Abstract
Herbivorous fishes play important functional roles in coral reef ecosystems, and their influence on mediating competitive dynamics between corals and macroalgae is well studied. Nonetheless, direct interactions between herbivorous fishes and corals may also be relevant, although these are less studied. Here, we describe a series of observations of schools of the herbivorous streaked rabbitfish (Siganus javus) nibbling on black corals (order Antipatharia) at the SS Yongala wreck, within the Great Barrier Reef Marine Park. We provide a hypothesis that may explain this behaviour, which, if confirmed, would represent a mechanism influencing the health of the corals. Moreover, this interaction extends the typical coral–algae competition for space paradigm and furthers knowledge of complex relationships between coral reef organisms.
1. Introduction
Coral reefs are among the most biodiverse and ecologically complex ecosystems on Earth. Understanding the myriad trophic interactions among coral reef species is important because these interactions mediate essential ecological processes and maintain reef ecosystem function (Brandl et al. 2019) [1]. For instance, herbivorous fishes influence the competitive dynamics between corals and macroalgae—the two dominant benthic taxa on shallow reefs—by consuming algae that compete for space with corals and other habitat-forming taxa (Bellwood et al. 2019) [2].
Rabbitfishes (family Siganidae) are among the most important herbivores on Indo-Pacific reefs, and are known to consume algae directly from the benthos and/or from the water column (e.g., Fox and Bellwood 2013, Streit et al. 2015) [3,4]. The herbivorous streaked rabbitfish (Siganus javus) occurs in low numbers on inshore and mid-shelf reefs across the Great Barrier Reef (GBR) (Cheal et al. 2012, Hoey et al. 2013) [5,6], and detailed studies of this species’ feeding behaviour are scarce. However, S. javus have been previously reported to feed on floating algal fragments and graze on algae attached to the substrate (Froese and Pauly 2023) [7]. Other Siganus species are thought to target epiphytes (finer algae on top of macroalgae); for instance, S. doliatus’ fast bite rates suggest that this species primarily targets epiphytes, while only incidentally removing macroalgae (Hoey and Bellwood 2009, Hoey et al. 2009) [6,8]. Similarly, schools of S. fuscenscens were observed grazing on turf algae growing on the surface of soft corals (Sarcophyton sp.) (Kuo et al. 2015) [9]. Despite Siganus being classified as herbivorous, in the Red Sea, S. rivulatus has been observed feeding on ctenophores and jellyfish (Aurelia aurita and Cephea cephea) (Cruz-Rivera and El-Regal 2016, Bos et al. 2017) [10,11].
Antipatharians—commonly known as black corals—are hexacorals (a group that includes hard corals, sea anemones and their relatives). Antipatharians inhabit most oceans at depths between 2 m and 8600 m (Roberts et al. 2009, Wagner et al. 2012) [12,13]. Empirical data on the habitat preferences of antipatharians are lacking, but they typically occur in moderate to strong current environments that provide abundant planktonic prey (Wagner et al. 2012) [13] and where sediment deposition is low (Grigg 1964, Fraser and Sedberry 2008) [14,15]. Antipatharians provide a habitat for numerous fishes and invertebrates (e.g., Boland and Parrish 2005, Tazioli et al. 2007, Suarez et al. 2015) [16,17,18], although details on the type of symbiosis or the interactions antipatharians have with fish and other invertebrates are very limited.
In general, direct interactions between the herbivorous fishes and sessile benthic invertebrates, in particular with black corals, have received little attention; however, such interactions likely play important functional roles in coral reef ecosystems. Here, we describe a series of observations of schools of the herbivorous S. javus nibbling on antipatharians and we provide a hypothesis that may explain this behaviour.
2. Materials and Methods
2.1. Field Site
The SS Yongala wreck sits between 14 m and 30 m depth ~ 90 km southeast of Townsville, Queensland, Australia (−19°18′16.20″ S, 147°37′18.59″ E). The shipwreck is a Maritime Heritage Site within the Great Barrier Reef Marine Park. The steamship sank in 1911, and over time, it has been colonised by a diverse and abundant benthic community dominated by antipatharians (Figure 1a) and a rich fish community (Malcolm et al. 1999) [19].
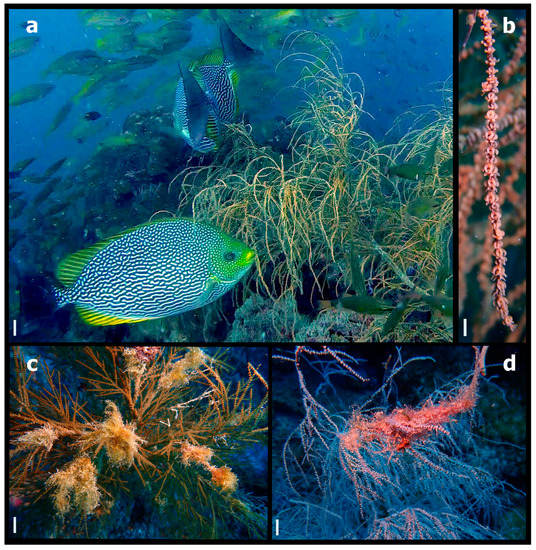
Figure 1.
(a) Rabbitfish (Siganus javus) nibbling on branches of a branching black coral colony (Antipathes cf. curvata). (b) Close-up of the polyps of one branch of a black coral colony (Antipathes cf. curvata) right after a rabbitfish (Siganus javus) had nibbled the branch. (c,d) Cyanobacteria and turf algae deposited on and smothering several branches of black coral colonies (Antipathes sp.). Scale bars: (a) = 2 cm, (b) = 1 cm, (c,d) = 5 cm.
2.2. Fish Observations
On a series of dives between May 2021 and January 2022 (~10 dives), we observed and took videos of schools of S. javus nibbling on the branches of antipatharians between 15 m and 26 m in depth (Figure 1b; Supplementary Materials, Video S1). These observations were opportunistic (i.e., the dives were conducted with other foremost aims).
2.3. Algae Coverage
We conducted visual transects to estimate the percentage cover of algae and/or cyanobacteria on antipatharians at the Yongala between May 2021 and January 2022. The transects were 110 m long and 2 m wide, and were carried out on both sides of the wreck at four depth ranges from the top of the wreck to the sea floor: 16–18 m, 19–21 m, 22–24 m, and 26–28 m. For each colony observed, we estimated the percentage cover of algae and/or cyanobacteria as one of five categories: <10%, 10–30%, 30–50%, 50–70%, and >70%.
3. Results and Discussion
The interaction between S. javus and antipatharians was observed on about 80% of the dives. S. javus schools ranged from two to ten fish and were observed nibbling on the coral branches for up to 2 min. Afterwards, the fish would swim away, although they would constantly return to the antipatharian colonies (Supplementary Materials, Video S1). Following fish departure, we examined the antipatharian branches in situ and we found that the polyps in the ‘grazed’ area remained intact (Figure 1b), suggesting that S. javus were not feeding on the coral polyps themselves. The area of the coral branches that the fish targeted was not overgrown by large algae or cyanobacteria, although the polyps could have been covered by fine epibiotic algae or biofilms—which are not always visible to the naked eye. Therefore, we propose that S. javus on the Yongala are feeding on epibiotic algae or biofilms deposited on the antipatharians’ polyps and/or the zooplankton that the polyp had trapped but not yet digested. To confirm this hypothesis, however, fish gut content and morphological analysis would be necessary. Additionally, feeding in very close proximity of coral tissues requires that fishes have a mechanism to protect themselves from the coral’s stinging nematocysts (Huertas and Bellwood 2017) [20], something that would also need to be investigated on S. javus.
Despite the Yongala often being exposed to strong currents, cyanobacteria and turf algae can accumulate on the corals (Figure 1c,d), and affect a large proportion of antipatharian colonies at all depths (Figure 2). Unlike scleractinian corals, antipatharians do not have corallites to retract their tentacles; therefore, sediments, algae and other particulate material can accumulate on top of the polyps (Wagner et al. 2012, Daly et al. 2003) [13,21] and smoother parts of or entire colonies (Figure 2). Thus, we posit that if the S. javus are targeting fouling material from the coral colonies, this would provide a service to antipatharians by preventing the further detrimental accumulation of filamentous algae and cyanobacteria on the polyps—which is usually the fate of initial depositions of fine films of algae or cyanobacteria (Diaz-Pulido and McCook 2002) [22]. At the Yongala, antipatharians are abundant and provide most of the structural habitat. Therefore, due to the importance of black corals for habitat provision at the site, this interaction would represent an important ecological process, even if not entirely preventing the deposition of algae and cyanobacteria on the colonies (Figure 2).
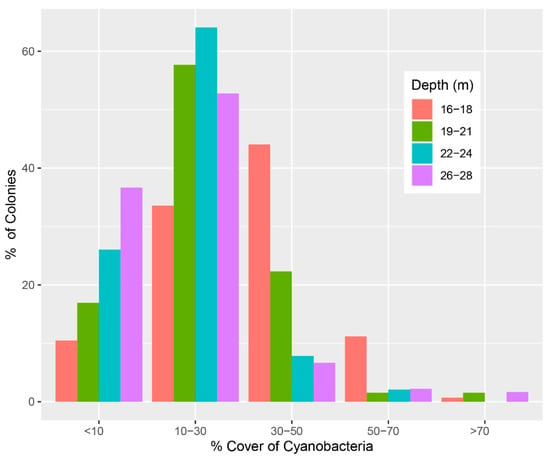
Figure 2.
Percentage of antipatharian (black coral) colonies at the SS Yongala with different percentages of turf algae and cyanobacteria coverage, across four depth ranges from the top of the wreck to the sea floor. The total number of colonies from all depths was used to estimate the percentage of colonies at each depth range. For brevity, only ‘Cyanobacteria’ is written on the x-axis label, referring to both cyanobacteria and turf algae.
On the other hand, it is possible that the fish are also taking zooplankton food from the polyps; this type of behaviour has been documented for fish from other groups. For instance, stomach content analysis proved that a Caristius sp. (Manfish) was taking food and eating pieces of its siphonophore host (Janssen et al. 1989) [23]. However, Caristius are carnivorous fish and our in situ observations of the coral polyps after S. javus had nibbled the branches corroborated that polyps were not being eaten or bitten (Figure 1b). Interestingly, we also did not observe grazing scars on the polyps (Figure 1b), as was the case for S. fuscensces, which left grazing scars on the soft corals after grazing the turf algae of the colonies (Kuo et al. 2015) [9]. Nonetheless, other Siganus species had been documented eating ctenophores and jellyfish, although they only appeared to be consuming pieces of the ctenophores and jellyfish, rather than stealing food and biting at the same time (Bos et al. 2017) [11]. While no stomach content examination was conducted in the study by Bos et al. (2017) [11] in the Red Sea, the rabbitfish only bite the bell of the jellyfish, not the tentacular area. Therefore, the hypothesis of the rabbitfish just targeting the epibiotic algae or biofilms on the polyps seems more plausible. We also observed S. javus feeding on floating algal fragments and grazing on algae attached to the substrate, a feeding behaviour that has previously been reported for this species (Froese and Pauly 2023) [7].
Notably, these adaptations in the feeding strategies of Siganus appear to be opportunistic, rather than a consequence of a shortage of food. Therefore, if S. javus were confirmed to also feed on epibiotic algae and/or zooplankton from the coral polyps, it would represent an ‘ecological opportunism’, which has been proposed as a major determinant of fish functional roles (Bellwood et al. 2019) [2]. Importantly, if zooplankton is confirmed to be part of the S. javus diet—and in addition to previous studies reporting other Siganus consuming ctenophores and jellyfish—their trophic group should be revised, and it will show that variations in the behavioural traits of rabbitfish individuals are still to be elucidated. While our hypothesis needs to be confirmed with further fish stomach content analysis, our observations suggest that the ecosystem services provided by herbivorous fish may extend beyond the typical coral–algae competition for space paradigm.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oceans4030016/s1, Video S1: Schools of rabbitfish (Siganus javus) nibbling on black coral (Antipathes cf. curvata) at the SS Yongala. Videographer: Erika Gress.
Author Contributions
Conceptualization, E.G.; data collection E.G. and J.F.; formal analysis, E.G., G.G. and T.C.B.; writing—original draft preparation, E.G.; writing—review and editing, E.G. and G.G.; funding acquisition, E.G. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for fieldwork contributing to this study was provided by the David Yellowlees Excellence in Research Award 2021 to E.G.
Institutional Review Board Statement
Ethical approval: No animal testing was performed during this study. Permit to conduct surveys: G20/44571.1.
Informed Consent Statement
Not applicable.
Data Availability Statement
Code and data are provided as private-for-peer-review via the following links, respectively: https://figshare.com/s/3fad35f48cb6b7d97105 (accessed on 9 July 2023), https://figshare.com/s/42563040adb4c4dfd088 (accessed on 9 July 2023).
Acknowledgments
We thank Paul Crocombe from Adrenalin Dive and the Great Barrier Reef Joint Field Management Program for providing vessel and logistical support. We also thank Victor Huertas for helpful comments during the early stages of manuscript preparation, and Aurora Philpin for support with data collection.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Brandl, S.J.; Rasher, D.B.; Côté, I.M.; Casey, J.M.; Darling, E.S.; Lefcheck, J.S.; Duffy, J.E. Coral reef ecosystem functioning: Eight core processes and the role of biodiversity. Front. Ecol. Environ. FEE 2019, 17, 445–454. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Streit, R.P.; Brandl, S.J.; Tebbett, S.B. The meaning of the term ‘function’ in ecology: A coral reef perspective. Funct. Ecol. 2019, 33, 948–961. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Niche partitioning of feeding microhabitats produces a unique function for herbivorous rabbitfishes (Perciformes, Siganidae) on coral reefs. Coral Reefs 2013, 32, 13–23. [Google Scholar] [CrossRef]
- Streit, R.P.; Hoey, A.S.; Bellwood, D.R. Feeding characteristics reveal functional distinctions among browsing herbivorous fishes on coral reefs. Coral Reefs 2015, 34, 1037–1047. [Google Scholar] [CrossRef]
- Cheal, A.; Emslie, M.; Miller, I.; Sweatman, H. The distribution of herbivorous fishes on the Great Barrier Reef. Mar. Biol. 2012, 159, 1143–1154. [Google Scholar] [CrossRef]
- Hoey, A.S.; Brandl, S.J.; Bellwood, D.R. Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: Implications for ecosystem function. Coral Reefs 2013, 32, 973–984. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Fishbase. World Wide Web Electronic Publication. 2023. Available online: https://www.fishbase.org (accessed on 15 February 2023).
- Hoey, A.S.; Bellwood, D.R. Limited Functional Redundancy in a High Diversity System: Single Species Dominates Key Ecological Process on Coral Reefs. Ecosystems 2009, 12, 1316–1328. [Google Scholar] [CrossRef]
- Kuo, F.W.; Kuo, C.Y.; Fan, T.Y.; Liu, M.C.; Chen, C.A. Hidden ecosystem function of rabbitfishes? Siganus fuscescens feeds on the soft coral, Sarcophyton sp. Coral Reefs 2015, 34, 57. [Google Scholar]
- Cruz-Rivera, E.; El-Regal, M.A. A bloom of an edible scyphozoan jellyfish in the Red Sea. Mar. Biodivers. 2016, 46, 515–519. [Google Scholar] [CrossRef]
- Bos, A.R.; Cruz-Rivera, E.; Sanad, A.M. Herbivorous fishes Siganus rivulatus (Siganidae) and Zebrasoma desjardinii (Acanthuridae) feed on Ctenophora and Scyphozoa in the Red Sea. Mar. Biodivers. 2017, 47, 243–246. [Google Scholar] [CrossRef]
- Roberts, M.J.; Wheeler, A.J.; Freiwald, A.; Cairns, S.D. Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Wagner, D.; Luck, D.G.; Toonen, R.J. The Biology and Ecology of Black Corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv. Mar. Biol. 2012, 63, 67–132. [Google Scholar]
- Grigg, R.W. A Contribution to the Biology and Ecology of the Black Coral, Antipathes grandis in Hawaii. Master’s Thesis, University of Hawai’i, Honolulu, HI, USA, 1964. [Google Scholar]
- Fraser, S.B.; Sedberry, G.R. Reef Morphology and Invertebrate Distribution at Continental Shelf Edge Reefs in the South Atlantic Bight. Southeast. Nat. 2008, 7, 191–206. [Google Scholar] [CrossRef]
- Boland, R.C.; Parrish, F.A. A Description of Fish Assemblages in the Black Coral Beds off Lahaina, Maui, Hawai‘i. Pac. Sci. 2005, 59, 411–420. [Google Scholar] [CrossRef]
- Tazioli, S.; Bo, M.; Boyer, M.; Rotinsulu, H.; Bavestrello, G. Ecological observations of some common antipatharian corals in the marine park of Bunaken (North Sulawesi, Indonesia). Zool. Stud. 2007, 46, 227–241. [Google Scholar]
- Suarez, H.N.; Dy, D.T.; Violanda, R.R. Density of associated macrofauna of black corals (Anthozoa: Antipatharia) in Jagna, Bohol, central Philippines. Philipp. J. Sci. 2015, 144, 107–115. [Google Scholar]
- Malcolm, H.A.; Cheal, A.J.; Thompson, A.A. Fishes of the Yongala Historic Shipwreck; CRC Reef Research Technical Report; CRC Reef Research Centre: Townsville, QLD, Australia, 1999. [Google Scholar]
- Huertas, V.; Bellwood, D.R. Mucus-Secreting Lips Offer Protection to Suction-Feeding Corallivorous Fishes. Curr. Biol. 2017, 33, R406–R407. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Fautin, D.G.; Cappola, V.A. Systematics of the Hexacorallia (Cnidaria: Anthozoa). Zool. J. Linn. Soc. 2003, 139, 419–437. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; McCook, L.J. The fate of bleached corals: Patterns and dynamics of algal recruitment. Mar. Ecol. Prog. Ser. 2002, 232, 115–128. [Google Scholar] [CrossRef]
- Janssen, J.; Gibbs, R.H.; Pugh, P.R. Association of Caristius Sp. (Pisces: Caristiidae) with a Siphonophore, Bathyphysa conifera. Copeia 1989, 1989, 198–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).