which reported mild-to-significant improvement in scarring.
With the development of technology, filtering narrow-spectrum intense pulsed light (DPL) of 500–600 nm through the spectrum at both ends can make treatment energy more concentrated; when the spot is large and uniform, the energy is lower and can more effectively protect normal skin tissue around the scar. This method offers the precision of a laser and the safety of strong pulsed light, greatly improving the curative effects. DPL still contains the absorption peak of hemoglobin, reduces the absorption of light by other tissue, and can use higher energy to block blood vessels; DPL can also inhibit angiogenesis, is more specific than IPL, and leads to less pain than PDL [72,79]. Zhang et al. [79] used DPL to treat 90 patients with scars after 3 weeks but within 1 year. After treatment for 3 months, the pruritus of scars was obviously alleviated. The degree of microvascular regeneration was related to the formation of erythema in the scar, which, in turn, became taller and harder, as well as the level of the hypertrophy of the scar [80]. As a result, we suggest that once the scar heals and appears red, photo-electric therapy that targets neovascularization should be started.
At the same time, the effectiveness of other methods cannot be ignored. Our study still showed a great effect of CO2AFL, PDL, LLLT, and RF on scar appearance. The CO2AFL can create 3D microthermal damage zones, thereby decreasing wound repair time and adverse reactions. Additionally, the use of fractional carbon dioxide laser treatment for hypertrophic scars can promote a decrease in type I collagen in scar tissue and an increase in type III collagen, which is closer to the collagen structure of normal skin tissue [81]. This treatment can also cause damage to blood vessels, producing scar ischemia and releasing collagenase to break down collagen, while the thermal effect of lasers can also stimulate collagen synthesis and remodeling, which helps to promote collagen remodeling as well as improve the appearance of scars [50]. A recent study compared the effects of starting CO2AFL treatment at multiple times after injury. One month after the last treatment, the results showed that CO2AFL was more effective for scars after more than 12 months in terms of height and pliability. However, for hardness and redness, scars at 1–3 months presented better results than other groups. The authors suggested that the ideal time point for the initiation of early fractional laser treatment could be within 1 month after injury [38]. However, there are some common side effects of CO2AFL, including erythema, seepage, bleeding, swelling, pigmentation, and deterioration of scarring [38,82]. Lower density with moderate laser energy for treating scars was proposed to avoid such problems.
PDL (pulsed dye laser) is the most widely used and effective type of laser for preventing early scarring [83]. According to the principle of selective photothermolysis, hemoglobin has two absorption peaks at 542 and 578 nm. Therefore, a laser at 585 nm will have a noticeable effect on eliminating blood vessels. Meanwhile, through the function of photothermolysis, collagen fibers are heated, and the disulfide bonds are broken, enabling them to be catabolized. In this way, collagen over-deposition can be prevented, stimulating collagen remodeling and allowing for the structure of scar epithelial tissue to be reconstructed [46,84]. However, the efficacy of PDL is limited by the thickness of the lesion. PDL penetrates to a depth of approximately 1.2 mm [85]. The most common side effect of PDL is postdelivery purpura, which persist for up to 7–10 days [41]. When the PDL energy is too high, pigment loss can easily occur [27]. Thus, to treat deeper lesions and reduce the side effects at the same time, more methods for combining other treatments with PDL need to be developed. Recently, Naoaki Rikihisa et al. [86] found that intravenous preadministration of carbonyl hemoglobin vesicles (CO-HbVs) followed by the application of vascular selective laser irradiation to the blood vessels of rabbit pinna reduced thermal damage to the perivascular tissue and partially enhanced vascular damage. In combination with Nd:YAG, PDL first changes hemoglobin into methemoglobin, which can absorb more energy from the Nd:YAG laser and thus penetrate more deeply [84].
LLLT treats scars in a different way by acting on the skin through photobiomodulation (PBM), which is an efficient and safe therapeutic modality for postburn scars. LLLT was found to suppress the viability of fibroblasts, inhibit the proliferation and formation of collagen in skin, and increase apoptosis of fibroblasts through mitochondria [87,88]. LLLT can also improve macrophage migration and phagocytosis independently of TGF-β signaling [89]. At present, LLLT is known to cause no side effects, which is a great advantage in early scar treatment [48]. It is known with certainty that LLLT promotes beneficial effects in the early stages of burn injury. However, we still need more basic and clinical studies to understand the relevant mechanisms and direct the best parameters for each type of burn, each type of skin, and each stage of scarring.
Finally, the mechanism underlying the RF stimulation of collagen fiber remodeling is likely protein denaturation caused by the effects of heat followed by the stimulation of collagen synthesis due to the increased expression of heat shock proteins [90]. With the development of fractional technology, in 2010, fractional microplasma radiofrequency technology (FMRT) was developed and initially used for the treatment of facial scars and rhytids [91]. Pinheiro et al. [92] compared the histological examination of postburn hypertrophic scar tissue treated with RF. The results showed that the treated area featured collagen fiber density in the papillary and reticular dermis similar to that of normal skin. This density was significantly greater in the area with no RF treatment [93]. However, the thermal effects of radiofrequency occur in the deeper layers of the skin, so short-term irritation, edema, and even burns can occur [94]. Additionally, numbness and sensory dullness can occur when thermal coagulation leads to demyelination of sensory nerves [95], so more experienced operators are needed for treatment.
5. Conclusions
In conclusion, this study indicated that treatment starting between six months and one year after injury had better outcomes in terms of the general presentation of scarring. Meanwhile, using IPL for burn scarring treatment seems to have better effects than other methods, especially for scarring within one year. We suggest using IPL and, especially, DPL for the treatment of early burn scarring. Notably, the scientific evidence in this area remains insufficient. We need more clinical trials of higher quality and less heterogeneity to confirm our results.
6. Limitations
There are still some limitations to this study. First, the majority of studies we included used pretreatment controls. Most burn scars improve over time, but concurrent control experiments in this area are extremely scarce. There is also a lack of higher-quality clinical trials, such as RCTs, and an inability to apply a double-blinded method for laser therapy. Meanwhile, some studies featured very short follow-ups. Significant heterogeneity was also observed among the studies included in this systematic review, including in the parameters, the application of different treatments, and the lack of general assessments evaluating burn scars. As a result, more well-performed, larger RCTs need to be carried out. The various evaluation criteria (POSAS for the patient and observer, VSS, etc.) also need to be standardized to further verify our results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ebj4020013/s1, Figure S1: Forest plot showing the effects of photo-electric therapy for burn scars according to VSS, POSAS, thickness, and VAS for the group with scarring for over one year and the group with scarring between six months and one year; File S1: the PRISMA-2020-Checklist [96,97,98].
Author Contributions
Conceptualization, M.Y.; methodology, Y.B. and Y.Z.; validation, Y.B., Y.Z. and W.N.; data curation, Y.B., Y.Z. and W.N.; data analysis, Y.B. and Y.Z.; writing—original draft preparation, Y.B.; writing—review and editing, Y.B.; Y.Z. and M.Y.; project administration, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Local High Level University Construction Program at Shanghai Jiao Tong University School of medicine.
Data Availability Statement
The original data are included in the article. Further information can be obtained from the corresponding author.
Acknowledgments
We would like to thank all the scientists who provided us with the detailed experimental data and Nicolas G. who helped us significantly.
Conflicts of Interest
The authors state no conflict of interest.
References
- Peck, M.D.; Kruger, G.E.; Van Der Merwe, A.E.; Godakumbura, W.; Ahuja, R.B. Burns and fires from non-electric domestic appliances in low and middle income countries Part I. The scope of the problem. Burns 2008, 34, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Forjuoh, S.N. Burns in low- and middle-income countries: A review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns 2006, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 2014, 40, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, M.C.; van der Veer, W.M.; Ulrich, M.M.; van Zuijlen, P.P.; Niessen, F.B.; Middelkoop, E. Prevention and curative management of hypertrophic scar formation. Burns 2009, 35, 463–475. [Google Scholar] [CrossRef]
- Oosterwijk, A.M.; Mouton, L.J.; Schouten, H.; Disseldorp, L.M.; van der Schans, C.P.; Nieuwenhuis, M.K. Prevalence of scar contractures after burn: A systematic review. Burns 2017, 43, 41–49. [Google Scholar] [CrossRef]
- Zuber, T.J.; DeWitt, D.E. Earlobe keloids. Am. Fam. Physician 1994, 49, 1835–1841. [Google Scholar]
- Hatamipour, E.; Mehrabi, S.; Hatamipour, M.; Ghafarian Shirazi, H.R. Effects of combined intralesional 5-Fluorouracil and topical silicone in prevention of keloids: A double blind randomized clinical trial study. Acta Med. Iran. 2011, 49, 127–130. [Google Scholar]
- Hassel, J.C.; Loser, C.; Koenen, W.; Kreuter, A.; Hassel, A.J. Promising results from a pilot study on compression treatment of ear keloids. J. Cutan. Med. Surg. 2011, 15, 130–136. [Google Scholar] [CrossRef]
- Nast, A.; Eming, S.; Fluhr, J.; Fritz, K.; Gauglitz, G.; Hohenleutner, S.; Panizzon, R.G.; Sebastian, G.; Sporbeck, B.; Koller, J.; et al. German S2k guidelines for the therapy of pathological scars (hypertrophic scars and keloids). J. Dtsch. Dermatol. Ges. 2012, 10, 747–762. [Google Scholar] [CrossRef]
- Robles, D.T.; Berg, D. Abnormal wound healing: Keloids. Clin. Dermatol. 2007, 25, 26–32. [Google Scholar] [CrossRef]
- Poochareon, V.N.; Berman, B. New therapies for the management of keloids. J. Craniofac. Surg. 2003, 14, 654–657. [Google Scholar] [CrossRef]
- Gold, M.H.; Berman, B.; Clementoni, M.T.; Gauglitz, G.G.; Nahai, F.; Murcia, C. Updated international clinical recommendations on scar management: Part 1—Evaluating the evidence. Dermatol. Surg. 2014, 40, 817–824. [Google Scholar]
- Gold, M.H.; McGuire, M.; Mustoe, T.A.; Pusic, A.; Sachdev, M.; Waibel, J.; Murcia, C.; International Advisory Panel on Scar, M. Updated international clinical recommendations on scar management: Part 2—Algorithms for scar prevention and treatment. Dermatol. Surg. 2014, 40, 825–831. [Google Scholar] [CrossRef]
- Ahuja, R.B.; Chatterjee, P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns 2014, 40, 583–588. [Google Scholar] [CrossRef]
- Grisolia, G.A.; Danti, D.A.; Santoro, S.; Panozzo, G.; Bonini, G.; Pampaloni, A. Injection therapy with triamcinolone hexacetonide in the treatment of burn scars in infancy: Results of 44 cases. Burns 1983, 10, 131–134. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, Y.K.; Qing, C.; Lu, S.L. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann. Plast. Surg. 2009, 63, 688–692. [Google Scholar]
- Trisliana Perdanasari, A.; Torresetti, M.; Grassetti, L.; Nicoli, F.; Zhang, Y.X.; Dashti, T.; Di Benedetto, G.; Lazzeri, D. Intralesional injection treatment of hypertrophic scars and keloids: A systematic review regarding outcomes. Burn. Trauma 2015, 3, 14. [Google Scholar] [CrossRef]
- Fredman, R.; Tenenhaus, M. Cushing’s syndrome after intralesional triamcinolone acetonide: A systematic review of the literature and multinational survey. Burns 2013, 39, 549–557. [Google Scholar] [CrossRef]
- Ragoowansi, R.; Cornes, P.G.; Moss, A.L.; Glees, J.P. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast. Reconstr. Surg. 2003, 111, 1853–1859. [Google Scholar] [CrossRef]
- Cho, Y.S.; Jeon, J.H.; Hong, A.; Yang, H.T.; Yim, H.; Cho, Y.S.; Kim, D.H.; Hur, J.; Kim, J.H.; Chun, W.; et al. The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: A randomized controlled trial. Burns 2014, 40, 1513–1520. [Google Scholar] [CrossRef]
- Willows, B.M.; Ilyas, M.; Sharma, A. Laser in the management of burn scars. Burns 2017, 43, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Williams, E.A.; Pham, C.H.; Collier, Z.J.; Dang, J.; Yenikomshian, H.A.; Gillenwater, T.J. Fractional CO2 laser treatment for burn scar improvement: A systematic review and meta-analysis. Burns 2021, 47, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Mahar, P.D.; Spinks, A.B.; Cleland, H.; Bekhor, P.; Waibel, J.S.; Lo, C.; Goodman, G. Improvement of Burn Scars Treated with Fractional Ablative CO2 Lasers-A Systematic Review and Meta-analysis Using the Vancouver Scar Scale. J. Burn Care Res. 2021, 42, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, J.; Ziolkowski, N.; Fish, J. A Systematic Review of the Effectiveness of Laser Therapy for Hypertrophic Burn Scars. Clin. Plast. Surg. 2017, 44, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.A.; Shupp, J.; Fernandez, S.; Prindeze, N.; DeKlotz, C.M.C. Effectiveness of Early Laser Treatment in Surgical Scar Minimization: A Systematic Review and Meta-analysis. Dermatol. Surg. 2020, 46, 402–410. [Google Scholar] [CrossRef]
- Behrouz-Pirnia, A.; Liu, H.; Peternel, S.; Dervishi, G.; Labeit, A.; Peinemann, F. Early laser intervention to reduce scar formation in wound healing by primary intention: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 528–536. [Google Scholar] [CrossRef]
- Brewin, M.P.; Lister, T.S. Prevention or treatment of hypertrophic burn scarring: A review of when and how to treat with the pulsed dye laser. Burns 2014, 40, 797–804. [Google Scholar] [CrossRef]
- Poetschke, J.; Gauglitz, G.G. Treatment of Immature Scars: Evidence-Based Techniques and Treatments. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Santer, M.; Kloppenburg, M.; Gottfried, T.A.-O.; Runge, A.; Schmutzhard, J.; Vorbach, S.M.; Mangesius, J.A.-O.; Riedl, D.A.-O.; Mangesius, S.A.-O.; Widmann, G.; et al. Current Applications of Artificial Intelligence to Classify Cervical Lymph Nodes in Patients with Head and Neck Squamous Cell Carcinoma—A Systematic Review. Cancers 2022, 14, 5397. [Google Scholar] [CrossRef]
- Chinese Association of Plastics; Aesthetics Scar Medicine Branch. National expert consensus on early management of scars (2020 version). Zhonghua Shao Shang Za Zhi 2021, 37, 113–125. [Google Scholar]
- Elrod, J.; Schiestl, C.; Neuhaus, D.; Mohr, C.; Neuhaus, K. Patient- and Physician-Reported Outcome of Combined Fractional CO2 and Pulse Dye Laser Treatment for Hypertrophic Scars in Children. Ann. Plast. Surg. 2020, 85, 237–244. [Google Scholar] [CrossRef]
- Ge, X.; Sun, Y.; Lin, J.; Zhou, F.; Yao, G.; Su, X. Effects of multiple modes of UltraPulse fractional CO2 laser treatment on extensive scarring: A retrospective study. Lasers Med. Sci. 2022, 37, 1575–1582. [Google Scholar] [CrossRef]
- Hultman, C.S.; Edkins, R.E.; Wu, C.; Calvert, C.T.; Cairns, B.A. Prospective, before-after cohort study to assess the efficacy of laser therapy on hypertrophic burn scars. Ann. Plast. Surg. 2013, 70, 521–526. [Google Scholar] [CrossRef]
- Khandelwal, A.; Yelvington, M.; Tang, X.; Brown, S. Ablative fractional photothermolysis for the treatment of hypertrophic burn scars in adult and pediatric patients: A single surgeon’s experience. J. Burn Care Res. 2014, 35, 455–463. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Cheng, J.; Han, J.; Yang, X.; Zheng, Z.; Guan, H.; Hu, D. A retrospective study to identify the optimal parameters for pulsed dye laser in the treatment of hypertrophic burn scars in Chinese children with Fitzpatrick skin types III and IV. Lasers Med. Sci. 2021, 36, 1671–1679. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Cheng, J.; Han, J.T.; Hu, D.H. Clinical comparative study of pulsed dye laser and ultra-pulsed fractional carbon dioxide laser in the treatment of hypertrophic scars after burns. Zhonghua Shao Shang Za Zhi 2018, 34, 603–607. [Google Scholar] [CrossRef]
- Patel, S.P.; Nguyen, H.V.; Mannschreck, D.; Redett, R.J.; Puttgen, K.B.; Stewart, F.D. Fractional CO2 Laser Treatment Outcomes for Pediatric Hypertrophic Burn Scars. J. Burn Care Res. 2019, 40, 386–391. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, J.; Huang, L.; Fu, Q.; Ao, M.; Yuan, L.; Luo, G. Hypertrophic Scar Improvement by Early Intervention with Ablative Fractional Carbon Dioxide Laser Treatment. Lasers Surg. Med. 2021, 53, 450–457. [Google Scholar] [CrossRef]
- Travis, T.E.; Allely, R.A.; Johnson, L.S.; Shupp, J.W. A Single-Institution Experience with Standardized Objective and Subjective Scar Evaluation While Undergoing Fractional Ablative Carbon Dioxide Laser Treatment. J. Burn Care Res. 2022, 43, 61–69. [Google Scholar] [CrossRef]
- Woods, J.F.C.; Kirkham, J.; Shelley, O.P. Treatment of Postburn Scar Erythema and Dyschromia with Pulsed Dye and Q-Switched KTP Laser. Dermatol. Surg. 2022, 48, 700–702. [Google Scholar] [CrossRef]
- Xie, W.G.; Lei, F.; Wang, J.; Xu, J.; Ruan, J.J.; Li, Z. Clinical effects of sequential laser treatments on early stage hypertrophic burn scars. Zhonghua Shao Shang Za Zhi 2018, 34, 615–623. [Google Scholar] [CrossRef]
- Zuccaro, J.; Kelly, C.; Perez, M.; Doria, A.; Fish, J.S. The Effectiveness of Laser Therapy for Hypertrophic Burn Scars in Pediatric Patients: A Prospective Investigation. J. Burn Care Res. 2021, 42, 847–856. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Cheng, J.; Han, J.; Hu, D. Early intervention by Z-plasty combined with fractional CO2 laser therapy as a potential treatment for hypertrophic burn scars. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 3087–3093. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shu, F.; Xu, H.; Ji, C.; Wang, Y.; Lou, X.; Luo, P.; Xiao, S.; Xia, Z.; Lv, K. Ablative fractional carbon dioxide laser improves quality of life in patients with extensive burn scars: A nested case-control study. Lasers Surg. Med. 2022, 54, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Issler-Fisher, A.C.; Fisher, O.M.; Haertsch, P.A.; Li, Z.; Maitz, P.K.M. Effectiveness and safety of ablative fractional CO2 laser for the treatment of burn scars: A case-control study. Burns 2021, 47, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, N.; Cheng, J.; Han, J.T.; Hu, D.H. A prospective randomized controlled clinical study on the optimal treatment interval of pulsed dye laser in treating hypertrophic scar after burn. Zhonghua Shao Shang Za Zhi 2021, 37, 57–63. [Google Scholar] [CrossRef]
- Tawfic, S.O.; Hassan, A.S.; El-Zahraa Sh Aly, F.; Elbendary, A.; Shaker, O.G.; AlOrbani, A.M. Fractional microneedle radiofrequency versus fractional carbon dioxide laser in the treatment of postburn hypertrophic scars. Lasers Surg. Med. 2022, 54, 1089–1098. [Google Scholar] [CrossRef]
- Alsharnoubi, J.; Shoukry, K.E.; Fawzy, M.W.; Mohamed, O. Evaluation of scars in children after treatment with low-level laser. Lasers Med. Sci. 2018, 33, 1991–1995. [Google Scholar] [CrossRef]
- Ebid, A.A.; Ibrahim, A.R.; Omar, M.T.; El Baky, A.M.A. Long-term effects of pulsed high-intensity laser therapy in the treatment of post-burn pruritus: A double-blind, placebo-controlled, randomized study. Lasers Med. Sci. 2017, 32, 693–701. [Google Scholar] [CrossRef]
- Douglas, H.; Lynch, J.; Harms, K.A.; Krop, T.; Kunath, L.; van Vreeswijk, C.; McGarry, S.; Fear, M.W.; Wood, F.M.; Murray, A.; et al. Carbon dioxide laser treatment in burn-related scarring: A prospective randomised controlled trial. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 863–870. [Google Scholar] [CrossRef]
- El-Zawahry, B.M.; Sobhi, R.M.; Bassiouny, D.A.; Tabak, S.A. Ablative CO2 fractional resurfacing in treatment of thermal burn scars: An open-label controlled clinical and histopathological study. J. Cosmet. Dermatol. 2015, 14, 324–331. [Google Scholar] [CrossRef]
- Gaida, K.; Koller, R.; Isler, C.; Aytekin, O.; Al-Awami, M.; Meissl, G.; Frey, M. Low Level Laser Therapy—A conservative approach to the burn scar? Burns 2004, 30, 362–367. [Google Scholar] [CrossRef]
- Joo, S.Y.; Cho, Y.S.; Lee, S.Y.; Seo, C.H. Regenerative effect of combined laser and human stem cell-conditioned medium therapy on hypertrophic burn scar. Burns 2022. [Google Scholar] [CrossRef]
- Kono, T.; Ercocen, A.R.; Nakazawa, H.; Nozaki, M. Treatment of hypertrophic scars using a long-pulsed dye laser with cryogen-spray cooling. Ann. Plast. Surg. 2005, 54, 487–493. [Google Scholar] [CrossRef]
- El-Hoshy, K.; Abdel-Halim, M.R.E.; Dorgham, D.; El-Din Sayed, S.S.; El-Kalioby, M. Efficacy of Fractional Carbon Dioxide Laser in the Treatment of Mature Burn Scars: A Clinical, Histopathological, and Histochemical Study. J. Clin. Aesthet. Dermatol. 2017, 10, 36–43. [Google Scholar]
- Elmelegy, N.G.; Hegazy, A.M.; Sadaka, M.S.; Abdeldaim, D.E. Electrophotobiomodulation in the treatment of facial post-burn hypertrophic scars in pediatric patients. Ann. Burn. Fire Disasters 2018, 31, 127–132. [Google Scholar]
- Huang, Z.; Chen, Y.; Wang, P.; Zheng, D.W.; Zong, Y.L.; Lyu, G.Z. A prospective randomized controlled clinical study on the treatment of hypertrophic scar after burn by fractional carbon dioxide laser combined with autologous fat injection. Zhonghua Shao Shang Za Zhi 2021, 37, 49–56. [Google Scholar] [CrossRef]
- Kemp Bohan, P.M.; Cooper, L.E.; Lu, K.N.; Raper, D.M.; Batchinsky, M.; Carlsson, A.H.; Cancio, L.C.; Chan, R.K. Fractionated Ablative Carbon Dioxide Laser Therapy Decreases Ultrasound Thickness of Hypertrophic Burn Scar: A Prospective Process Improvement Initiative. Ann. Plast. Surg. 2021, 86, 273–278. [Google Scholar] [CrossRef]
- Khedr, M.M.; Mahmoud, W.H.; Sallam, F.A.; Elmelegy, N. Comparison of Nd: YAG Laser and Combined Intense Pulsed Light and Radiofrequency in the Treatment of Hypertrophic Scars: A Prospective Clinico-Histopathological Study. Ann. Plast. Surg. 2020, 84, 518–524. [Google Scholar] [CrossRef]
- Lei, J.; Hao, Z.; Yu, L.; Duan, P.; Meng, Y. Clinical observation of the effects of lattice ultra pulse carbon dioxide laser combined with traditional Chinese medicine on the treatment of hyperplastic scar. Chin. J. Burn. 2015, 31, 164–167. [Google Scholar] [CrossRef]
- Majid, I.; Imran, S. Fractional Carbon Dioxide Laser Resurfacing in Combination with Potent Topical Corticosteroids for Hypertrophic Burn Scars in the Pediatric Age Group: An Open Label Study. Derm. Surg. 2018, 44, 1102–1108. [Google Scholar] [CrossRef]
- Matuszczak, E.; Weremijewicz, A.; Koper-Lenkiewicz, O.M.; Kaminska, J.; Hermanowicz, A.; Debek, W.; Komarowska, M.; Tylicka, M. Effects of combined Pulsed Dye Laser and Fractional CO2 Laser treatment of burn scars and correlation with plasma levels of collagen type I, MMP-2 and TIMP-1. Burns 2021, 47, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Miletta, N.; Siwy, K.; Hivnor, C.; Clark, J.; Shofner, J.; Zurakowski, D.; Anderson, R.R.; Lee, K.; Donelan, M. Fractional Ablative Laser Therapy is an Effective Treatment for Hypertrophic Burn Scars: A Prospective Study of Objective and Subjective Outcomes. Ann. Surg. 2021, 274, e574–e580. [Google Scholar] [CrossRef] [PubMed]
- Poetschke, J.; Dornseifer, U.; Clementoni, M.T.; Reinholz, M.; Schwaiger, H.; Steckmeier, S.; Ruzicka, T.; Gauglitz, G.G. Ultrapulsed fractional ablative carbon dioxide laser treatment of hypertrophic burn scars: Evaluation of an in-patient controlled, standardized treatment approach. Lasers Med. Sci. 2017, 32, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Tawfic, S.; Sayed, S.; Nada, A.; Manaa, D.; Shalaby, S. High-Versus Low-Density Fractional Laser in the Treatment of Hypertrophic Postburn Scars: A Randomized Clinical Trial. Dermatol. Surg. 2020, 46, e38–e44. [Google Scholar] [CrossRef]
- Wang, S.; Mi, J.; Li, Q.; Jin, R.; Dong, J. Fractional microplasma radiofrequency technology for non-hypertrophic post-burn scars in Asians: A prospective study of 95 patients. Lasers Surg. Med. 2017, 49, 563–569. [Google Scholar] [CrossRef]
- Weshahy, R.H.; Aly, D.G.; Shalaby, S.; Mohammed, F.N.; Sayed, K.S. Clinical and Histological Assessment of Combined Fractional CO2 Laser and Growth Factors Versus Fractional CO2 Laser Alone in the Treatment of Facial Mature Burn Scars: A Pilot Split-Face Study. Lasers Surg. Med. 2020, 52, 952–958. [Google Scholar] [CrossRef]
- Yang, J.; Shi, S.; Wang, L.; Li, N.; Han, J.T.; Hu, D.H. A prospective randomized controlled study on the effects of compound analgesia in ultra-pulsed fractional carbon dioxide laser treatment of post-burn hypertrophic scars in children. Zhonghua Shao Shang Za Zhi 2022, 38, 683–690. [Google Scholar] [CrossRef]
- Zuccaro, J.; Muser, I.; Singh, M.; Yu, J.; Kelly, C.; Fish, J. Laser Therapy for Pediatric Burn Scars: Focusing on a Combined Treatment Approach. J. Burn Care Res. 2018, 39, 457–462. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Cancer as an overhealing wound: An old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 2008, 9, 628–638. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Yang, R.; Ding, X.; Li, Y.; Liu, H.; Yan, H. Blood perfusion in hypertrophic scars and keloids studied by laser speckle contrast imaging. Skin Res. Technol. 2021, 27, 789–796. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Li, X. Research progress of DPL in early prevention and treatment of hypertrophic scar after burn in children. J. Tissue Eng. Reconstr. Surg. 2021, 17, 497–502. [Google Scholar] [CrossRef]
- Fajardo, L.F.; Kwan, H.H.; Kowalski, J.; Prionas, S.D.; Allison, A.C. Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 1992, 140, 539–544. [Google Scholar]
- Jaspers, M.E.H.; Stekelenburg, C.M.; Simons, J.M.; Brouwer, K.M.; Vlig, M.; van den Kerckhove, E.; Middelkoop, E.; van Zuijlen, P.P.M. Assessing blood flow, microvasculature, erythema and redness in hypertrophic scars: A cross sectional study showing different features that require precise definitions. Burns 2017, 43, 1044–1050. [Google Scholar] [CrossRef]
- Li, N.; Han, J.; Hu, D.; Cheng, J.; Wang, H.; Wang, Y.; Yang, X.; Liu, J.; Li, T.; Zhao, W. Intense pulsed light is effective in treating postburn hyperpigmentation and telangiectasia in Chinese patients. J. Cosmet. Laser Ther. 2018, 20, 436–441. [Google Scholar] [CrossRef]
- Vrijman, C.; van Drooge, A.M.; Limpens, J.; Bos, J.D.; van der Veen, J.P.; Spuls, P.I.; Wolkerstorfer, A. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: A systematic review. Br. J. Dermatol. 2011, 165, 934–942. [Google Scholar] [CrossRef]
- Cao, Y.; Huo, R.; Feng, Y.; Li, Q.; Wang, F. Effects of intense pulsed light on the biological properties and ultrastructure of skin dermal fibroblasts: Potential roles in photoaging. Photomed. Laser Surg. 2011, 29, 327–332. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, J.; Gold, M.H. Skin rejuvenation in Asian skin: The analysis of clinical effects and basic mechanisms of intense pulsed light. J. Drugs Dermatol. 2008, 7, 273–279. [Google Scholar]
- Zhang, Y.; Dong, J.; Wang, C.; Yan, M.; Yao, M. Clinical effects of a combination treatment with narrow-spectrum intense pulsed light and fractional carbon dioxide laser on hypertrophic scar pruritus. Chin. J. Burns 2018, 34, 608–614. [Google Scholar] [CrossRef]
- Allison, K.P.; Kiernan, M.N.; Waters, R.A.; Clement, R.M. Pulsed dye laser treatment of burn scars. Alleviation or irritation? Burns 2003, 29, 207–213. [Google Scholar] [CrossRef]
- Makboul, M.; Makboul, R.; Abdelhafez, A.H.; Hassan, S.S.; Youssif, S.M. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-beta1 expression in hypertrophic scar. J. Cosmet. Dermatol. 2014, 13, 169–179. [Google Scholar] [CrossRef]
- Baroni, A.; Verolino, P. Plasma Radiofrequency Ablation for Scar Treatment. J. Clin. Med. 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Huang, X.; Li, H.; Yuan, Y.; Li, B.; Cheng, C.; Li, Q. Laser therapy for prevention and treatment of pathologic excessive scars. Plast. Reconstr. Surg. 2013, 132, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Jinping, D.; Bo, C.; Yilin, C. Research progress of laser treatment for pathological scar. J. Tissue Eng. Reconstr. Surg. 2016, 12, 141–143. [Google Scholar]
- Pan, L.; Qin, H.; Li, C.; Zhang, G.; Yang, L.; Zhang, L. Efficacy of the Neodymium-Doped Yttrium Aluminum Garnet Laser in the Treatment of Keloid and Hypertrophic Scars: A Systematic Review and Meta-analysis. Aesthet. Plast. Surg. 2022, 46, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, N.; Shimanouchi, K.; Saito, Y.; Sakai, H.; Mitsukawa, N. Carbon monoxide combined with artificial blood cells acts as an antioxidant for tissues thermally-damaged by dye laser irradiation. Burns 2023, 49, 388–400. [Google Scholar] [CrossRef]
- Shu, B.; Ni, G.X.; Zhang, L.Y.; Li, X.P.; Jiang, W.L.; Zhang, L.Q. High-power helium-neon laser irradiation inhibits the growth of traumatic scars in vitro and in vivo. Lasers Med. Sci. 2013, 28, 693–700. [Google Scholar] [CrossRef]
- Lev-Tov, H.; Brody, N.; Siegel, D.; Jagdeo, J. Inhibition of fibroblast proliferation in vitro using low-level infrared light-emitting diodes. Dermatol. Surg. 2013, 39, 422–425. [Google Scholar] [CrossRef]
- Khan, I.; Rahman, S.U.; Tang, E.; Engel, K.; Hall, B.; Kulkarni, A.B.; Arany, P.R. Author Correction: Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-beta1. Sci. Rep. 2021, 11, 17706. [Google Scholar] [CrossRef]
- Hantash, B.M.; Ubeid, A.A.; Chang, H.; Kafi, R.; Renton, B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg. Med. 2009, 41, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Wang, Y.; Du, Y.; Yu, S. Effectiveness and safety of fractional micro-plasma radio-frequency treatment combined with ablative fractional carbon dioxide laser treatment for hypertrophic scar: A retrospective study. Ann. Palliat. Med. 2021, 10, 9800–9809. [Google Scholar] [CrossRef]
- Vestita, M.; Filoni, A.; Elia, R.; Bonamonte, D.; Giudice, G. 595 nm Pulsed Dye Laser for Hypetrophic and Keloid Scars Treatment. A Randomized-Controlled Study. Plast. Reconstr. Surg. Glob. Open 2017, 5, 86–87. [Google Scholar] [CrossRef]
- Pinheiro, N.M.; Melo, P.R.; Crema, V.O.; Mendonça, A.C. Effects of radiofrequency procedure on hypertrophic scar due to burns. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 187–189. [Google Scholar] [CrossRef]
- Swanson, E. A Systematic Review of Subsurface Radiofrequency Treatments in Plastic Surgery. Ann. Plast. Surg. 2022, 89, 274–285. [Google Scholar] [CrossRef]
- Mulholland, R.S. The Science and Art of Radiofrequency Assisted Lipocoagulation (RFAL) in Body Contouring Surgery. In The Art of Body Contouring; Books on Demand: Paris, France, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence Work Group. The Oxford Levels of Evidence 2. Oxford Center of Evidence Based Medicine. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 20 January 2023).
- Cooper, L.E.; Nuutila, K.; Kemp Bohan, P.M.; Diaz, V.; Batchinsky, M.; Carlsson, A.H.; Cancio, L.C.; Chan, R.K. Analysis of the Utility of CO2 and Pulse-Dye Lasers Together and Separately in the Treatment of Hypertrophic Burn Scars. Ann. Plast. Surg. 2022, 89, 166–172. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).